Sulfonylurea Atieh Amouzegar MD Endocrine Research Center Research



















- Slides: 46

Sulfonylurea Atieh Amouzegar MD Endocrine Research Center, Research Institute For Endocrine Science Shahid Beheshti University

Objectives • • • Case presentation Mechanism of action ADA guideline focus on Sus Pros and Cons of therapy Choice of Sus Conclusion

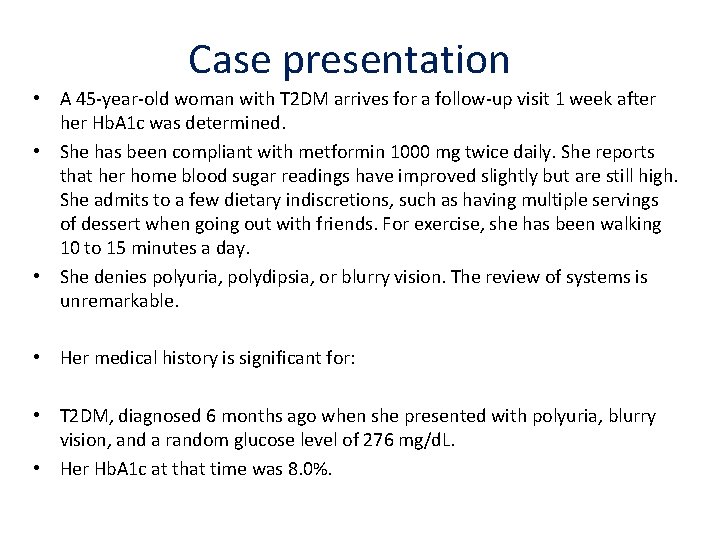
Case presentation • A 45‐year‐old woman with T 2 DM arrives for a follow‐up visit 1 week after her Hb. A 1 c was determined. • She has been compliant with metformin 1000 mg twice daily. She reports that her home blood sugar readings have improved slightly but are still high. She admits to a few dietary indiscretions, such as having multiple servings of dessert when going out with friends. For exercise, she has been walking 10 to 15 minutes a day. • She denies polyuria, polydipsia, or blurry vision. The review of systems is unremarkable. • Her medical history is significant for: • T 2 DM, diagnosed 6 months ago when she presented with polyuria, blurry vision, and a random glucose level of 276 mg/d. L. • Her Hb. A 1 c at that time was 8. 0%.

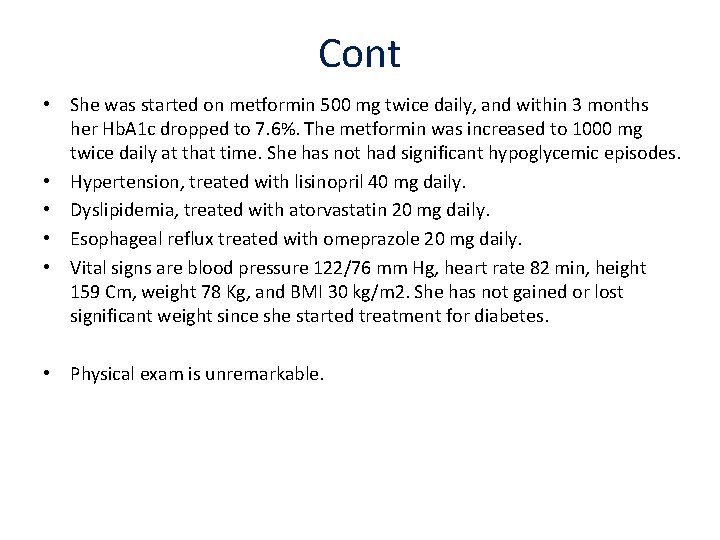
Cont • She was started on metformin 500 mg twice daily, and within 3 months her Hb. A 1 c dropped to 7. 6%. The metformin was increased to 1000 mg twice daily at that time. She has not had significant hypoglycemic episodes. • Hypertension, treated with lisinopril 40 mg daily. • Dyslipidemia, treated with atorvastatin 20 mg daily. • Esophageal reflux treated with omeprazole 20 mg daily. • Vital signs are blood pressure 122/76 mm Hg, heart rate 82 min, height 159 Cm, weight 78 Kg, and BMI 30 kg/m 2. She has not gained or lost significant weight since she started treatment for diabetes. • Physical exam is unremarkable.

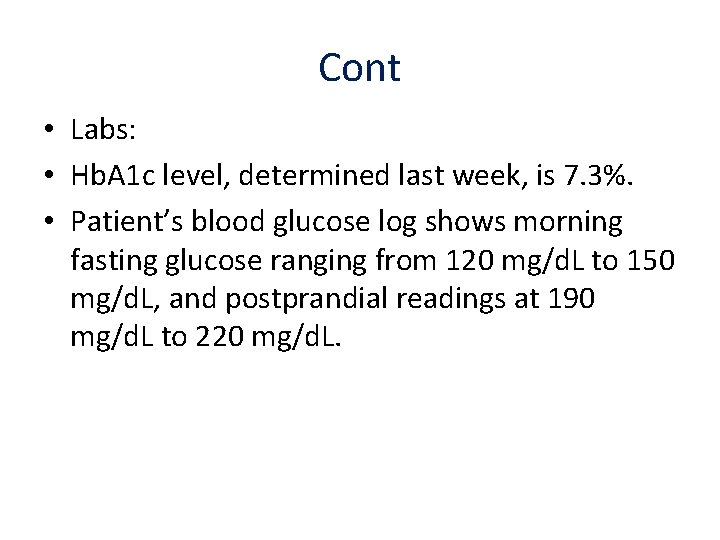
Cont • Labs: • Hb. A 1 c level, determined last week, is 7. 3%. • Patient’s blood glucose log shows morning fasting glucose ranging from 120 mg/d. L to 150 mg/d. L, and postprandial readings at 190 mg/d. L to 220 mg/d. L.

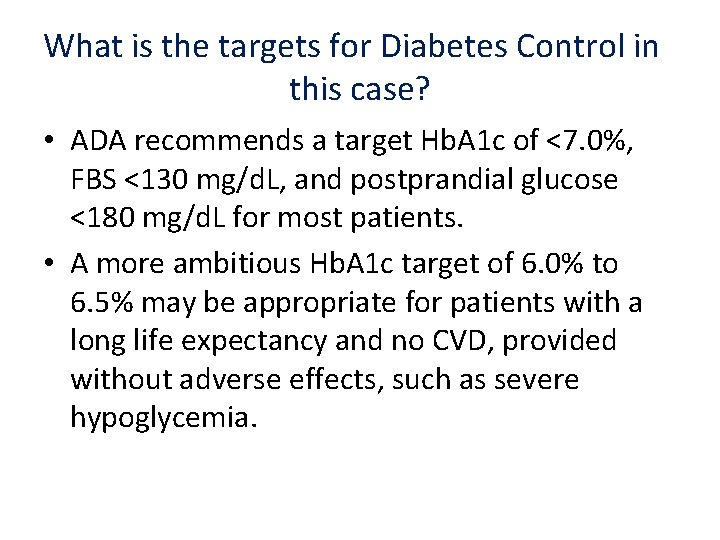
What is the targets for Diabetes Control in this case? • ADA recommends a target Hb. A 1 c of <7. 0%, FBS <130 mg/d. L, and postprandial glucose <180 mg/d. L for most patients. • A more ambitious Hb. A 1 c target of 6. 0% to 6. 5% may be appropriate for patients with a long life expectancy and no CVD, provided without adverse effects, such as severe hypoglycemia.

Assessment • The patient’s Hb. A 1 c has improved since starting metformin, but is still not at target. • Her fasting and postprandial glucose levels are also too high. • The underlying causes for hyperglycemia in this patient include dietary factors, inadequate exercise, and obesity. She has no signs or symptoms of an acute illness that could cause hyperglycemia. • The maximum recommended dose of metformin for adults is 2000 to 2500 mg daily, depending on the formulation. Her current total daily dose is 2000 mg, and it is unlikely that her glycemic control will improve significantly just by adding another 500 mg of metformin.

What do you do next? • The patient is referred to a diabetes education and support class. • She is briefly counseled on lifestyle changes to improve her diet and increase her physical activity. • Diabetic individuals in the intensive lifestyle intervention arm of the Look AHEAD study lost 8. 6% of their weight in the first year, with an average reduction in fasting glucose from 152 mg/d. L to 130 mg/d. L and reduction in Hb. A 1 c from 7. 3% to 6. 6%. • If a similarly intensive program is available, this patient should be referred to it. • If Hb. A 1 c is not in the target range on metformin alone (as in this patient), an additional medication would be beneficial. • The Look AHEAD trial. Diabetes Care. 2007; 30: 1374‐ 1383.

Which OHA is the best for this step? • Sulfonylurea • Thiazolidinedione • Glucagon‐like peptide (GLP)‐ 1 agonist • Dipeptidyl peptidase (DPP)‐ 4 inhibitor • SGLT 2 I • Insulin

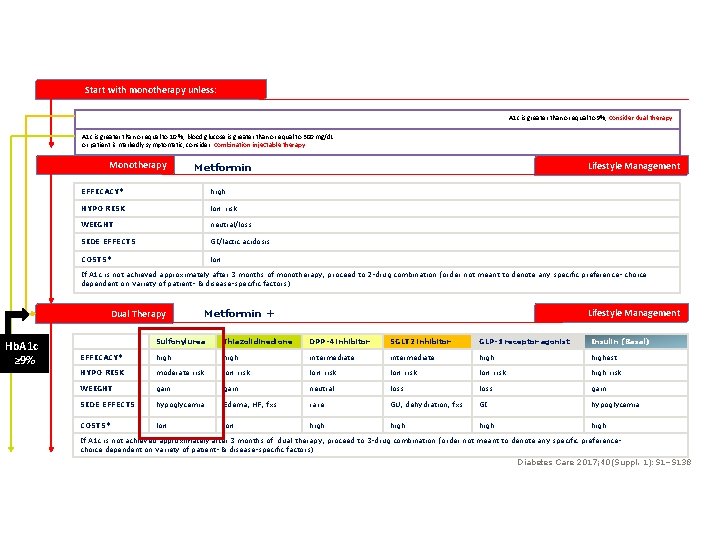
Start with monotherapy unless: A 1 c is greater than or equal to 9%, consider dual therapy A 1 c is greater than or equal to 10 %, blood glucose is greater than or equal to 300 mg/d. L or patient is markedly symptomatic, consider combination injectable therapy Monotherapy Lifestyle Management Metformin EFFICACY* high HYPO RISK low risk WEIGHT neutral/loss SIDE EFFECTS GI/lactic acidosis COSTS* low If A 1 c is not achieved approximately after 3 months of monotherapy, proceed to 2 -drug combination (order not meant to denote any specific preference- choice dependent on variety of patient- & disease-specific factors) Dual Therapy Hb. A 1 c ≥ 9% Metformin + Lifestyle Management Sulfonylurea Thiazolidinedione DPP-4 inhibitor SGLT 2 inhibitor GLP-1 receptor agonist Insulin (Basal) EFFICACY* high intermediate highest HYPO RISK moderate risk low risk high risk WEIGHT gain neutral loss gain SIDE EFFECTS hypoglycemia Edema, HF, fxs rare GU, dehydration, fxs GI hypoglycemia COSTS* low high If A 1 c is not achieved approximately after 3 months of dual therapy, proceed to 3 -drug combination (order not meant to denote any specific preferencechoice dependent on variety of patient- & disease-specific factors) Diabetes Care 2017; 40(Suppl. 1): S 1–S 138

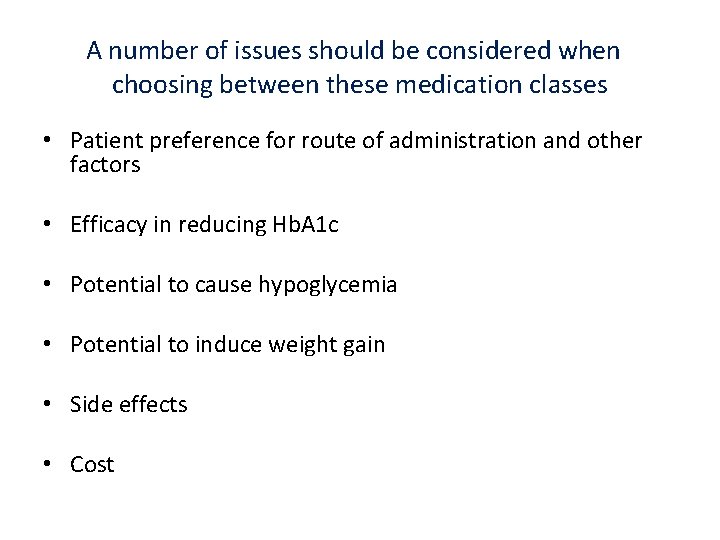
A number of issues should be considered when choosing between these medication classes • Patient preference for route of administration and other factors • Efficacy in reducing Hb. A 1 c • Potential to cause hypoglycemia • Potential to induce weight gain • Side effects • Cost

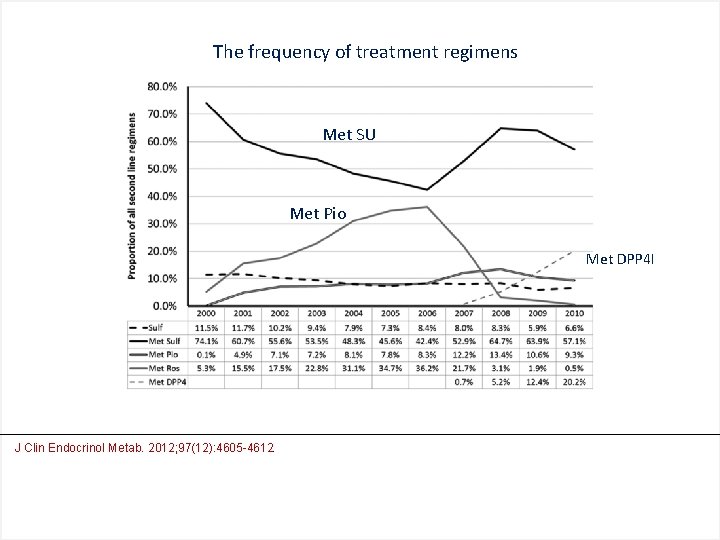
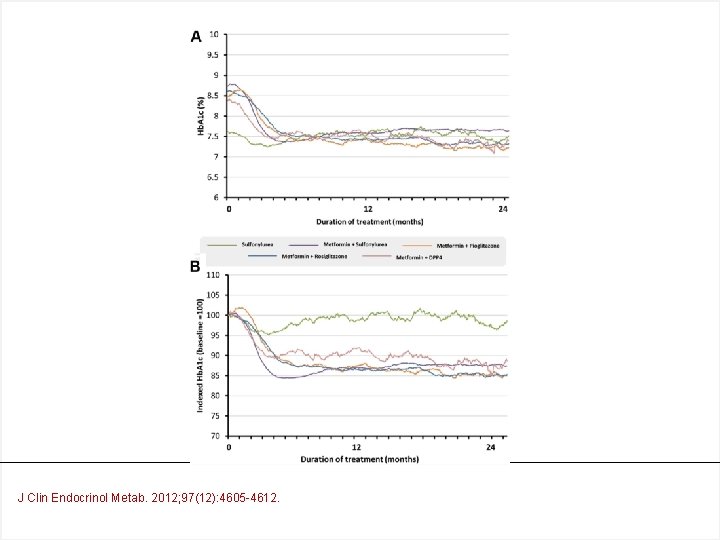
The frequency of treatment regimens Met SU Met Pio Met DPP 4 I J Clin Endocrinol Metab. 2012; 97(12): 4605 -4612.

• Which drug is the choice as the second line treatment?

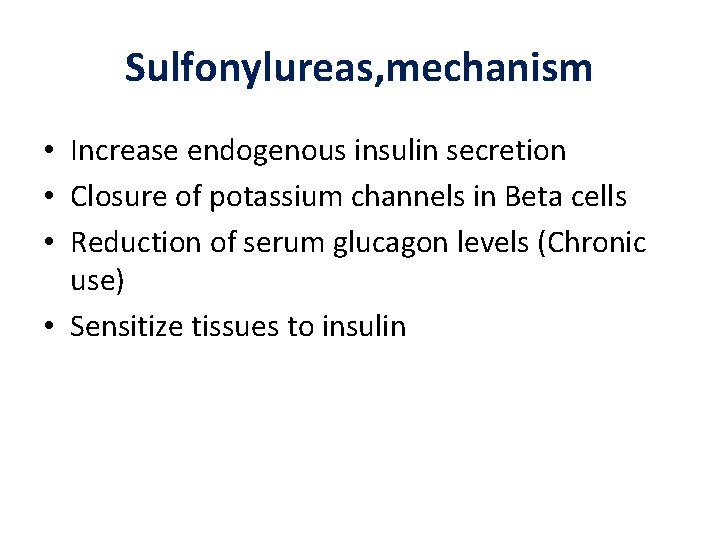
Sulfonylureas, mechanism • Increase endogenous insulin secretion • Closure of potassium channels in Beta cells • Reduction of serum glucagon levels (Chronic use) • Sensitize tissues to insulin

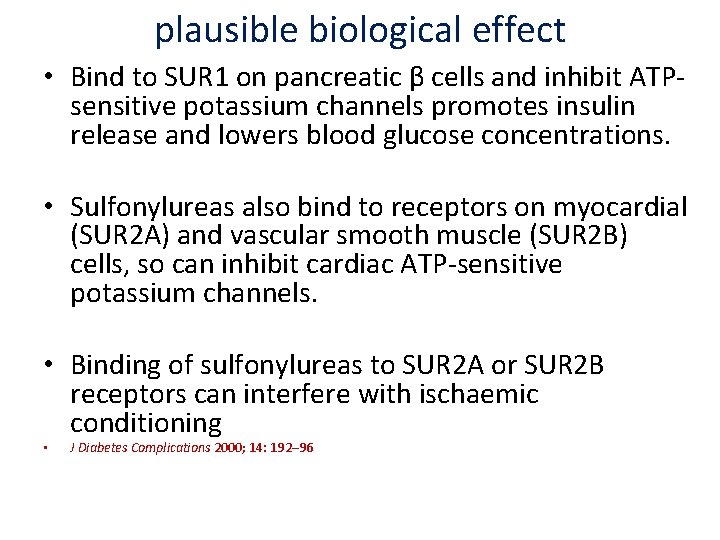
plausible biological effect • Bind to SUR 1 on pancreatic β cells and inhibit ATP‐ sensitive potassium channels promotes insulin release and lowers blood glucose concentrations. • Sulfonylureas also bind to receptors on myocardial (SUR 2 A) and vascular smooth muscle (SUR 2 B) cells, so can inhibit cardiac ATP‐sensitive potassium channels. • Binding of sulfonylureas to SUR 2 A or SUR 2 B receptors can interfere with ischaemic conditioning • J Diabetes Complications 2000; 14: 192– 96


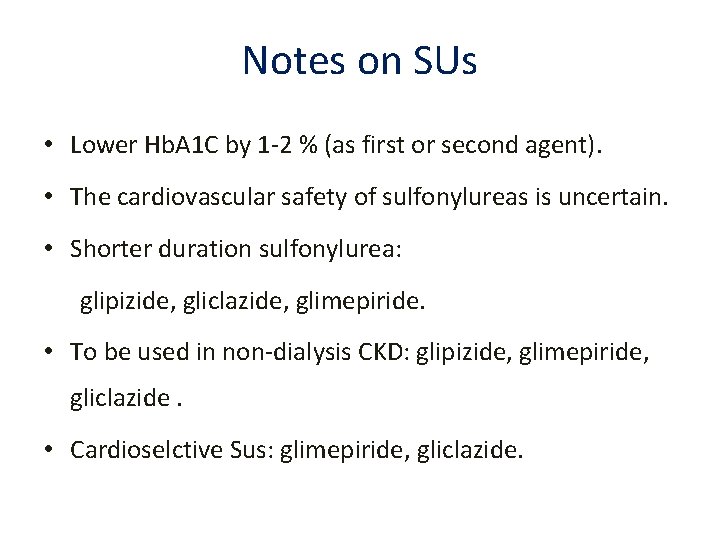
Notes on SUs • Lower Hb. A 1 C by 1‐ 2 % (as first or second agent). • The cardiovascular safety of sulfonylureas is uncertain. • Shorter duration sulfonylurea: glipizide, gliclazide, glimepiride. • To be used in non‐dialysis CKD: glipizide, glimepiride, gliclazide. • Cardioselctive Sus: glimepiride, gliclazide.

Sulfonylureas • First generation: Tolbutamide, Chlorpropamide, Tolazamide • Second generation: Glibenclamide (Glyburide), Glipizide, Gliclazide, Glimepiride

Sulfonylureas • Efficay • Adverse effects – Hypoglycemia – Weight gain – CV Event

J Clin Endocrinol Metab. 2012; 97(12): 4605 -4612.

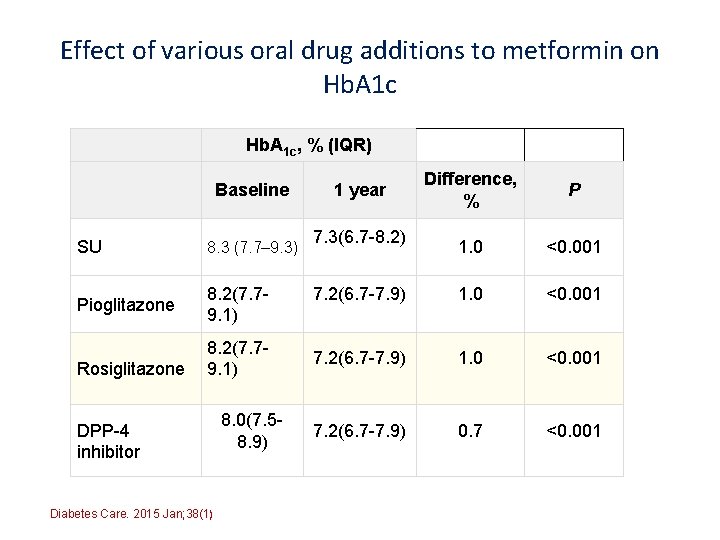
Effect of various oral drug additions to metformin on Hb. A 1 c, % (IQR) Baseline SU 8. 3 (7. 7– 9. 3) Pioglitazone 8. 2(7. 79. 1) Rosiglitazone 8. 2(7. 79. 1) DPP-4 inhibitor Diabetes Care. 2015 Jan; 38(1) 8. 0(7. 58. 9) Difference, % P 1. 0 <0. 001 7. 2(6. 7 -7. 9) 0. 7 <0. 001 1 year 7. 3(6. 7 -8. 2)

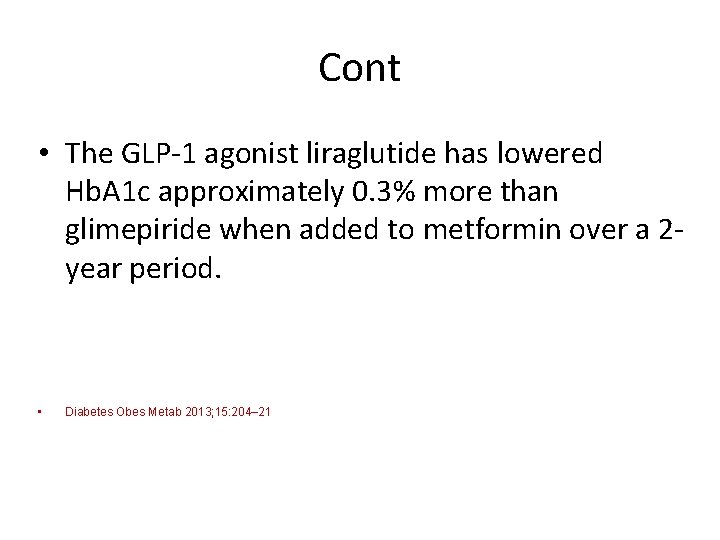
Cont • The GLP‐ 1 agonist liraglutide has lowered Hb. A 1 c approximately 0. 3% more than glimepiride when added to metformin over a 2‐ year period. • Diabetes Obes Metab 2013; 15: 204– 21

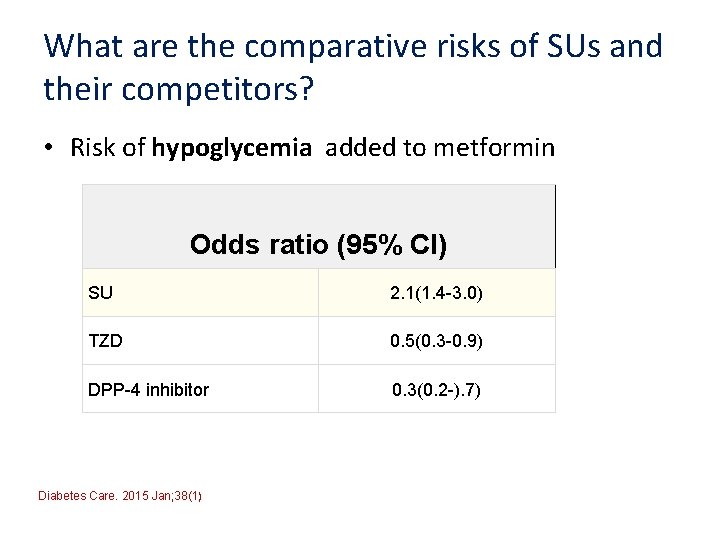
What are the comparative risks of SUs and their competitors? • Risk of hypoglycemia added to metformin Odds ratio (95% CI) SU 2. 1(1. 4 -3. 0) TZD 0. 5(0. 3 -0. 9) DPP-4 inhibitor 0. 3(0. 2 -). 7) Diabetes Care. 2015 Jan; 38(1)

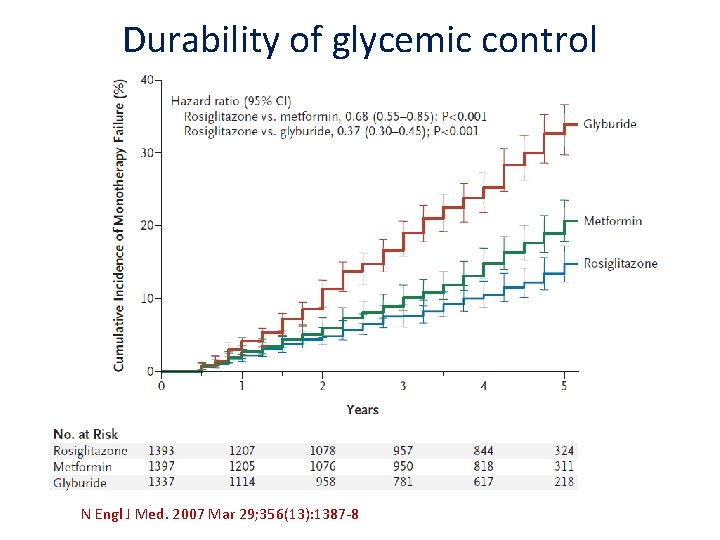
Durability of glycemic control N Engl J Med. 2007 Mar 29; 356(13): 1387‐ 8.

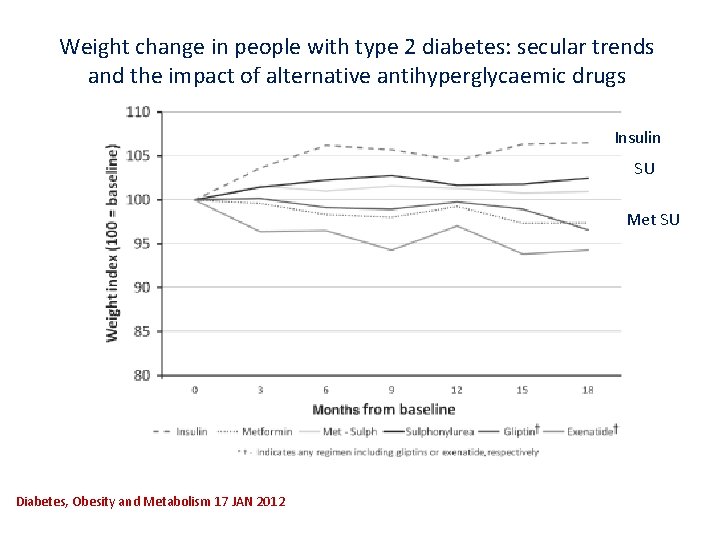
Weight change in people with type 2 diabetes: secular trends and the impact of alternative antihyperglycaemic drugs Insulin SU Met SU Diabetes, Obesity and Metabolism 17 JAN 2012

Cost • Another “adverse effect” of drug use can be their costs. • SUs enjoy a major advantage. • For patients lacking adequate insurance coverage, all the SUs are the choice.

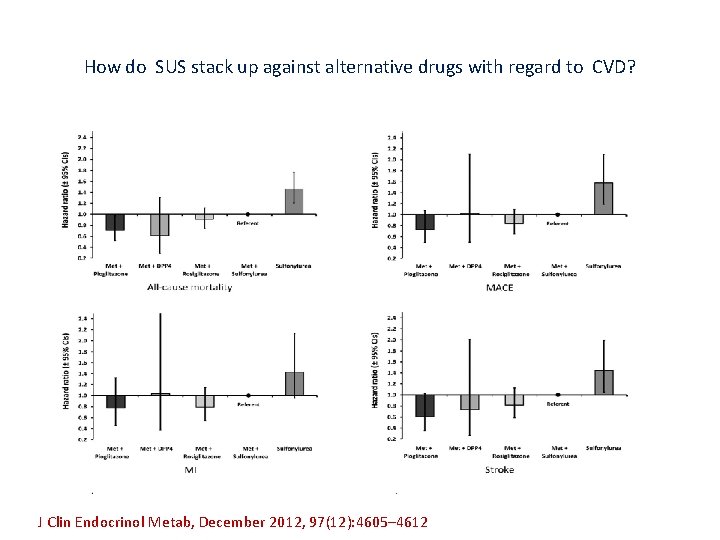
How do SUS stack up against alternative drugs with regard to CVD? J Clin Endocrinol Metab, December 2012, 97(12): 4605– 4612

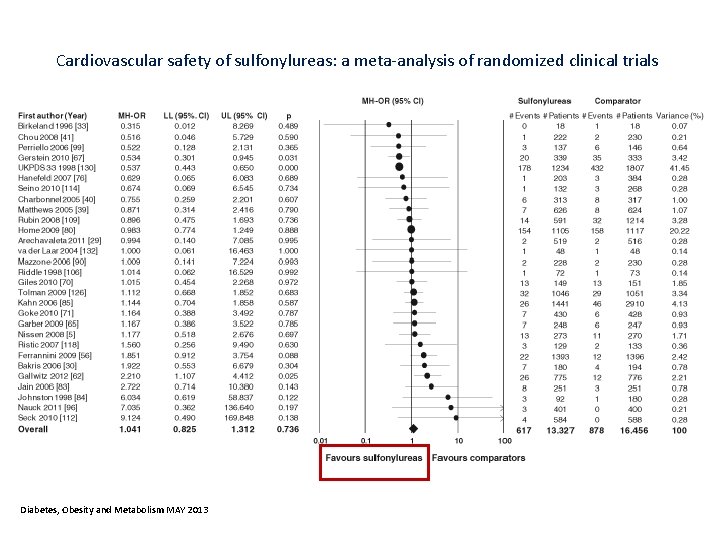
Cardiovascular safety of sulfonylureas: a meta‐analysis of randomized clinical trials Diabetes, Obesity and Metabolism MAY 2013

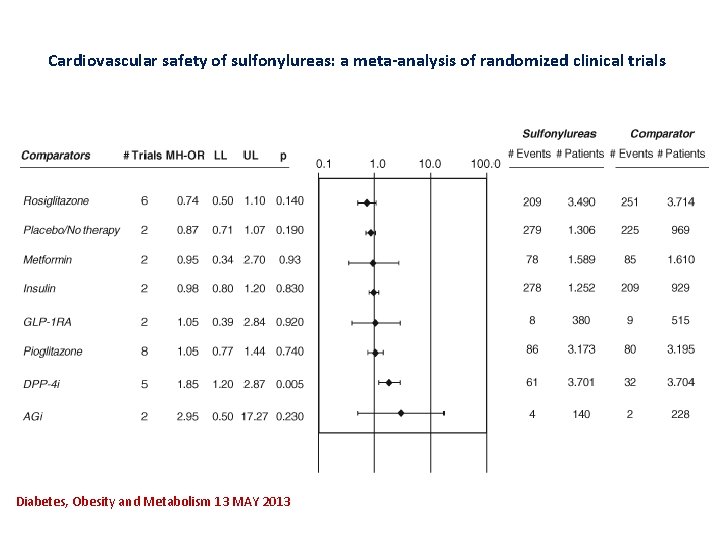
Cardiovascular safety of sulfonylureas: a meta‐analysis of randomized clinical trials Diabetes, Obesity and Metabolism 13 MAY 2013

Which Sus?

Which SU Should Be Used? • Individual SUs express a different selectivity for pancreatic and myocardial SU receptors. • Gliclazide seems the most selective with respect to pancreatic receptor stimulation. • Amongst other SUs, specifically advising Gliclazide is based on evidence from observational studies showing cardiovascular benefits of Gliclazide over other SUs. 31

Glycemic efficacy of SUF 9/24/2020 32

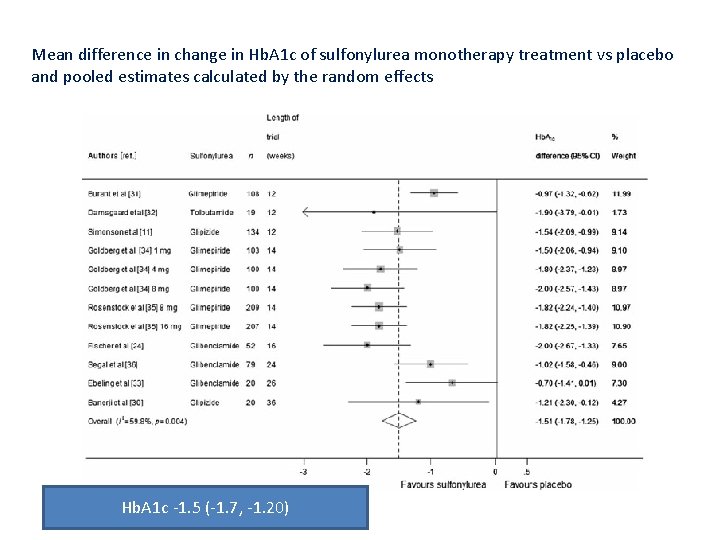
Mean difference in change in Hb. A 1 c of sulfonylurea monotherapy treatment vs placebo and pooled estimates calculated by the random effects Hb. A 1 c ‐ 1. 5 (‐ 1. 7, ‐ 1. 20)

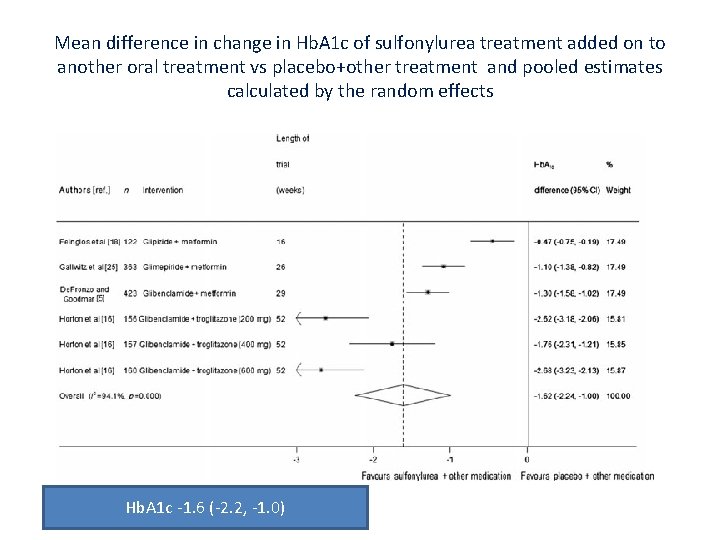
Mean difference in change in Hb. A 1 c of sulfonylurea treatment added on to another oral treatment vs placebo+other treatment and pooled estimates calculated by the random effects Hb. A 1 c ‐ 1. 6 (‐ 2. 2, ‐ 1. 0)

Hypoglycemia • The second plausible mechanism for the higher risk of adverse CV effects associated. • Can prolong the QT interval and are associated with cardiac ischaemia. • A prolonged QT interval and cardiac ischamia can increase the risk of adverse CVE, such as ventricular arrhythmias, MI, and sudden cardiac death. • Differences in SUR 1 receptor affinity and pharmacokinetic properties seem to create differences in the risk of hypoglycamia. • Glibenclamide, which has the highest affinity for SUR 1 having the highest risk among the Sus.

Lancet Diabetes Endocrinol 2015; 3: 43‐ 51.

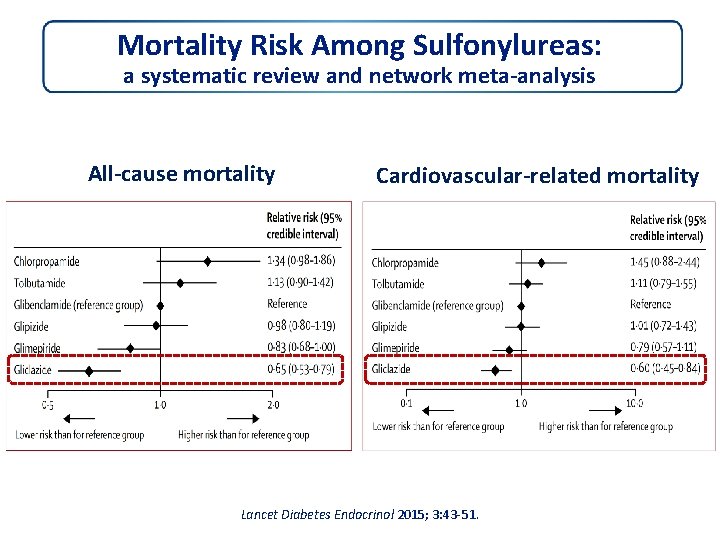
Mortality Risk Among Sulfonylureas: a systematic review and network meta‐analysis All‐cause mortality Cardiovascular‐related mortality Lancet Diabetes Endocrinol 2015; 3: 43‐ 51.



Glibenclamide • Glibenclamide has been associated with an increased risk of severe hypoglycemia, especially in the elderly. • Evidence show the increased relative risk of hypoglycemia and the resulting harm with use of glibenclamide versus any of the other second generation SFUs, particularly gliclazide and glipizide. WHO; EML Section 18. 5 – Insulin and other medicines used for diabetes

Gliclazide • Gliclazide is the first choice sulphonylurea for type 2 diabetes in many countries as it is associated with a lower risk of hypoglycemia. • When added to metformin, gliclazide was associated with the lowest risk of hypoglycaemia between the newer generation SUFs. • Gliclazide reduces platelet adhesion, aggregation and hyperactivity and increases fibrinolysis. These actions, thought to be independent of its hypoglycaemic activity, may make gliclazide useful in halting the progression of diabetic microangiopathy BMJ 2016; 354: i 3625 Br J Clin Pharmacol (2016) 82 1291– 1302 Journal of Applied Pharmaceutical Science 01 (09); 2011: 11‐ 19

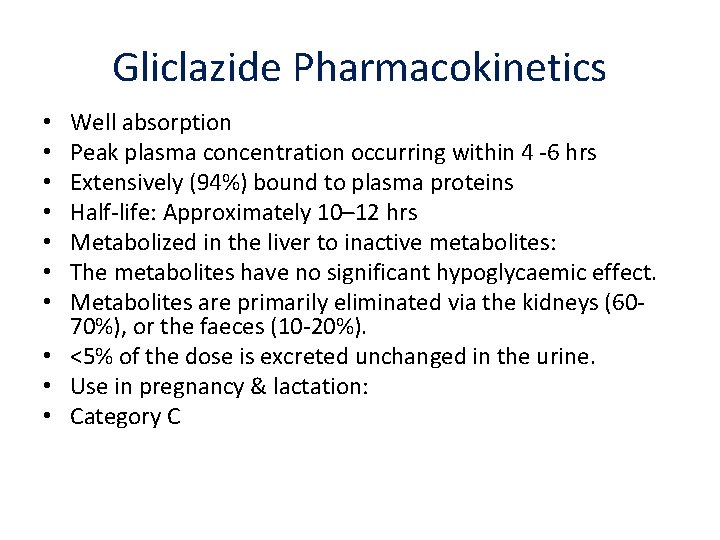
Gliclazide Pharmacokinetics Well absorption Peak plasma concentration occurring within 4 ‐ 6 hrs Extensively (94%) bound to plasma proteins Half‐life: Approximately 10– 12 hrs Metabolized in the liver to inactive metabolites: The metabolites have no significant hypoglycaemic effect. Metabolites are primarily eliminated via the kidneys (60‐ 70%), or the faeces (10‐ 20%). • <5% of the dose is excreted unchanged in the urine. • Use in pregnancy & lactation: • Category C • •

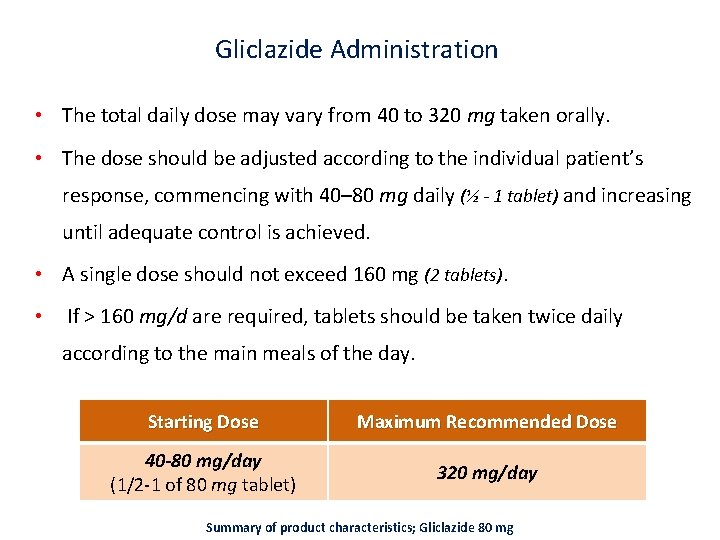
Gliclazide Administration • The total daily dose may vary from 40 to 320 mg taken orally. • The dose should be adjusted according to the individual patient’s response, commencing with 40– 80 mg daily (½ - 1 tablet) and increasing until adequate control is achieved. • A single dose should not exceed 160 mg (2 tablets). • If > 160 mg/d are required, tablets should be taken twice daily according to the main meals of the day. Starting Dose Maximum Recommended Dose 40 -80 mg/day (1/2‐ 1 of 80 mg tablet) 320 mg/day Summary of product characteristics; Gliclazide 80 mg

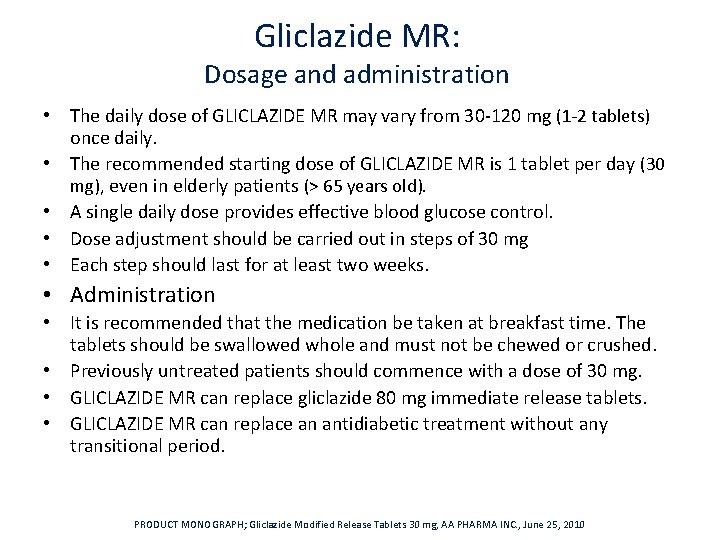
Gliclazide MR: Dosage and administration • The daily dose of GLICLAZIDE MR may vary from 30‐ 120 mg (1‐ 2 tablets) once daily. • The recommended starting dose of GLICLAZIDE MR is 1 tablet per day (30 mg), even in elderly patients (> 65 years old). • A single daily dose provides effective blood glucose control. • Dose adjustment should be carried out in steps of 30 mg • Each step should last for at least two weeks. • Administration • It is recommended that the medication be taken at breakfast time. The tablets should be swallowed whole and must not be chewed or crushed. • Previously untreated patients should commence with a dose of 30 mg. • GLICLAZIDE MR can replace gliclazide 80 mg immediate release tablets. • GLICLAZIDE MR can replace an antidiabetic treatment without any transitional period. PRODUCT MONOGRAPH; Gliclazide Modified Release Tablets 30 mg, AA PHARMA INC. , June 25, 2010

conclusion • • Consider individualization for your patients Consider all factors for the selection Sus are as effective as other OHA Consider risk of hypoglycemia and weight gain Cost of the drug We are not sure for cardiovascular unsafety Short acting drugs such as gliclazide are the best

 Atieh madani
Atieh madani Jamshid amouzegar
Jamshid amouzegar Sulfonylurea drugs
Sulfonylurea drugs Biguanides moa
Biguanides moa Sulfonylureas drugs
Sulfonylureas drugs Repaglinide mechanism of action
Repaglinide mechanism of action Insulin sensitivity factor
Insulin sensitivity factor Disease artinya
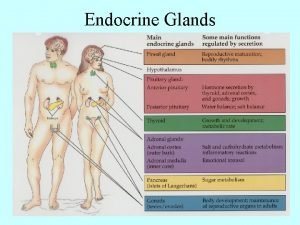
Disease artinya Major endocrine glands male and female
Major endocrine glands male and female Chapter 16 matching questions 20-24
Chapter 16 matching questions 20-24 Endocrine system and reproductive system
Endocrine system and reproductive system Kelenjar endokrin
Kelenjar endokrin Parts of the endocrine system
Parts of the endocrine system Endocrine system vs nervous system
Endocrine system vs nervous system Mechanisms of hypothalamic control over endocrine function
Mechanisms of hypothalamic control over endocrine function The body's speedy electrochemical communication network
The body's speedy electrochemical communication network Endocrine anatomy
Endocrine anatomy Comparison of endocrine and nervous system
Comparison of endocrine and nervous system Gonads glands
Gonads glands Facts about the endocrine system
Facts about the endocrine system Difference between endocrine and exocrine glands
Difference between endocrine and exocrine glands Lympathic
Lympathic Endocrine glands of rat
Endocrine glands of rat Chapter 46 digestive and endocrine disorders
Chapter 46 digestive and endocrine disorders Calcitonin and pth are antagonistic hormones
Calcitonin and pth are antagonistic hormones Chapter 39 endocrine and reproductive systems
Chapter 39 endocrine and reproductive systems Chapter 29 endocrine and metabolic disorders
Chapter 29 endocrine and metabolic disorders Chapter 19 endocrine and hematologic emergencies
Chapter 19 endocrine and hematologic emergencies Metabolic action of growth hormone
Metabolic action of growth hormone Endocrine and nervous system comparison
Endocrine and nervous system comparison Chapter 7 13 endocrine system
Chapter 7 13 endocrine system Mammillary body
Mammillary body Glisson's capsule
Glisson's capsule Stimulus humoral
Stimulus humoral Janos lobe
Janos lobe Endocrine axis
Endocrine axis Pituitary gland
Pituitary gland Endo crine gland
Endo crine gland Endocrine exocrine
Endocrine exocrine Goiter
Goiter Whats the difference between endocrine and exocrine glands
Whats the difference between endocrine and exocrine glands Chapter 11 endocrine system
Chapter 11 endocrine system Biology 30 endocrine system
Biology 30 endocrine system Endocrine exocrine
Endocrine exocrine Estrogen effect
Estrogen effect Endocrine system
Endocrine system Endocrine
Endocrine