DSQ 4 Al 3 O 2 2 Al






- Slides: 21

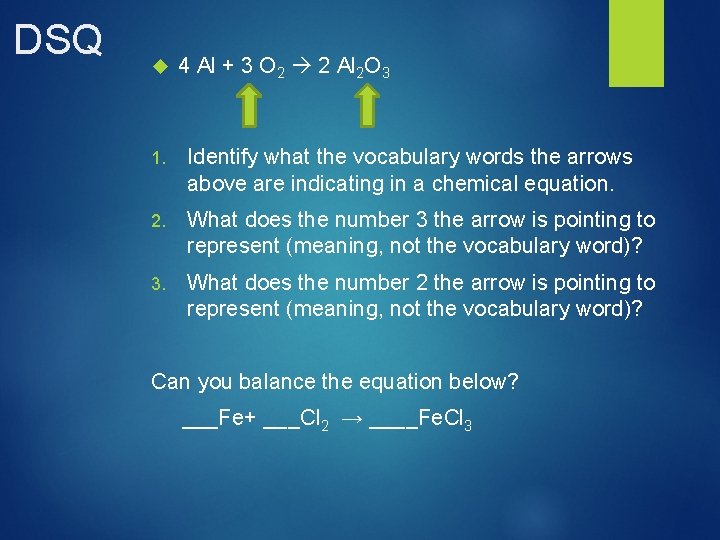
DSQ 4 Al + 3 O 2 2 Al 2 O 3 1. Identify what the vocabulary words the arrows above are indicating in a chemical equation. 2. What does the number 3 the arrow is pointing to represent (meaning, not the vocabulary word)? 3. What does the number 2 the arrow is pointing to represent (meaning, not the vocabulary word)? Can you balance the equation below? ___Fe+ ___Cl 2 → ____Fe. Cl 3

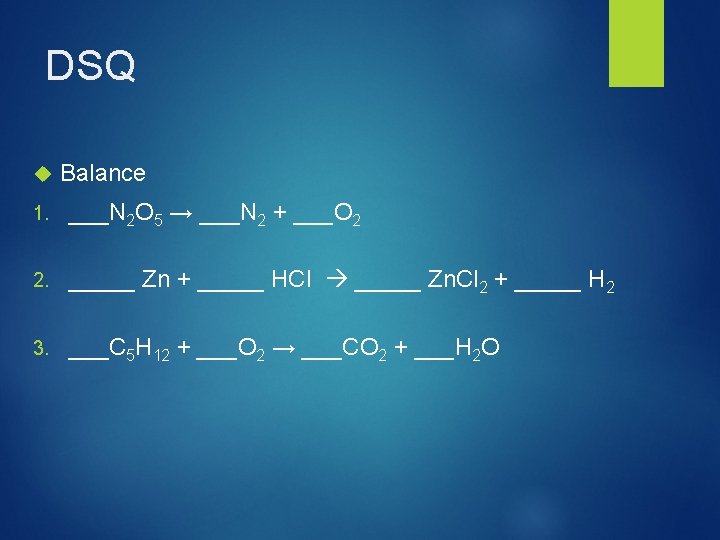
DSQ Balance 1. ___N 2 O 5 → ___N 2 + ___O 2 2. _____ Zn + _____ HCl _____ Zn. Cl 2 + _____ H 2 3. ___C 5 H 12 + ___O 2 → ___CO 2 + ___H 2 O


Types of Reactions 1. Synthesis reactions 2. Decomposition reactions 3. Single displacement reactions 4. Double displacement reactions 5. Combustion reactions You need to be able to identify each type.

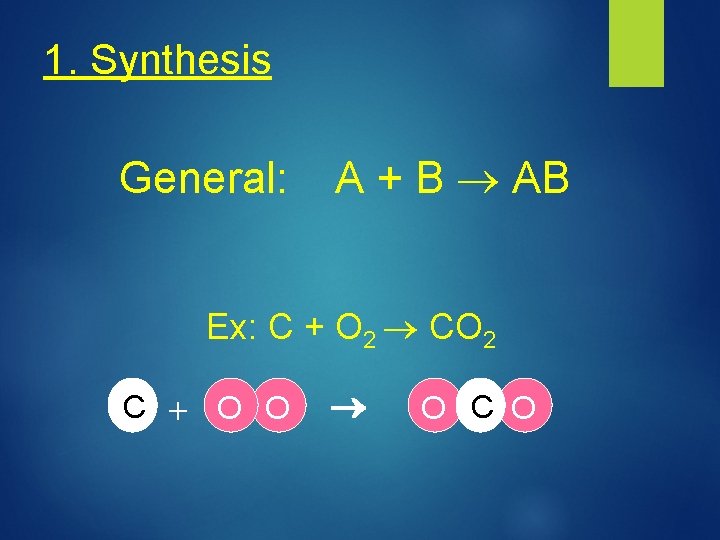
1. Synthesis General: A + B AB Ex: C + O 2 CO 2 C + O O O C O

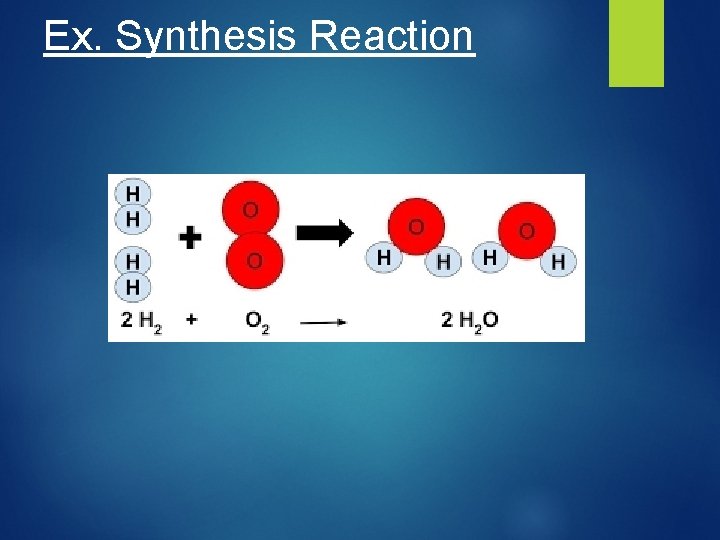
Ex. Synthesis Reaction

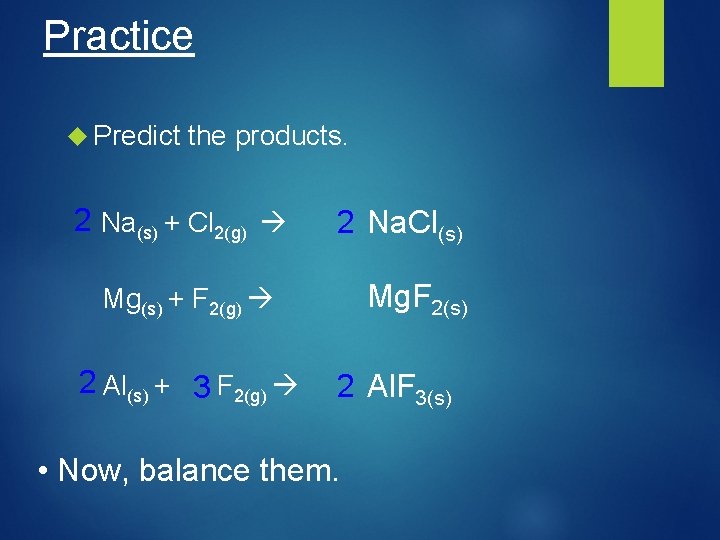
Practice Predict the products. 2 Na(s) + Cl 2(g) 2 Na. Cl(s) Mg. F 2(s) Mg(s) + F 2(g) 2 Al(s) + 3 F 2(g) 2 Al. F 3(s) • Now, balance them.

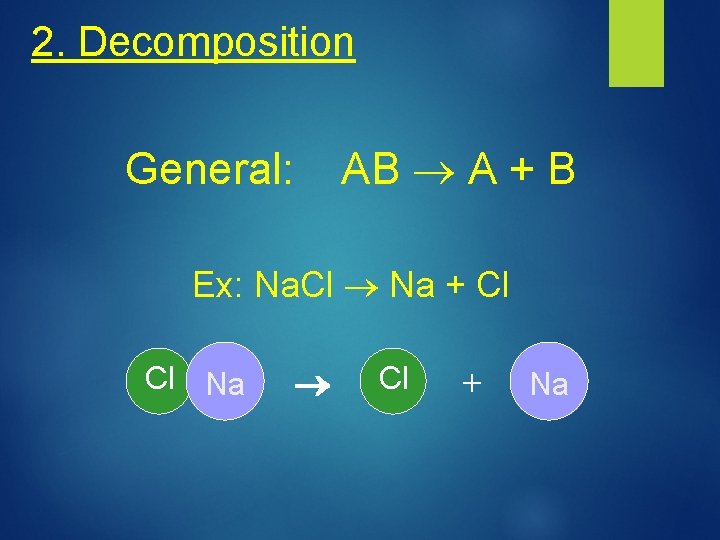
2. Decomposition AB A + B General: Ex: Na. Cl Na + Cl Cl Na Cl + Na

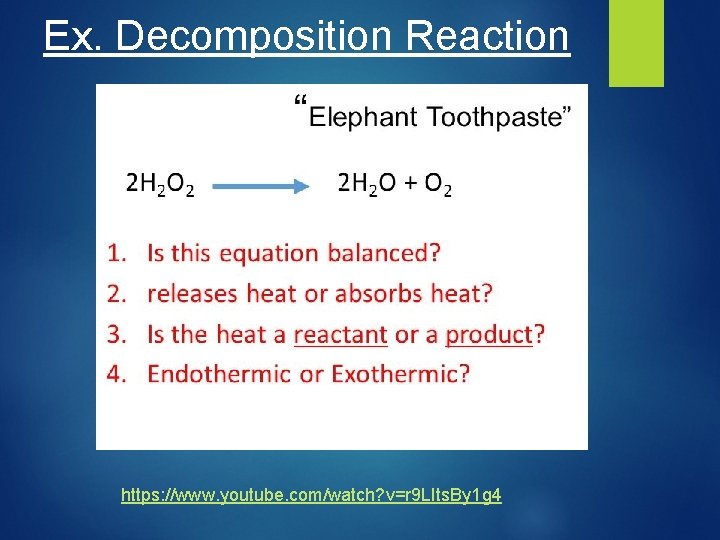
Ex. Decomposition Reaction https: //www. youtube. com/watch? v=r 9 LIts. By 1 g 4

Exothermic Reaction – a reaction that release heat Endothermic Reaction – a reaction that absorbs heat.

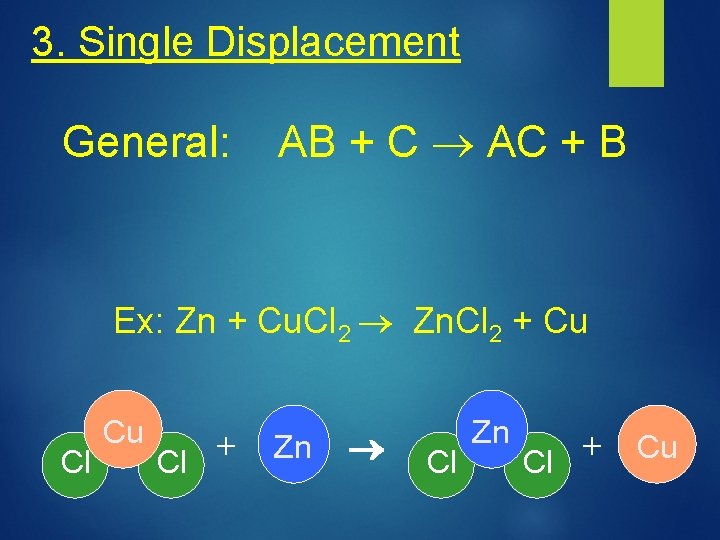
3. Single Displacement General: AB + C AC + B Ex: Zn + Cu. Cl 2 Zn. Cl 2 + Cu Cl Cu + Cl Zn + Cu Cl

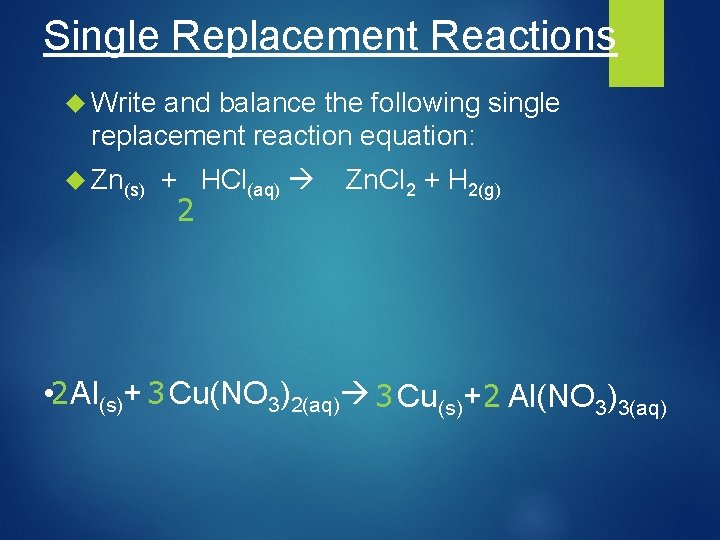
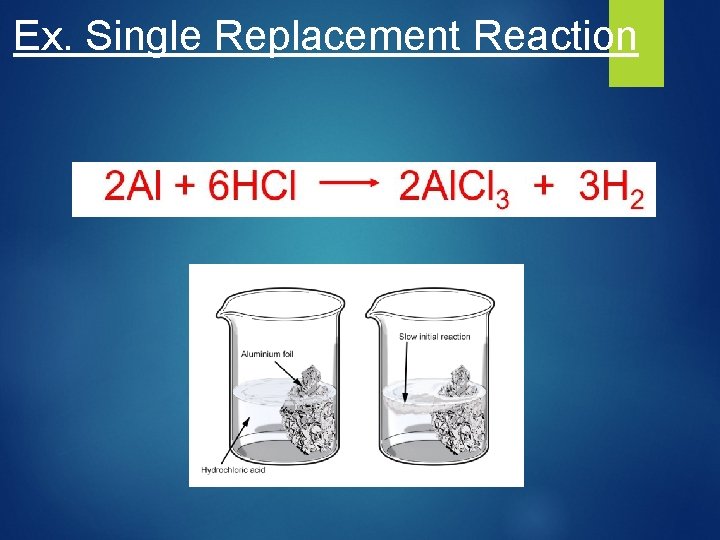
Single Replacement Reactions Write and balance the following single replacement reaction equation: Zn(s) + HCl(aq) 2 Zn. Cl 2 + H 2(g) • 2 Al(s)+ 3 Cu(NO 3)2(aq) 3 Cu(s)+ 2 Al(NO 3)3(aq)

Ex. Single Replacement Reaction

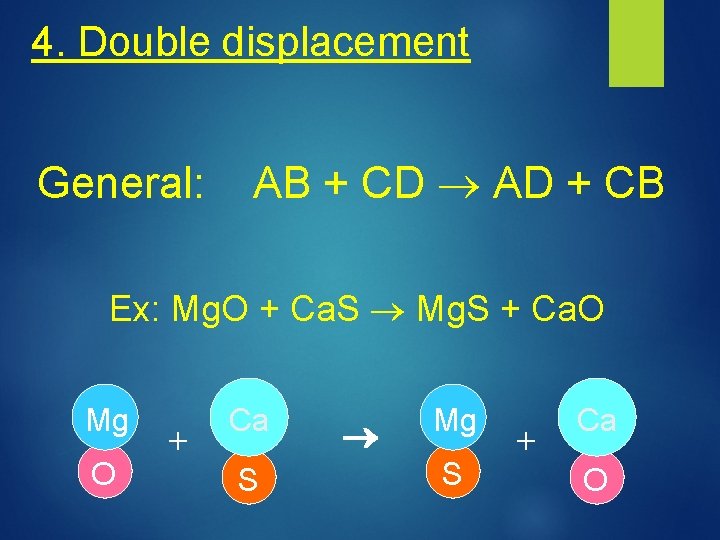
4. Double displacement General: AB + CD AD + CB Ex: Mg. O + Ca. S Mg. S + Ca. O Mg O + Ca S Mg S + Ca O

5. Combustion Reactions Combustion reactions - a hydrocarbon reacts with oxygen gas. This is also called burning!!! In order to burn something you need the 3 things in the “fire triangle”: 1) Fuel (hydrocarbon) 2) Oxygen 3) Something to ignite the reaction (spark)

Combustion Reactions In general: Cx. Hy + O 2 CO 2 + H 2 O Products are ALWAYS carbon dioxide and water. (although incomplete burning does cause some by-products like carbon monoxide) Combustion is used to heat homes and run automobiles (octane, as in gasoline, is C 8 H 18)

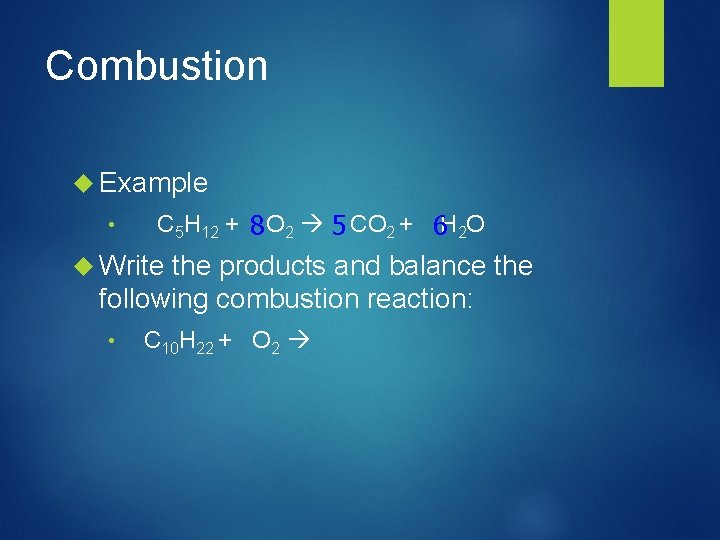
Combustion Example • C 5 H 12 + 8 O 2 5 CO 2 + 6 H 2 O Write the products and balance the following combustion reaction: • C 10 H 22 + O 2

Demonstrations Butane bubbles Alcohol in a jar

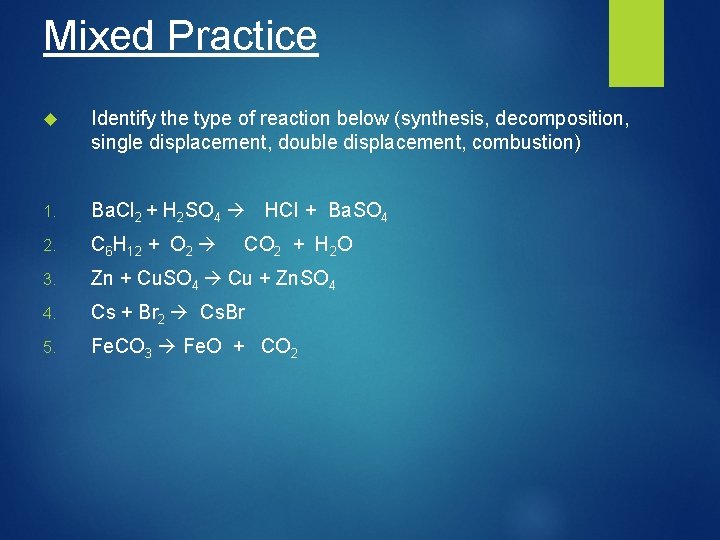
Mixed Practice Identify the type of reaction below (synthesis, decomposition, single displacement, double displacement, combustion) 1. Ba. Cl 2 + H 2 SO 4 HCl + Ba. SO 4 2. C 6 H 12 + O 2 3. Zn + Cu. SO 4 Cu + Zn. SO 4 4. Cs + Br 2 Cs. Br 5. Fe. CO 3 Fe. O + CO 2 + H 2 O

Oxidation of Steel Wool What will happens when something rusts, will it weigh more? Less? https: //www. youtube. com/watch? v=gn. Rc. Dt. Mni. VE

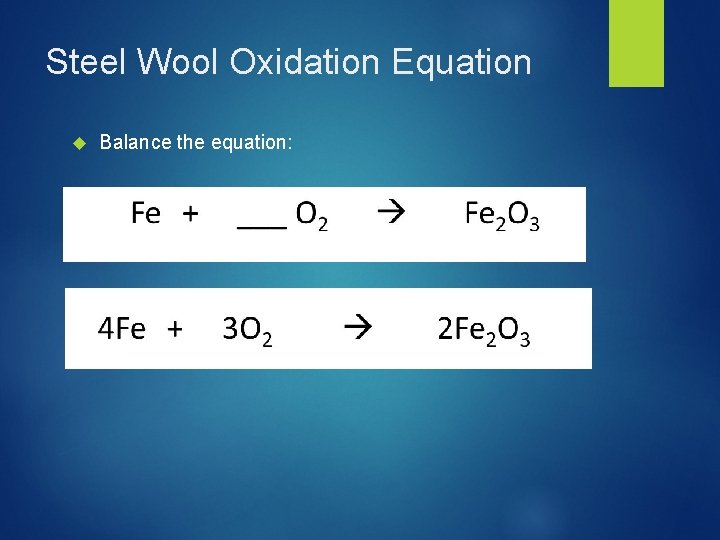
Steel Wool Oxidation Equation Balance the equation: