Primary lipid disorders A Amouzegar MD Research Institute





























- Slides: 66

Primary lipid disorders A. Amouzegar. MD Research Institute For Endocrine Sciences Endocrine Research Center

AGENDA • • • Familial hypercholesterolemia Familial defective Apo B 100 Polygenic hypercholesterolemia Familial combined hyperlipidemia Familial dysbetalipoproteinemia Lipoprotein Lipase deficiency Apo Lipoprotein Cll deficiency Familial hyper triglyceridemia Familial Hypoalphalipoproteinemia

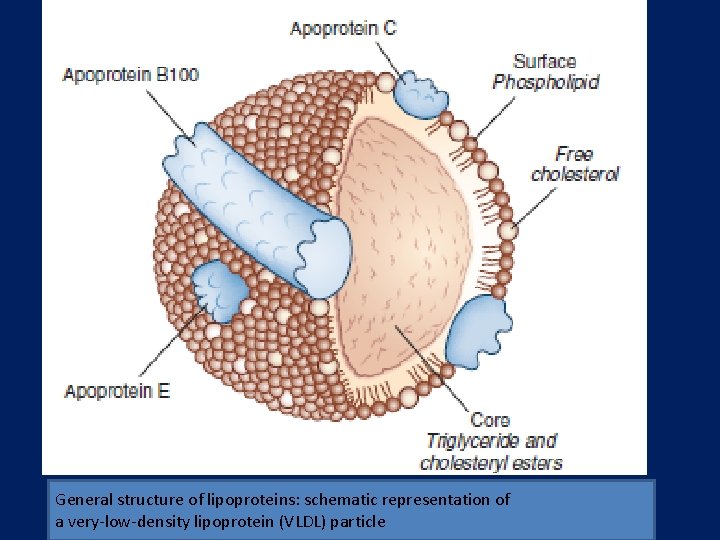
General structure of lipoproteins: schematic representation of a very-low-density lipoprotein (VLDL) particle

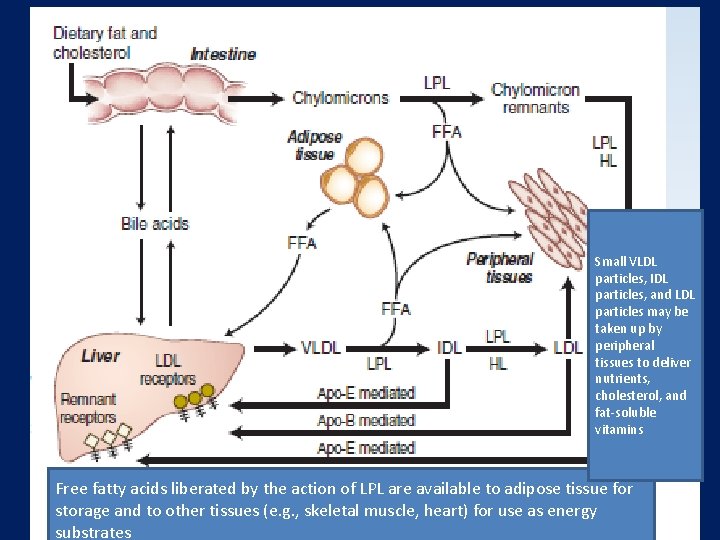
Small VLDL particles, IDL particles, and LDL particles may be taken up by peripheral tissues to deliver nutrients, cholesterol, and fat-soluble vitamins Free fatty acids liberated by the action of LPL are available to adipose tissue for storage and to other tissues (e. g. , skeletal muscle, heart) for use as energy substrates


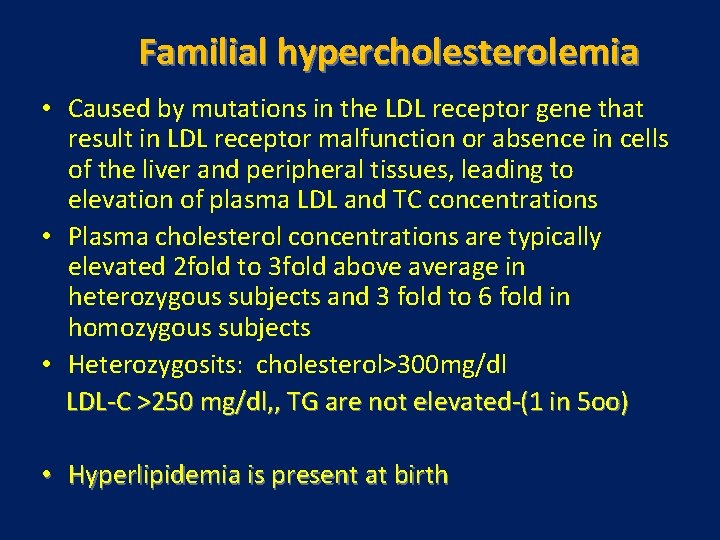
Familial hypercholesterolemia • Caused by mutations in the LDL receptor gene that result in LDL receptor malfunction or absence in cells of the liver and peripheral tissues, leading to elevation of plasma LDL and TC concentrations • Plasma cholesterol concentrations are typically elevated 2 fold to 3 fold above average in heterozygous subjects and 3 fold to 6 fold in homozygous subjects • Heterozygosits: cholesterol>300 mg/dl LDL-C >250 mg/dl, , TG are not elevated-(1 in 5 oo) • Hyperlipidemia is present at birth

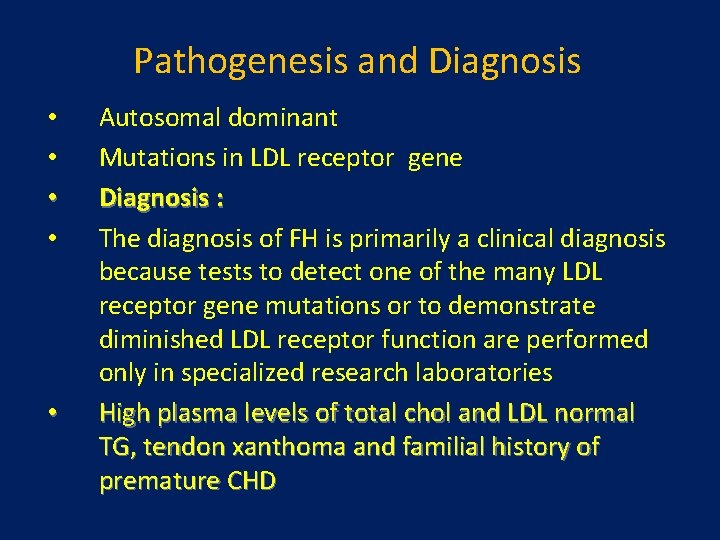
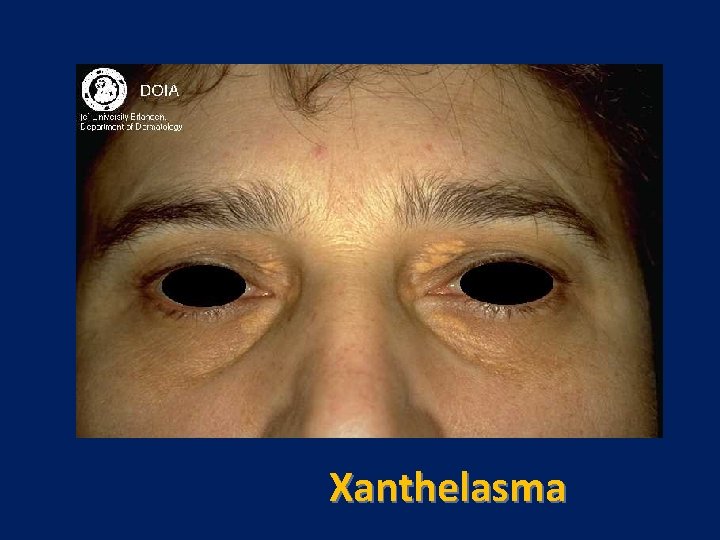
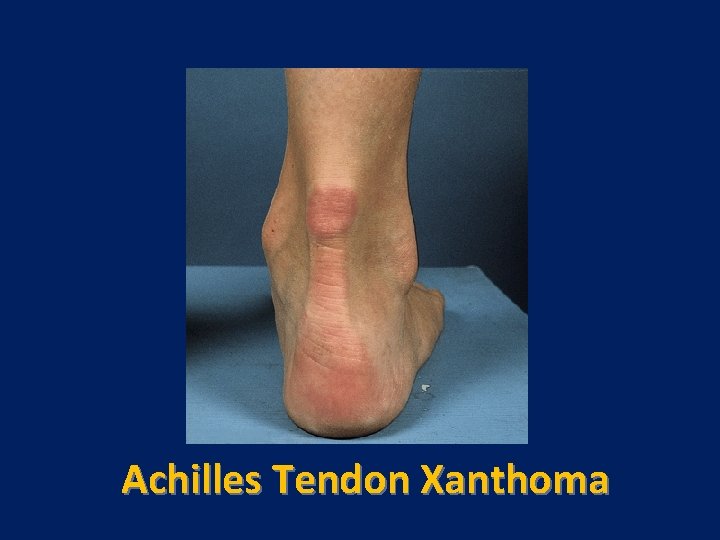
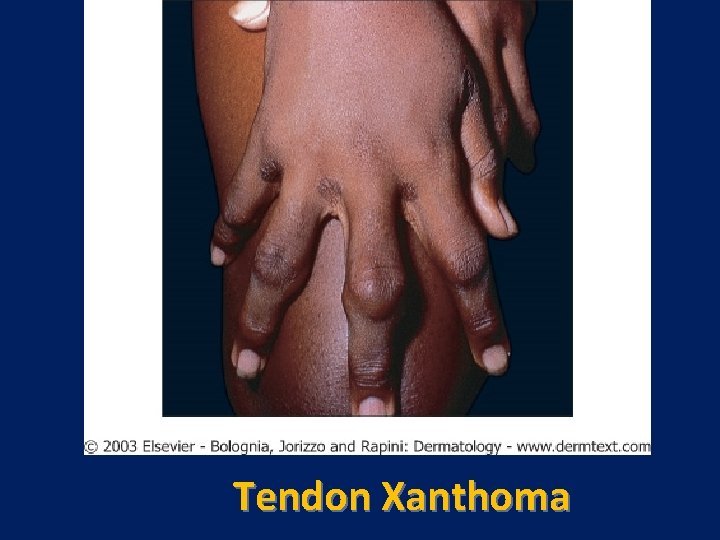
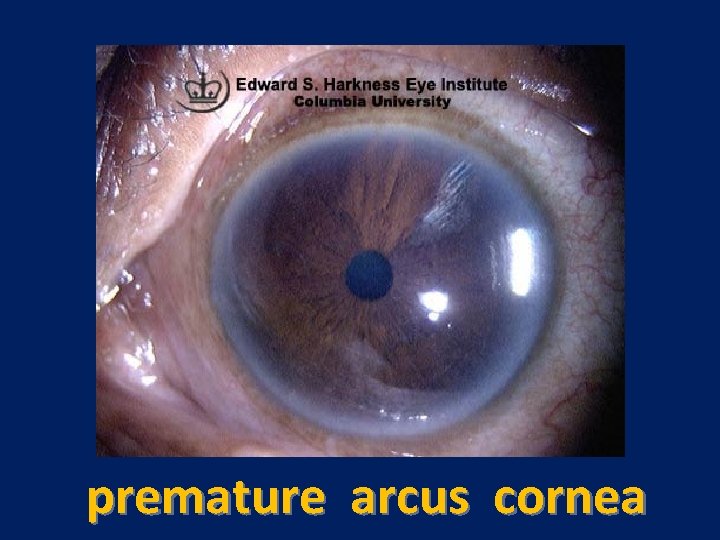
Characteristic physical finding: • Tendon xanthoma usually on the Achilles tendons or extensor tendons of the hands • Xanthelasma • premature arcus cornea • Many affected subjects have no physical findings • Premature CAD is common • The age of onset of coronary disease is about 45 years in men and women who are heterozygous for FH

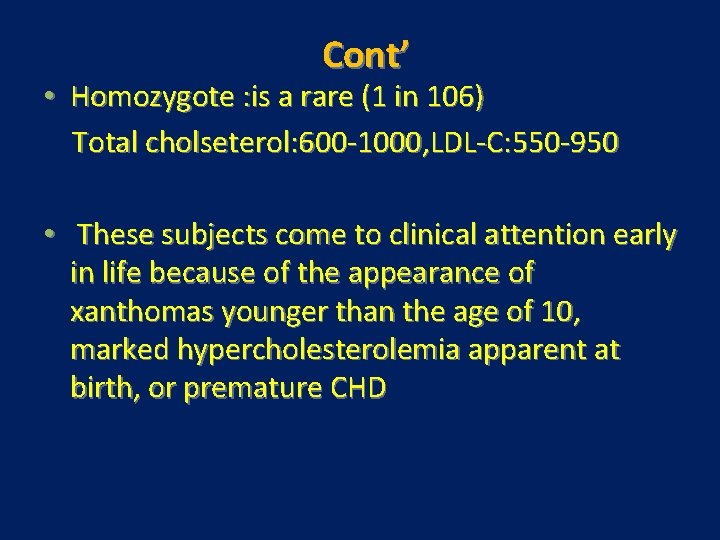
Cont’ • Homozygote : is a rare (1 in 106) Total cholseterol: 600 -1000, LDL-C: 550 -950 • These subjects come to clinical attention early in life because of the appearance of xanthomas younger than the age of 10, marked hypercholesterolemia apparent at birth, or premature CHD

Pathogenesis and Diagnosis • • • Autosomal dominant Mutations in LDL receptor gene Diagnosis : The diagnosis of FH is primarily a clinical diagnosis because tests to detect one of the many LDL receptor gene mutations or to demonstrate diminished LDL receptor function are performed only in specialized research laboratories High plasma levels of total chol and LDL normal TG, tendon xanthoma and familial history of premature CHD

Treatment Diet modification(5 -15%) Drug therapy HMG-COA reductase inhibitor - Bile Acid sequestrants-Niacin • LDL apheresis • • •

Xanthelasma





Achilles Tendon Xanthoma


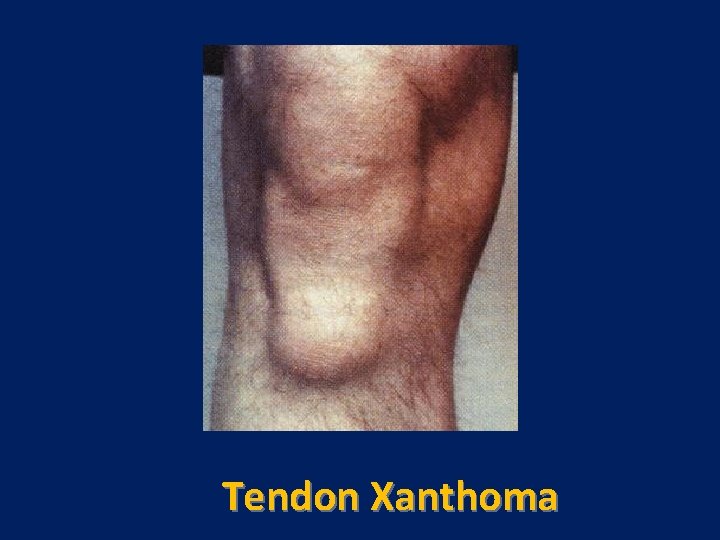
Tendon Xanthoma

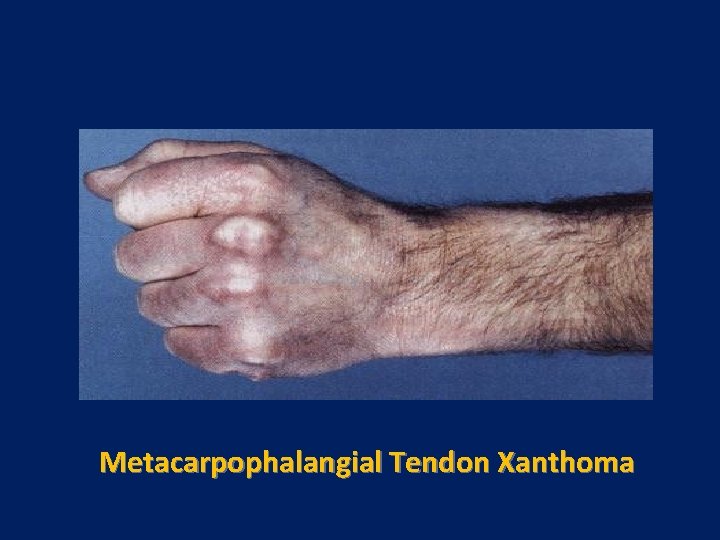
Metacarpophalangial Tendon Xanthoma

Tendon Xanthoma

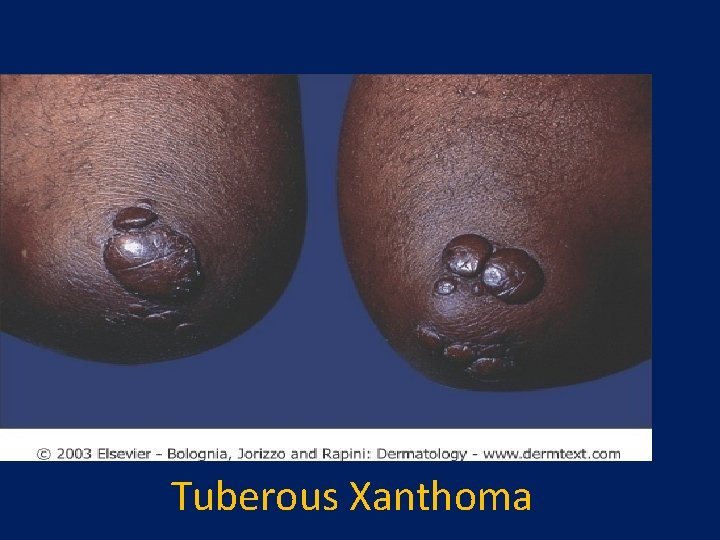
Tuberous xanthomas

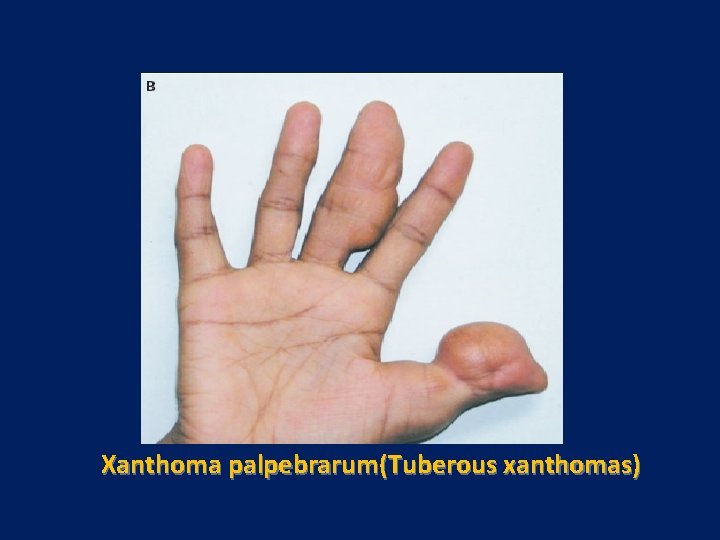
Xanthoma palpebrarum(Tuberous xanthomas)

premature arcus cornea

Familial defective Apolipoprotein. B 100 • AD • Frequency (1 in 500 to 1 in 750) • The clinical features of heterozygous familial defective apo-B 100 overlap extensively with those of heterozygous FH and include isolated elevations of plasma LDL, tendon xanthomas, xanthelasmas, and premature CHD

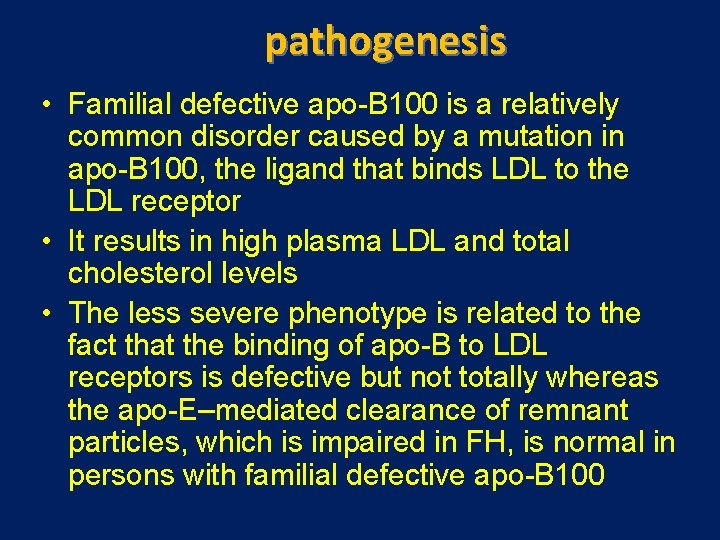
pathogenesis • Familial defective apo-B 100 is a relatively common disorder caused by a mutation in apo-B 100, the ligand that binds LDL to the LDL receptor • It results in high plasma LDL and total cholesterol levels • The less severe phenotype is related to the fact that the binding of apo-B to LDL receptors is defective but not totally whereas the apo-E–mediated clearance of remnant particles, which is impaired in FH, is normal in persons with familial defective apo-B 100

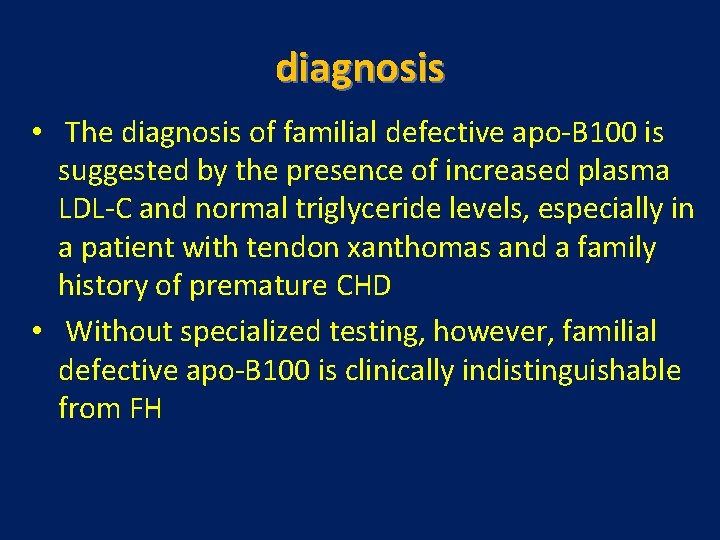
diagnosis • The diagnosis of familial defective apo-B 100 is suggested by the presence of increased plasma LDL-C and normal triglyceride levels, especially in a patient with tendon xanthomas and a family history of premature CHD • Without specialized testing, however, familial defective apo-B 100 is clinically indistinguishable from FH

Treatment • Similar to that heterozygots FH • Diet modification( low fat-low cholesterol) • Drug therapy • HMG-COA reductase inhibitor

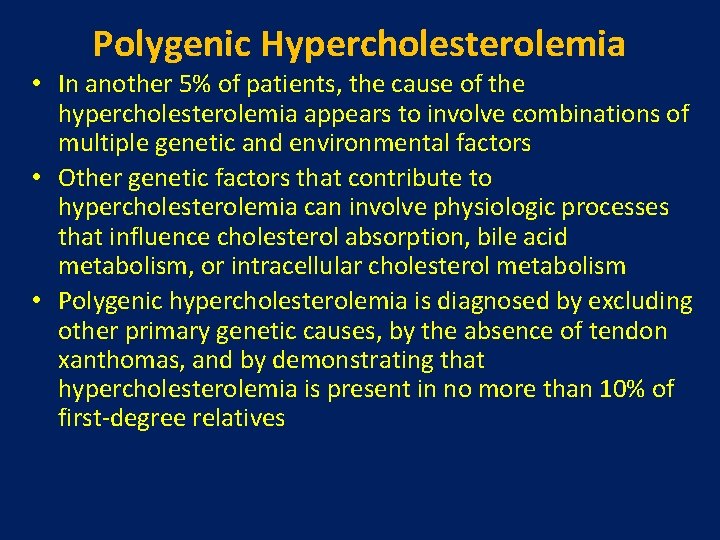
Polygenic Hypercholesterolemia • In another 5% of patients, the cause of the hypercholesterolemia appears to involve combinations of multiple genetic and environmental factors • Other genetic factors that contribute to hypercholesterolemia can involve physiologic processes that influence cholesterol absorption, bile acid metabolism, or intracellular cholesterol metabolism • Polygenic hypercholesterolemia is diagnosed by excluding other primary genetic causes, by the absence of tendon xanthomas, and by demonstrating that hypercholesterolemia is present in no more than 10% of first-degree relatives

Familial combined Hyperlipidemia • A commo n disorder (0. 5%-2%) - AD co • A common disorder of unknown genetic cause associated with elevations of plasma cholesterol and triglyceride levels and increased susceptibility to CHD • The most common dyslipidemia syndrome in families of survivors of premature MI • Association with metabolic syndrome • Neither its genetic cause nor its metabolic pathogenesis is clear

diagnosis • The features include moderate elevations of plasma cholesterol or triglycerides, or both, within subjects of an affected kindred • The predominant lipid abnormality (hypertriglyceridemia, hypercholesterolemia, or both) can vary among affected family members or in a single person over time

Cont • Variability in the type of dyslipidemia is a useful hint that the subject might have this disorder • Levels of HDL-C are often moderately decreased, especially in the setting of increased plasma triglycerides • Xanthomas are not a feature of familial combined hyperlipidemia

Treatment • Weight reduction • Diet modification • Drug therapy: directed at the predominant lipid abnormality

Familial dysbetalipoproteinemia • An uncommon disorder(1 in 10. 000)- AR • Moderate to severe hypertriglyceridemia and hypercholesterolemia (300 -400 mg) • LDL is always reduced- HDL is normal • Premature vascular disease and CAD • Requires a secondary exacerbating metabolic factor (either genetic or environmental) for expression of the phenotype

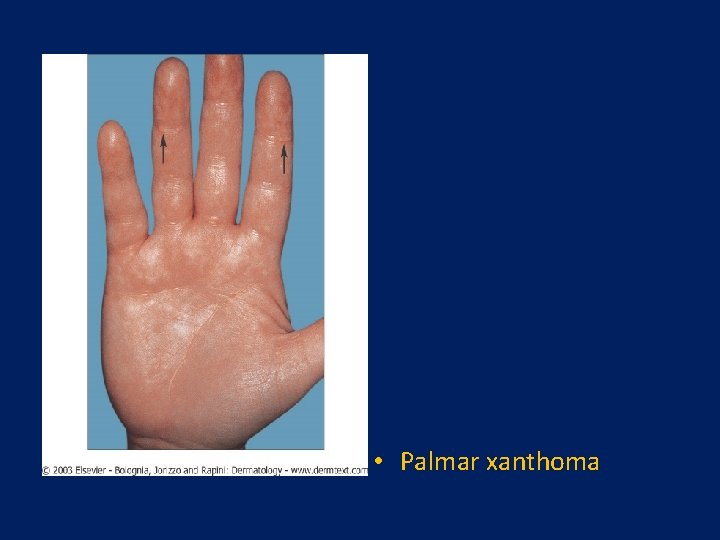
Clinical Features • More common in men • Not manifested in women until after menopause • Tendon xanthomas and xanthelasma occur in some • Tuberus or tubero eruptive xanthoma • Palmar xanthoma : pathogonomic • Coexisting metabolic conditions that exacerbate such as obesity, alcohol consumption, diabetes mellitus, and hypothyroidism, are often present

pathogenesis • Caused by mutant apo. E • The clearance defect is caused by mutant apo. E that binds defectively to remnant receptors, including LDL receptors • Electrophoresis(broad band B - lipoprotein) • Plasma accumulation of cholesterol rich remnants of VLDL, IDL and chylomicron particles

Diagnosis • Should be suspected in patients with moderately severe, nearly equal (based on values expressed as mg/d. L) elevations in both plasma triglyceride and cholesterol concentrations • Typically the cholesterol and triglyceride levels are in the range of 300 -400 mg/d. L • There is often no family history of hyperlipidemia or premature CHD • The presence of palmar or tuberous xanthomas makes the diagnosis highly likely

• The directly measured VLDL cholesterol-toplasma triglyceride ratio (lipid values in mg/d. L) is a useful screen; this ratio is usually greater than 0. 3 The normal ratio is typically about 0. 2

Treatment • • • Treatment of coexisting condition (obesity-Dm-hypothyroidism-alcohol) Dietary therapy Estrogen therapy Drug therapy(niacin-fibric acid)

• Palmar xanthoma

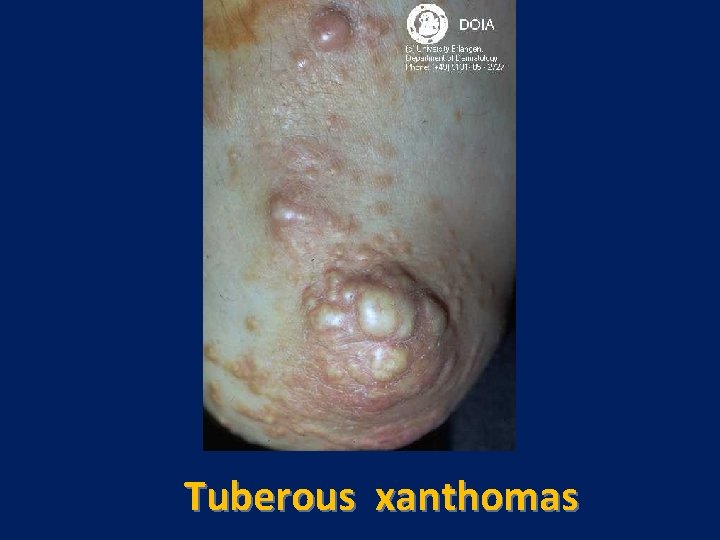
Tuberous Xanthoma

Tuberous xanthomas


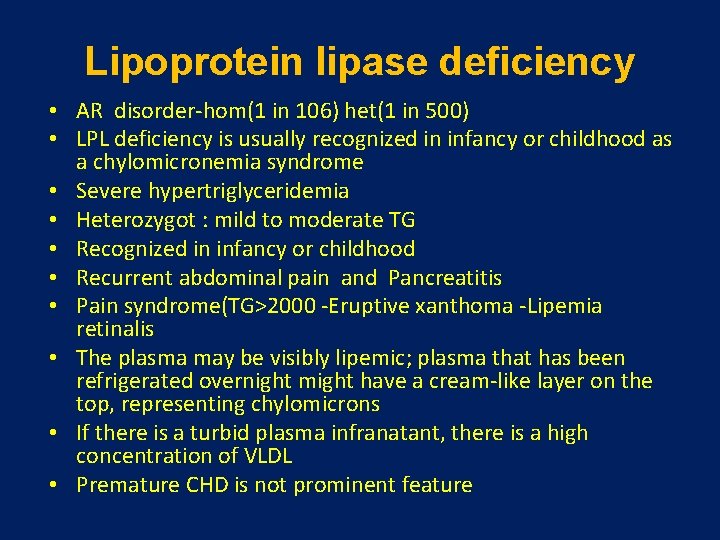
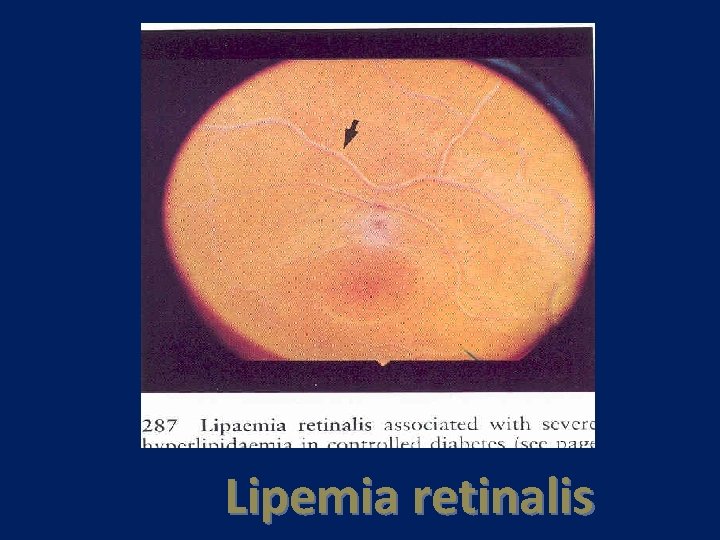
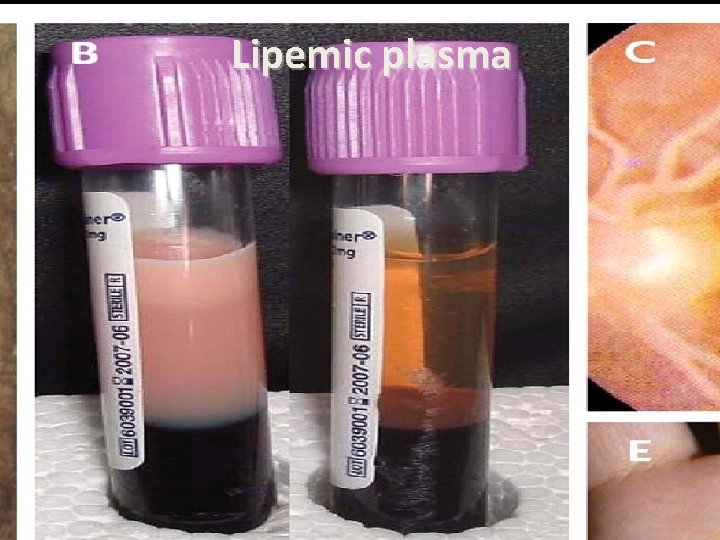
Lipoprotein lipase deficiency • AR disorder-hom(1 in 106) het(1 in 500) • LPL deficiency is usually recognized in infancy or childhood as a chylomicronemia syndrome • Severe hypertriglyceridemia • Heterozygot : mild to moderate TG • Recognized in infancy or childhood • Recurrent abdominal pain and Pancreatitis • Pain syndrome(TG>2000 -Eruptive xanthoma -Lipemia retinalis • The plasma may be visibly lipemic; plasma that has been refrigerated overnight might have a cream-like layer on the top, representing chylomicrons • If there is a turbid plasma infranatant, there is a high concentration of VLDL • Premature CHD is not prominent feature

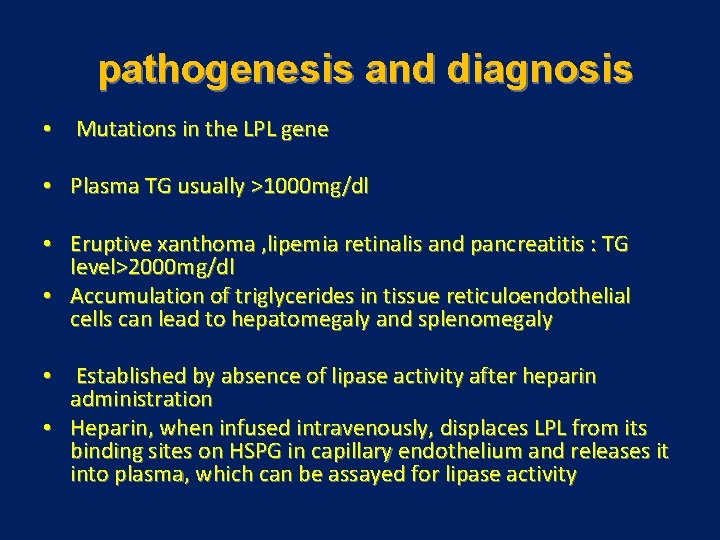
pathogenesis and diagnosis • Mutations in the LPL gene • Plasma TG usually >1000 mg/dl • Eruptive xanthoma , lipemia retinalis and pancreatitis : TG level>2000 mg/dl • Accumulation of triglycerides in tissue reticuloendothelial cells can lead to hepatomegaly and splenomegaly • Established by absence of lipase activity after heparin administration • Heparin, when infused intravenously, displaces LPL from its binding sites on HSPG in capillary endothelium and releases it into plasma, which can be assayed for lipase activity

Treatment • Initial stage of treatment : fat free diet • Goal of therapy : TG<1000 mg/dl • Secondary causes should be screened and treated • Drug therapy : largely ineffective • however, clofibrate, gemfibrozil, fenofibrate, or niacin can lower VLDL production and lessen the severity of the hypertriglyceridemia • Orlistat lowers triglyceride levels significantly in some patients with severe hypertriglyceridemia



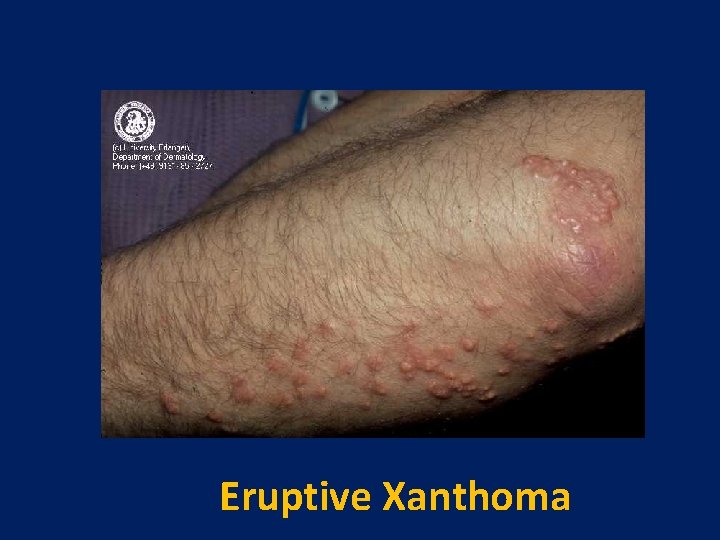
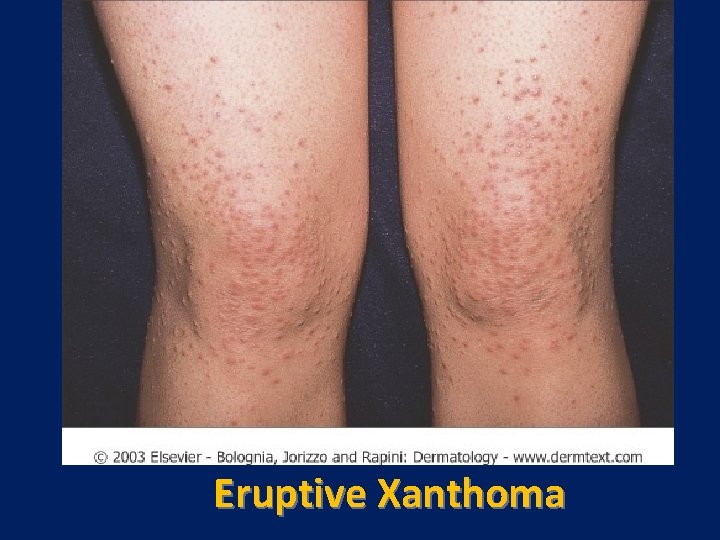
Eruptive Xanthoma

Eruptive Xanthoma

Lipemia retinalis


Lipemic plasma

Apo lipoprotein CII deficiency disorder • • AR disorder(1 in 106) Causes a chylomicronemia syndrome similar to that in LPL deficiency Plasma triglyceride levels are usually severely elevated ( 1000 mg/d. L), reflecting the accumulation of chylomicrons, VLDL, or both lipoproteins in the plasma This hyperlipoproteinemia results from the lack of apo-CII, an activating cofactor for LPL, which causes a functional LPL deficiency

The features: • • • As in LPL deficiency, the features include pancreatitis or recurrent bouts of abdominal pain in children or young adults and, after a 12 -hour fast, lipemic serum with chylomicrons that rise to the surface of serum that has stood undisturbed for 10 to 12 hours TG usually>1000 mg/dl Pancreatitis

Diagnosis • Requires specialized testing to demonstrate that apo-CII is absent on electrophoresis of the plasma apolipoproteins or that the plasma is incapable of activating LPL in vitro

Treatment • Is identical to that of primary LPL deficiency with the exception that severe hypertriglyceridemia in apo-CII-deficient patients with pancreatitis can be treated with transfusions of plasma, which contains apo-CII

Familial hypertriglyceridemia • Familial hypertriglyceridemia is characterized by increased plasma concentrations of triglyceriderich VLDLs, which cause elevations of plasma triglycerides but not plasma cholesterol levels • Appears to be caused by overproduction of VLDL triglycerides in the presence of near-normal apo. B production, which leads to the secretion of large, triglyceride-rich

Clinical Features • TG(200 -500) & LDL N & HDL L • TG-rich VLDL • Elevated TG usually not evident until adulthood • Exacerbated by secondary factors (e. g. , insulin resistance) that lead to VLDL overproduction can exacerbate the syndrome • Xanthoma usually not present. • Obesity & insulin resistance are common

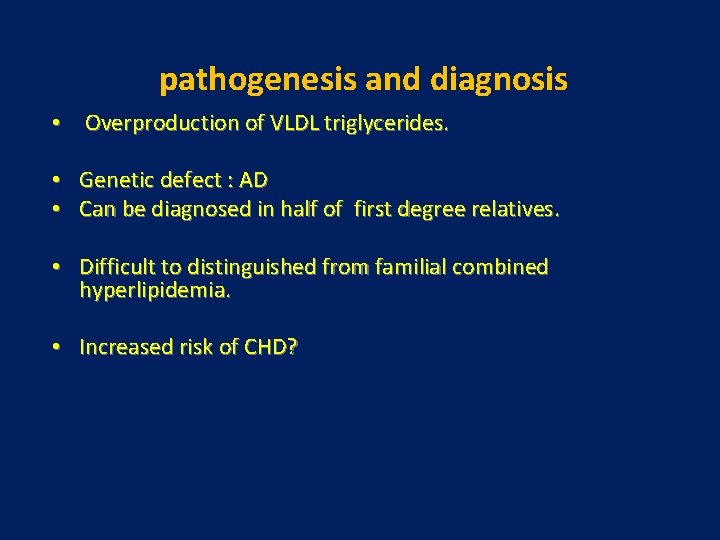
pathogenesis and diagnosis • Overproduction of VLDL triglycerides. • • Genetic defect : AD Can be diagnosed in half of first degree relatives. • Difficult to distinguished from familial combined hyperlipidemia. • Increased risk of CHD?

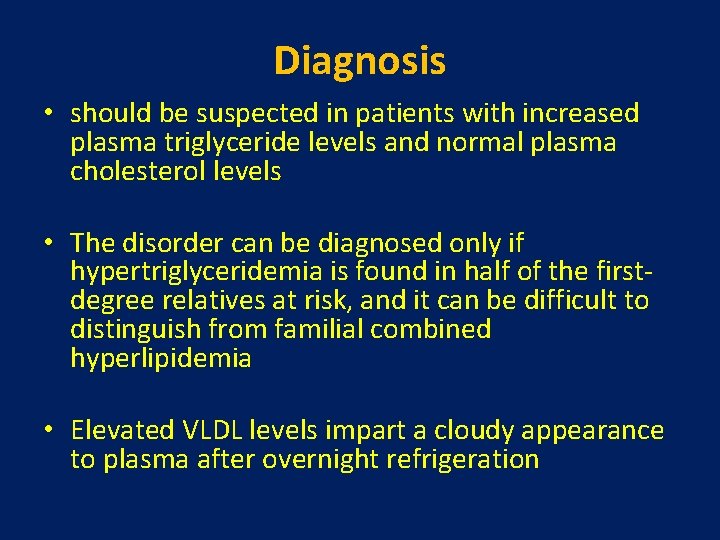
Diagnosis • should be suspected in patients with increased plasma triglyceride levels and normal plasma cholesterol levels • The disorder can be diagnosed only if hypertriglyceridemia is found in half of the firstdegree relatives at risk, and it can be difficult to distinguish from familial combined hyperlipidemia • Elevated VLDL levels impart a cloudy appearance to plasma after overnight refrigeration

Treatment • • Dietary fat restriction. Secondary disorders should be screened Drug may be useful (Niacin-Gemfibrozil)

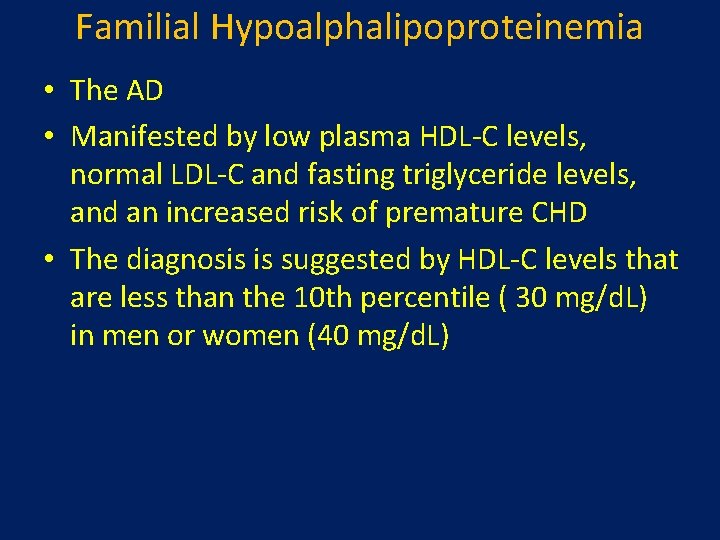
Familial Hypoalphalipoproteinemia • The AD • Manifested by low plasma HDL-C levels, normal LDL-C and fasting triglyceride levels, and an increased risk of premature CHD • The diagnosis is suggested by HDL-C levels that are less than the 10 th percentile ( 30 mg/d. L) in men or women (40 mg/d. L)

physical findings • There are no characteristic physical findings, but there is often a family history of low HDL-C levels and premature CHD • The genetic and metabolic defects that lead to low plasma HDL levels are unknown, but it appears that up to 50% of low HDL-C levels can be linked to the hepatic lipase or apo-AI/apo. CIII/apo-AIV/apo-AV gene locus

Con, t • The lack of HDL in the plasma accelerates the development of atherosclerosis, presumably because reverse cholesterol transport or other protective effects of HDL are impaired

Treatments • Drug therapy should be aimed at raising the plasma HDL concentration or lowering the plasma LDL concentration with statins Fibrates (gemfibrozil, fenofibrate) raise • HDL-C levels about 5% to 20%, similar to the effects of statins. Niacin is the most effective drug currently available to raise HDL-C levels

 Jamshid amouzegar
Jamshid amouzegar Australian primary health care research institute
Australian primary health care research institute Telethon institute for child health research
Telethon institute for child health research Assistive technology implementation plan
Assistive technology implementation plan Croatian forest research institute
Croatian forest research institute Where is happiness
Where is happiness Bill harris centerpointe
Bill harris centerpointe Primary control vs secondary control
Primary control vs secondary control Samsung biomedical research institute
Samsung biomedical research institute Sagar institute of research and technology
Sagar institute of research and technology Isgemiese hartsiekte
Isgemiese hartsiekte National human genome research institute
National human genome research institute National human genome research institute
National human genome research institute Kavi kenya
Kavi kenya Esri redlands, ca
Esri redlands, ca Electronics and telecommunications research institute
Electronics and telecommunications research institute Digital enterprise research institute
Digital enterprise research institute Turner syndrome
Turner syndrome Encode
Encode Jon keating oxford
Jon keating oxford Target institute of medical education and research
Target institute of medical education and research World research institute
World research institute Environmental systems research institute
Environmental systems research institute National research institute of brewing
National research institute of brewing International institute of health management research
International institute of health management research Prof ram meghe institute of technology and research
Prof ram meghe institute of technology and research Korea automobile testing & research institute
Korea automobile testing & research institute Kncap test protocol
Kncap test protocol Ivl swedish environmental research institute
Ivl swedish environmental research institute Hong kong institute of educational research
Hong kong institute of educational research Ozeanklima
Ozeanklima Cfa research challenge report template
Cfa research challenge report template Swedish national road and transport research institute
Swedish national road and transport research institute Institute for urban policy research
Institute for urban policy research Commerce development research institute
Commerce development research institute Sproel
Sproel Electric power research institute
Electric power research institute Research institute
Research institute Nina trondheim
Nina trondheim National research center kurchatov institute
National research center kurchatov institute National research center kurchatov institute
National research center kurchatov institute E learning agriculture portal
E learning agriculture portal Scottish institute for policing research
Scottish institute for policing research Scott thumma
Scott thumma Peace research institute frankfurt
Peace research institute frankfurt Northern institute for cancer research
Northern institute for cancer research Netherlands institute for social research
Netherlands institute for social research Manjushree research institute of ayurvedic science
Manjushree research institute of ayurvedic science Kumc research institute
Kumc research institute Kirdi logo
Kirdi logo Kazusa dna research institute
Kazusa dna research institute Communication research
Communication research Precision fluency shaping program review
Precision fluency shaping program review Trend research institute
Trend research institute Role of steroid hormone
Role of steroid hormone Are lipids energy storage
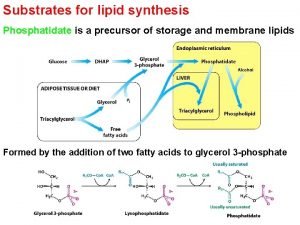
Are lipids energy storage Phosphatidate is formed from
Phosphatidate is formed from Qualitative analysis of lipids
Qualitative analysis of lipids Nonpolar lipids
Nonpolar lipids Lipids elements
Lipids elements Elements found in lipid
Elements found in lipid Oxidation of lipids
Oxidation of lipids Alpha lipid lifeline walmart
Alpha lipid lifeline walmart Jenis lipid
Jenis lipid Lipid rancidity
Lipid rancidity Lipid soluble hormones examples
Lipid soluble hormones examples Kemoototrof bakteri
Kemoototrof bakteri