Insulin Initiation Atieh Amouzegar MD Research Institute For

























- Slides: 45

Insulin Initiation Atieh Amouzegar MD Research Institute For Endocrine Sciences Endocrine Research Center Shahid Beheshti University 04. 1397

Outlines • • Case presentation Barriers of insulin therapy Action-profile Types of basal insulins How to begin basal regimen How to titrate basal regimen OHA and insulin

• Based on one recent study, the A 1 C level that triggers glucose-lowering action is > 9% Am J Manag Care 9: 213– 217, 2003

“patient-centered” approach • Individualizing therapy and goals for pts With T 2 DM • The individualized targets should take into account not only clinical conditions, such as relevant comorbid conditions, age, duration of diabetes, and history of sever hypoglycemia, but also the patient-specific psychosocioeconomic context

Barriers • Insulin causes blindness, renal failure, amputations, heart attacks, strokes, or early death • Sense of personal failure • Low self-confidence • Low confidence in therapy • Injection phobia • Hypoglycemia concerns • Feeling that diabetes is a serious cause of concern • Negative impact on social life and job • Inadequate health literacy • Health care provider inadequately explaining risks/benefits • Limited insulin self-management training

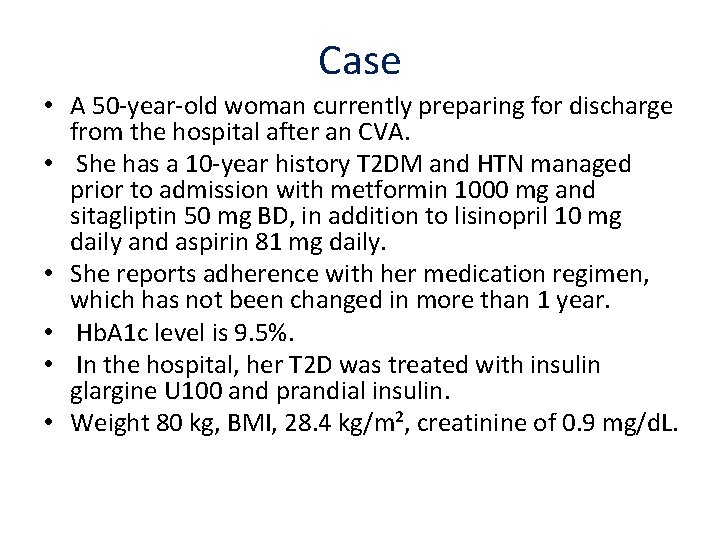
Case • A 50 -year-old woman currently preparing for discharge from the hospital after an CVA. • She has a 10 -year history T 2 DM and HTN managed prior to admission with metformin 1000 mg and sitagliptin 50 mg BD, in addition to lisinopril 10 mg daily and aspirin 81 mg daily. • She reports adherence with her medication regimen, which has not been changed in more than 1 year. • Hb. A 1 c level is 9. 5%. • In the hospital, her T 2 D was treated with insulin glargine U 100 and prandial insulin. • Weight 80 kg, BMI, 28. 4 kg/m², creatinine of 0. 9 mg/d. L.

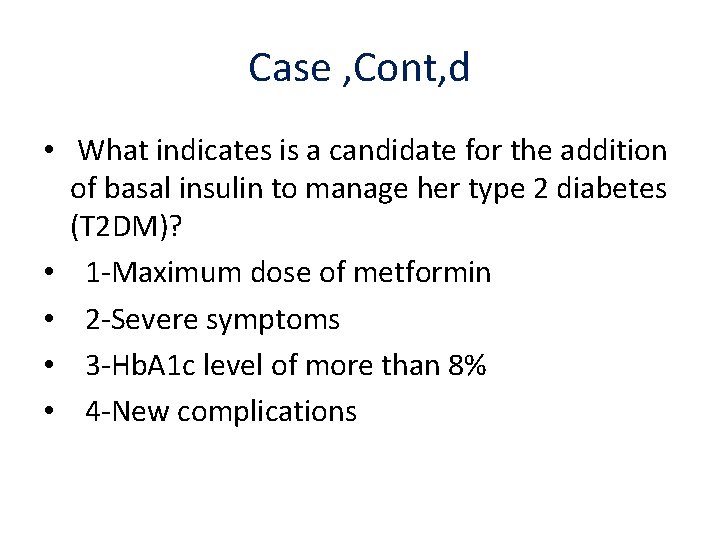
Case , Cont, d • What indicates is a candidate for the addition of basal insulin to manage her type 2 diabetes (T 2 DM)? • 1 -Maximum dose of metformin • 2 -Severe symptoms • 3 -Hb. A 1 c level of more than 8% • 4 -New complications

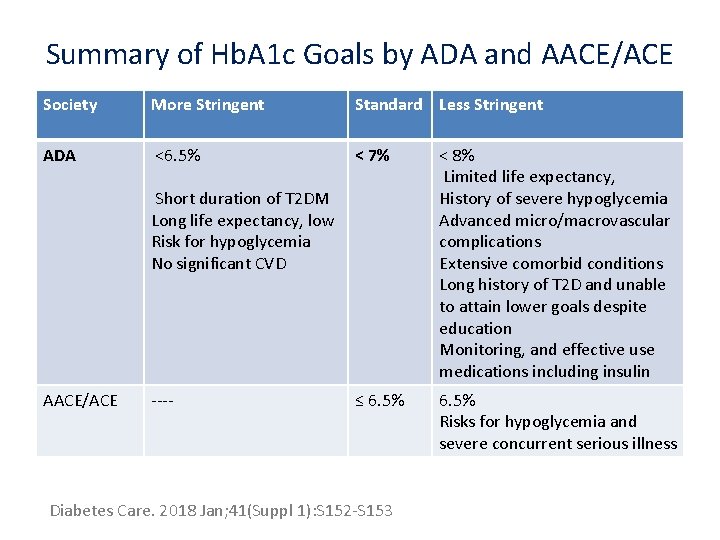
Summary of Hb. A 1 c Goals by ADA and AACE/ACE Society More Stringent Standard Less Stringent ADA <6. 5% < 7% < 8% Limited life expectancy, History of severe hypoglycemia Advanced micro/macrovascular complications Extensive comorbid conditions Long history of T 2 D and unable to attain lower goals despite education Monitoring, and effective use medications including insulin ≤ 6. 5% Risks for hypoglycemia and severe concurrent serious illness Short duration of T 2 DM Long life expectancy, low Risk for hypoglycemia No significant CVD AACE/ACE ---- Diabetes Care. 2018 Jan; 41(Suppl 1): S 152 -S 153

Answer • Basal insulin becomes an option when: • 1 -patients do not reach therapeutic Hb. A 1 c goals after 3 months of oral medications titrated to maximum doses. • 2 - If severe symptoms are present such as ketosis or weight loss. • Although metformin and sitagliptin are at maximum doses, there are other oral medications she could use. • However, in patients who are already taking maximum doses of 2 OHA and who have an Hb. A 1 c >8%, the chance of reaching their goal Hb. A 1 c level is lower with adding another oral agent vs adding insulin.


Notice! • Avoid using insulin as a threat or describing it as a sign of personal failure or punishment.

The Role of Basal Insulin • ADA recommends starting basal insulin in Pts with newly diagnosed T 2 DM • With symptoms • Hb. A 1 c > than 10% • Blood glucose level ≥ 300 mg/d. L

Consider • 1 - Weight gain • 2 -Hypoglycemia

Balancing Good Glycemic Control with a Low Risk of Hypoglycemia… Glycemic control Hypoglycemia

Patient Counseling Topics • Review symptoms and treatment of hypoglycemia • Proper training and correct use of glucose monitor • Target desired glycemic levels for each patient

Barriers to initiating Insulin • Patients may have fear—founded or unfounded—of injections, hypoglycemia, or weight gain. • There are limitations after you start treatment, such as hypoglycemia, nonadherence due to complexity of the regimen, and weight gain.

what would be an appropriate treatment plan for her to follow at home? • Long acting Insulin (glargine , determir, Abasaglar ) at 10 units daily • Metformin 1000 mg and sitagliptin 100 mg twice daily plus basal insulin 10 U • Metformin 1000 mg and sitagliptin 50 mg twice daily plus Long acting Insulin (glargine or determir )at 20 units daily • Metformin 1000 mg and sitagliptin 50 mg twice daily plus insulin NPH 16 units daily

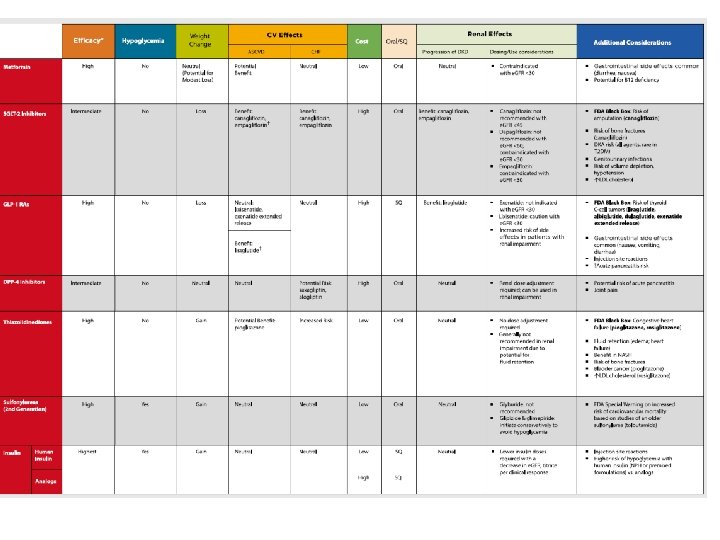
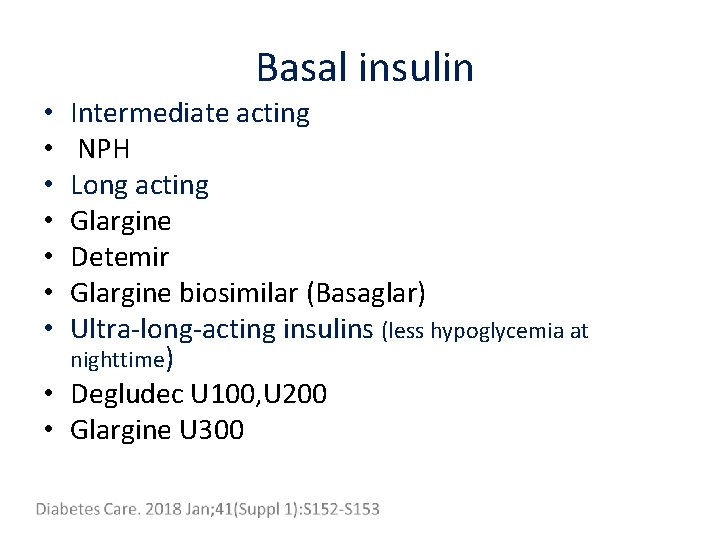
Basal insulin Intermediate acting NPH Long acting Glargine Detemir Glargine biosimilar (Basaglar) Ultra-long-acting insulins (less hypoglycemia at nighttime) • Degludec U 100, U 200 • Glargine U 300 • •

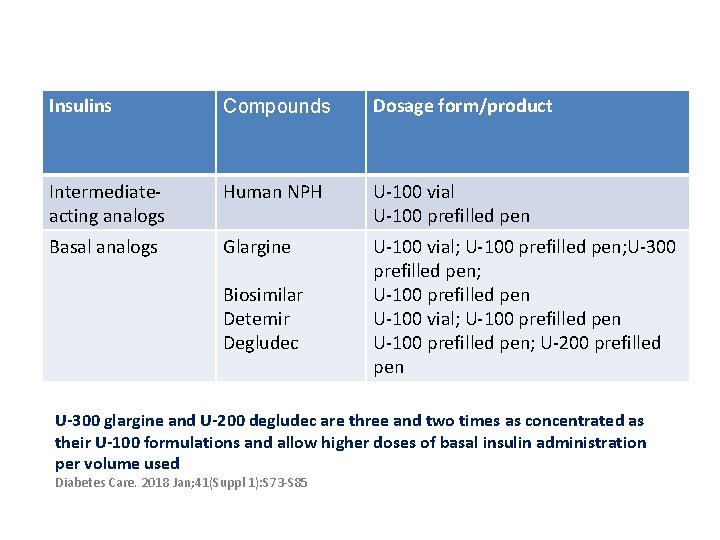
Insulins Compounds Dosage form/product Intermediateacting analogs Human NPH U-100 vial U-100 prefilled pen Basal analogs Glargine U-100 vial; U-100 prefilled pen; U-300 prefilled pen; U-100 prefilled pen U-100 vial; U-100 prefilled pen; U-200 prefilled pen Biosimilar Detemir Degludec U-300 glargine and U-200 degludec are three and two times as concentrated as their U-100 formulations and allow higher doses of basal insulin administration per volume used Diabetes Care. 2018 Jan; 41(Suppl 1): S 73 -S 85



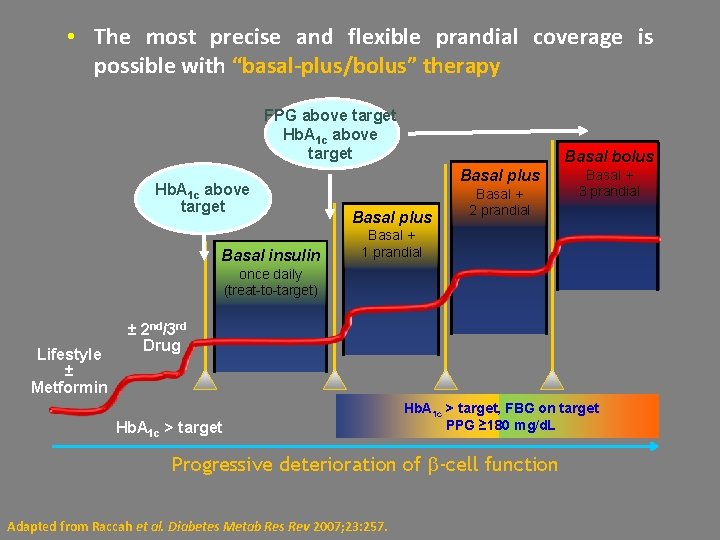
• The most precise and flexible prandial coverage is possible with “basal-plus/bolus” therapy FPG above target Hb. A 1 c above target Basal insulin Basal bolus Basal plus Basal + 2 prandial Basal + 3 prandial Basal + 1 prandial once daily (treat-to-target) Lifestyle ± Metformin ± 2 nd/3 rd Drug Hb. A 1 c > target, FBG on target PPG ≥ 180 mg/d. L Progressive deterioration of -cell function Adapted from Raccah et al. Diabetes Metab Res Rev 2007; 23: 257.

How do you pick basal insulin ? • The most affordable for the patient • Insulin will be covered by the patient's insurance • Available and accessible • How often the patient is able to administer insulin • Diabetes Care. 2018 Jan; 41(Suppl 1): S 73 -S 85

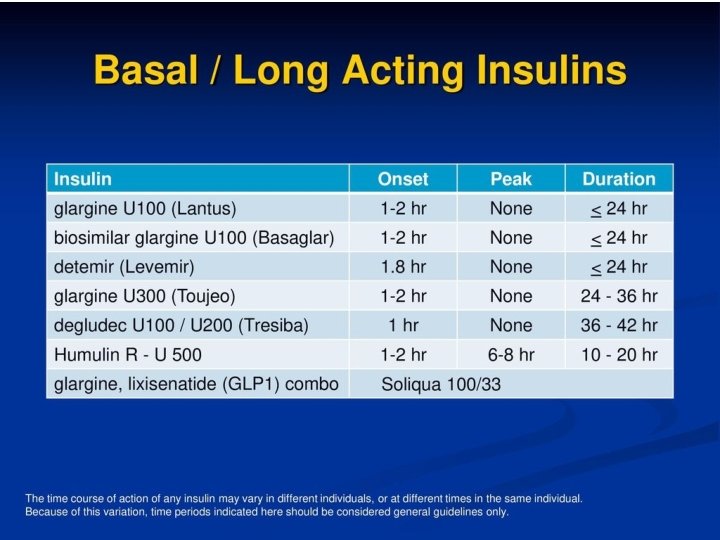
Basal Insulin Therapy and Hypoglycemia • The introduction of long-acting basal analogs, which have relatively flat and predictable time -action profiles compared with NPH, offers options for achieving glucose control with fewer episodes of hypoglycemia in the management of T 2 DM • Least hypoglycemia risk • It would be for patients who are experiencing nocturnal hypoglycemia • Diabetes Care. 2018 Jan; 41(Suppl 1): S 73 -S 85

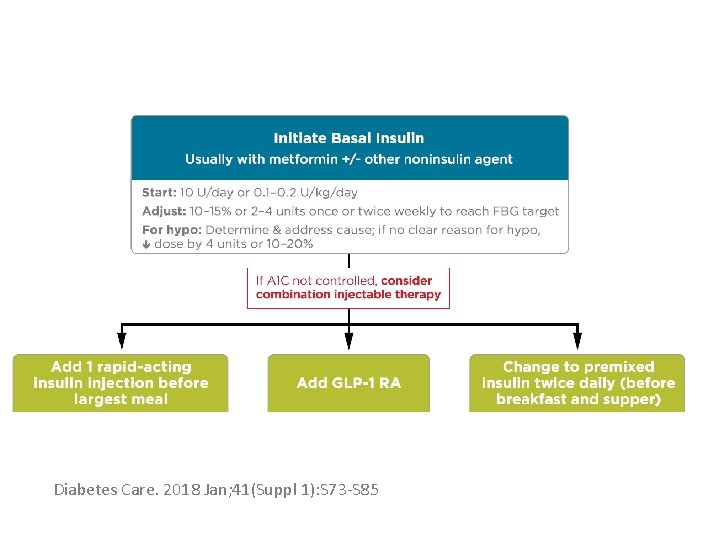
How to begin Basal • Two accepted approaches for choosing a dose of basal insulin include : • Starting with a fixed dose of 10 units per day • Determining a weight-based dose of 0. 1 - 0. 2 U/kg of body weight (in patients with usual insulin sensitivity and renal and hepatic function/day) • Although larger amounts (0. 3– 0. 4 U/kg/day) are reasonable in the more severely hyperglycemic

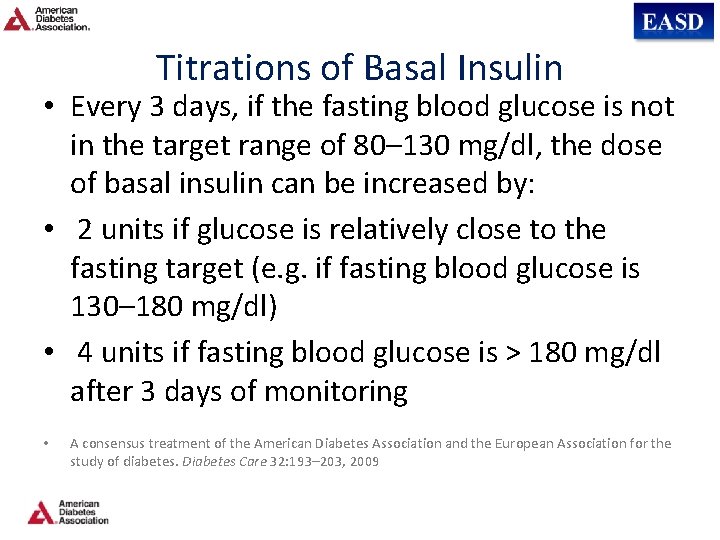
Titrations of Basal Insulin • Every 3 days, if the fasting blood glucose is not in the target range of 80– 130 mg/dl, the dose of basal insulin can be increased by: • 2 units if glucose is relatively close to the fasting target (e. g. if fasting blood glucose is 130– 180 mg/dl) • 4 units if fasting blood glucose is > 180 mg/dl after 3 days of monitoring • A consensus treatment of the American Diabetes Association and the European Association for the study of diabetes. Diabetes Care 32: 193– 203, 2009

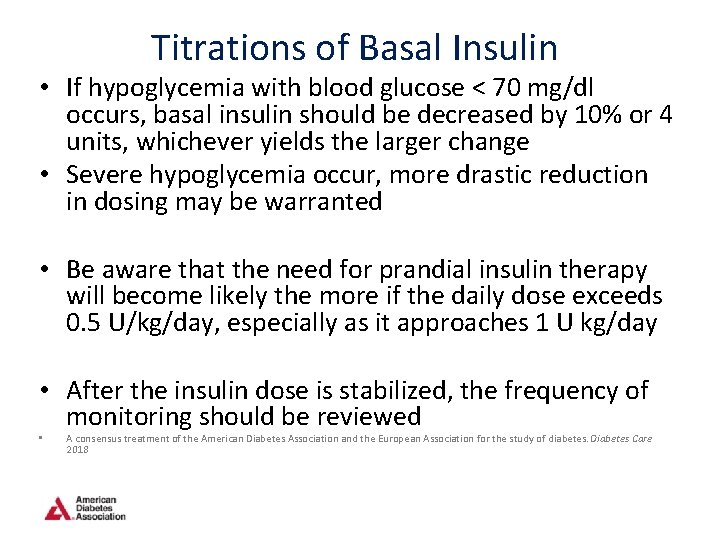
Titrations of Basal Insulin • If hypoglycemia with blood glucose < 70 mg/dl occurs, basal insulin should be decreased by 10% or 4 units, whichever yields the larger change • Severe hypoglycemia occur, more drastic reduction in dosing may be warranted • Be aware that the need for prandial insulin therapy will become likely the more if the daily dose exceeds 0. 5 U/kg/day, especially as it approaches 1 U kg/day • After the insulin dose is stabilized, the frequency of monitoring should be reviewed • A consensus treatment of the American Diabetes Association and the European Association for the study of diabetes. Diabetes Care 2018

Cont’d • If basal insulin has been titrated to an acceptable fasting blood glucose level (or if the dose is 0. 5 units/kg/day) and A 1 C remains above target, consider advancing to combination injectable therapy Diabetes Care. 2018 Jan; 41(Suppl 1): S 73 -S 85

• Diabetes Care. 2018 Jan; 41(Suppl 1): S 73 -S 85

• A common pitfall with basal insulin dosing is increasing the dose too much before adding prandial insulin • Often, providers may note continual “fasting” hyperglycemia and titrate glargine up to 60, 80, or 100 units per day before adding prandial insulin • A consensus treatment of the American Diabetes Association and the European Association for the study of diabetes. Diabetes Care 32: 193– 203, 2009

Basal Insulin and Oral Antidiabetic Agents • Basal insulin is usually prescribed in conjunction with metformin and sometimes one additional noninsulin agent. • In general, GLP-1 receptor agonists should not be discontinued with the initiation of basal insulin. • • Diabetes Care. 2006; 29: 1269 -1274. Diabetologia. 2006; 49: 442 -451

Cont’d • When initiating combination injectable therapy, metformin therapy should be maintained while other oral agents may be discontinued on an individual basis to avoid unnecessarily complex or costly regimens (i. e. , adding a fourth antihyperglycemic agent).

Cont’d • Sulfonylureas, DPP-4 inhibitors, and GLP- 1 receptor agonists are typically stopped once more complex insulin regimens beyond basal are used.

Adding rapid acting Vs GLP 1 agonists • Studies have demonstrated the noninferiority of basal insulin plus a single injection of rapidacting insulin at the largest meal relative to basal insulin plus a GLP-1 receptor agonist • Basal insulin plus GLP-1 agonists are associated with less hypoglycemia and with weight loss instead of weight gain but may be less tolerable and have a greater cost • • Lancet 2014; 384: 2228– 2234 Diabetes Care 2014; 37: 2763– 2773

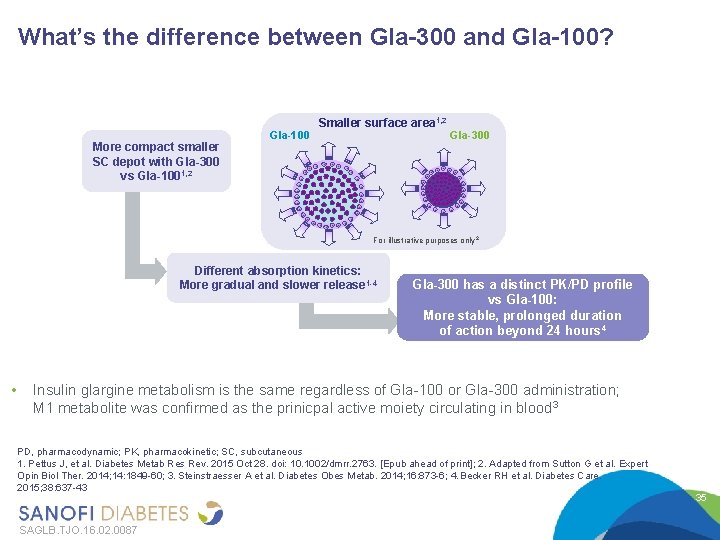
What’s the difference between Gla-300 and Gla-100? More compact smaller SC depot with Gla-300 vs Gla-1001, 2 Gla-100 Smaller surface area 1, 2 Gla-300 For illustrative purposes only 2 Different absorption kinetics: More gradual and slower release 1 -4 • Gla-300 has a distinct PK/PD profile vs Gla-100: More stable, prolonged duration of action beyond 24 hours 4 Insulin glargine metabolism is the same regardless of Gla-100 or Gla-300 administration; M 1 metabolite was confirmed as the prinicpal active moiety circulating in blood 3 PD, pharmacodynamic; PK, pharmacokinetic; SC, subcutaneous 1. Pettus J, et al. Diabetes Metab Res Rev. 2015 Oct 28. doi: 10. 1002/dmrr. 2763. [Epub ahead of print]; 2. Adapted from Sutton G et al. Expert Opin Biol Ther. 2014; 14: 1849 -60; 3. Steinstraesser A et al. Diabetes Obes Metab. 2014; 16: 873 -6; 4. Becker RH et al. Diabetes Care. 2015; 38: 637 -43 SAGLB. TJO. 16. 02. 0087 35

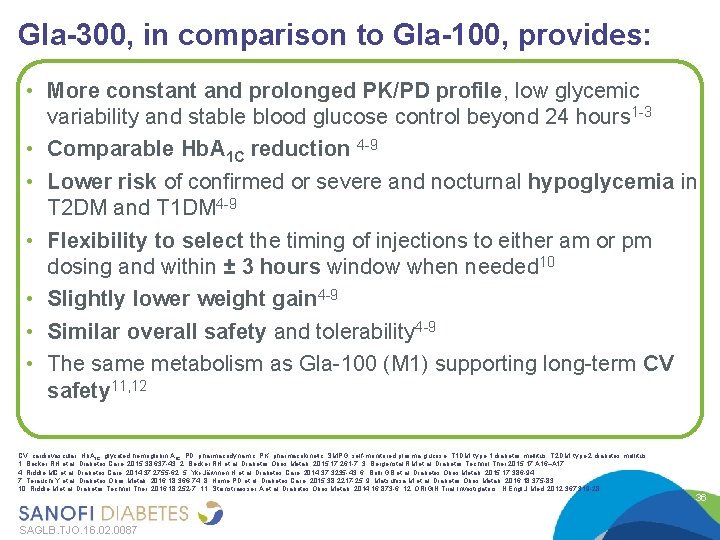
Gla-300, in comparison to Gla-100, provides: Age 20 to 79 years old • More constant and prolonged PK/PD profile, low glycemic variability and stable blood glucose control beyond 24 hours 1 -3 • Comparable Hb. A 1 C reduction 4 -9 • Lower risk of confirmed or severe and nocturnal hypoglycemia in T 2 DM and T 1 DM 4 -9 • Flexibility to select the timing of injections to either am or pm dosing and within ± 3 hours window when needed 10 • Slightly lower weight gain 4 -9 • Similar overall safety and tolerability 4 -9 • The same metabolism as Gla-100 (M 1) supporting long-term CV safety 11, 12 CV, cardiovascular; Hb. A 1 C, glycated hemoglobin A 1 C; PD, pharmacodynamic; PK, pharmacokinetic; SMPG, self-monitored plasma glucose; T 1 DM, type 1 diabetes mellitus; T 2 DM, type 2 diabetes mellitus 1. Becker RH et al. Diabetes Care. 2015; 38: 637 -43; 2. Becker RH et al. Diabetes Obes Metab. 2015; 17: 261 -7; 3. Bergenstal RM et al. Diabetes Technol Ther 2015; 17: A 16–A 17; 4. Riddle MC et al. Diabetes Care. 2014; 37: 2755 -62; 5. Yki-Järvinen H et al. Diabetes Care. 2014; 37: 3235 -43; 6. Bolli GB et al. Diabetes Obes Metab. 2015; 17: 386 -94; 7. Terauchi Y et al. Diabetes Obes Metab. 2016; 18: 366 -74; 8. Home PD et al. Diabetes Care. 2015; 38: 2217 -25; 9. Matsuhisa M et al. Diabetes Obes Metab. 2016; 18: 375 -83; 10. Riddle M et al. Diabetes Technol Ther. 2016; 18: 252 -7; 11. Steinstraesser A et al. Diabetes Obes Metab. 2014; 16: 873 -6; 12. ORIGIN Trial Investigators. N Engl J Med. 2012; 367: 319 -28 SAGLB. TJO. 16. 02. 0087 36

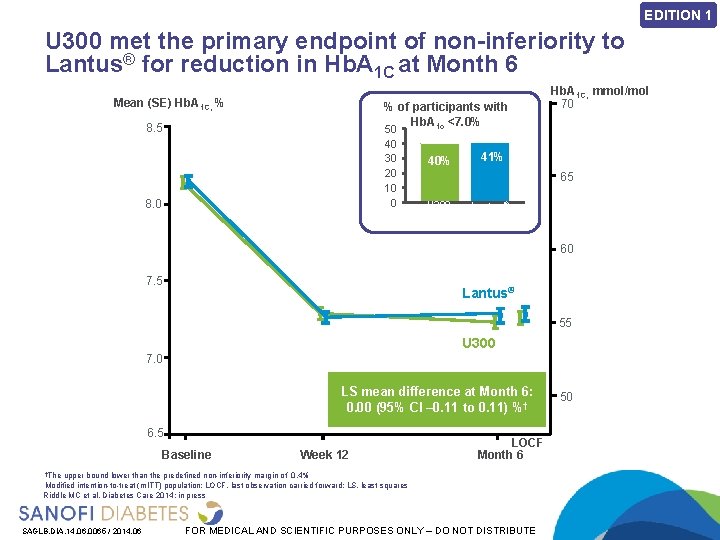
EDITION 1 U 300 met the primary endpoint of non-inferiority to Lantus® for reduction in Hb. A 1 C at Month 6 Mean (SE) Hb. A 1 C, % % of participants with Hb. A 1 c <7. 0% 8. 5 50 40 30 20 10 0 8. 0 40% Hb. A 1 C, mmol/mol 70 41% 65 U 300 Lantus® 60 7. 5 Lantus® 55 U 300 7. 0 LS mean difference at Month 6: 0. 00 (95% CI – 0. 11 to 0. 11) %† 6. 5 Baseline Week 12 LOCF Month 6 †The upper bound lower than the predefined non-inferiority margin of 0. 4% Modified intention-to-treat (m. ITT) population; LOCF, last observation carried forward; LS, least squares Riddle MC et al. Diabetes Care 2014: in press SAGLB. DIA. 14. 06. 0065 / 2014. 06 FOR MEDICAL AND SCIENTIFIC PURPOSES ONLY – DO NOT DISTRIBUTE 50

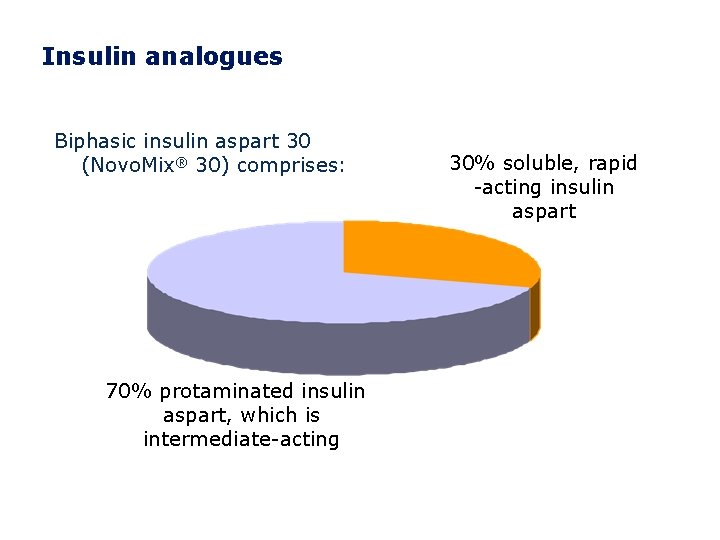
Insulin analogues Biphasic insulin aspart 30 (Novo. Mix® 30) comprises: 70% protaminated insulin aspart, which is intermediate-acting 30% soluble, rapid -acting insulin aspart

• How to begin: Premix

Start and stay with Novo. Mix® 30 1. 2. 3. Novo. Mix® 30 Summary of Product Characteristics. Garber A et al. The 1 -2 -3 study. Diabetes, Obesity and Metabolism 2006; 0: 1– 10. Raskin P et al. On behalf of the INITIATE Study Group. Basal insulin or premix analogue therapy in type 2 diabetes patients. Euro J Int Med 2007; 18: 56– 62.

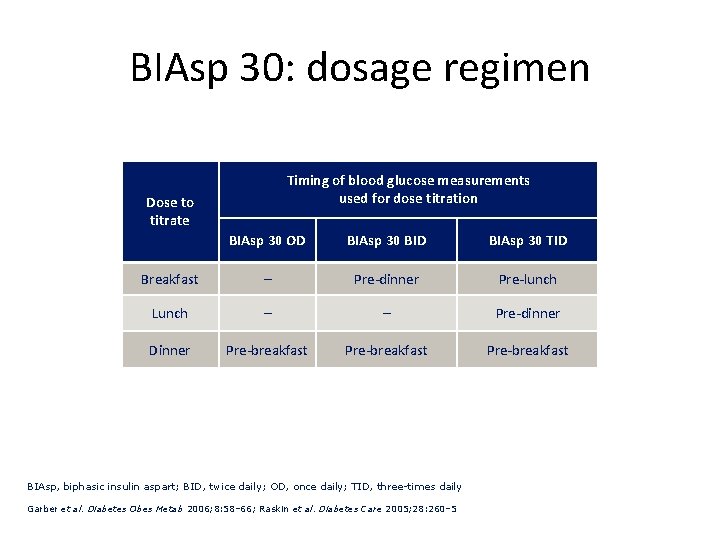
BIAsp 30: dosage regimen Timing of blood glucose measurements used for dose titration Dose to titrate BIAsp 30 OD BIAsp 30 BID BIAsp 30 TID Breakfast – Pre-dinner Pre-lunch Lunch – – Pre-dinner Dinner Pre-breakfast BIAsp, biphasic insulin aspart; BID, twice daily; OD, once daily; TID, three-times daily Garber et al. Diabetes Obes Metab 2006; 8: 58– 66; Raskin et al. Diabetes Care 2005; 28: 260– 5

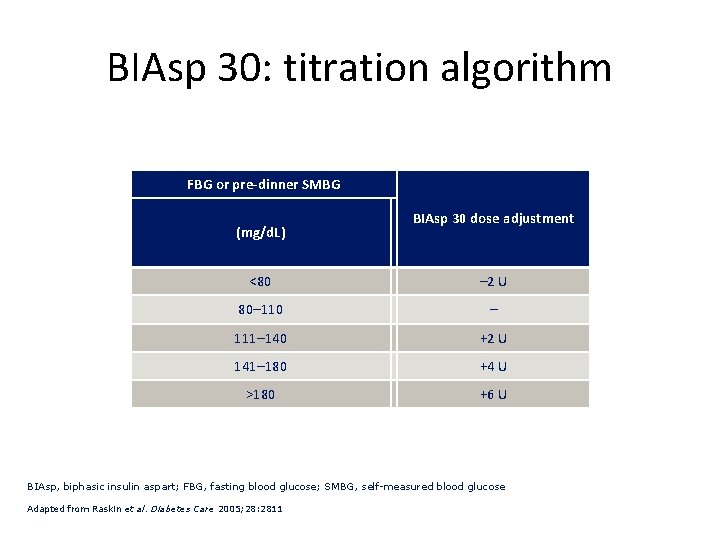
BIAsp 30: titration algorithm FBG or pre-dinner SMBG (mg/d. L) BIAsp 30 dose adjustment <80 – 2 U 80– 110 – 111– 140 +2 U 141– 180 +4 U >180 +6 U BIAsp, biphasic insulin aspart; FBG, fasting blood glucose; SMBG, self-measured blood glucose Adapted from Raskin et al. Diabetes Care 2005; 28: 2811

Recommendations • Perform the A 1 C test at least two times a year in patients who are meeting treatment goals (and who have stable glycemic control). • Perform the A 1 C test quarterly in patients whose therapy has changed or who are not meeting glycemic goals.

CONCLUSIONS • The importance of individualizing treatment in pts with T 2 DM is established • Note the barriers which are very important • Initiate with a basal insulin • Among the available insulins, long acting insulins cover all the items that have been mentioned • Titrate appropriately • Act in the way that be treat to target

 Atieh madani
Atieh madani Jamshid amouzegar
Jamshid amouzegar Insulin and insulin receptor
Insulin and insulin receptor Insulin and insulin receptor
Insulin and insulin receptor Clozapine titration chart
Clozapine titration chart Transcription initiation in eukaryotes
Transcription initiation in eukaryotes Supreme ordeal in the odyssey
Supreme ordeal in the odyssey Initiation radical reaction
Initiation radical reaction Raci 차트
Raci 차트 Importance of project initiation
Importance of project initiation Initiation à la recherche en soins infirmiers
Initiation à la recherche en soins infirmiers Site initiation visit powerpoint presentation
Site initiation visit powerpoint presentation Contoh inisiasi proyek sistem informasi
Contoh inisiasi proyek sistem informasi Phases of home visit in community health nursing
Phases of home visit in community health nursing In file organization a fixed format is used for records
In file organization a fixed format is used for records Divergent diagram
Divergent diagram Desert archetype examples
Desert archetype examples Sop sign off sheet
Sop sign off sheet Sensibilisation et initiation à la cybersécurité
Sensibilisation et initiation à la cybersécurité Experimenting relationship stage
Experimenting relationship stage Initiation and maintenance of callus culture
Initiation and maintenance of callus culture A quoi sert la qualité
A quoi sert la qualité L'initiation
L'initiation Initiation à la démonstration 5ème
Initiation à la démonstration 5ème Ccc 1326
Ccc 1326 Separation initiation return
Separation initiation return Revelation hero's journey
Revelation hero's journey Peace amplifier
Peace amplifier Pre initiation phase project management
Pre initiation phase project management Disjunctive topic shift
Disjunctive topic shift Initiation promotion progression
Initiation promotion progression Initiation complex
Initiation complex Central dogma
Central dogma Project selection process
Project selection process Kontinuitetshantering i praktiken
Kontinuitetshantering i praktiken Novell typiska drag
Novell typiska drag Nationell inriktning för artificiell intelligens
Nationell inriktning för artificiell intelligens Vad står k.r.å.k.a.n för
Vad står k.r.å.k.a.n för Varför kallas perioden 1918-1939 för mellankrigstiden?
Varför kallas perioden 1918-1939 för mellankrigstiden? En lathund för arbete med kontinuitetshantering
En lathund för arbete med kontinuitetshantering Underlag för särskild löneskatt på pensionskostnader
Underlag för särskild löneskatt på pensionskostnader Tidbok yrkesförare
Tidbok yrkesförare Anatomi organ reproduksi
Anatomi organ reproduksi Förklara densitet för barn
Förklara densitet för barn Datorkunskap för nybörjare
Datorkunskap för nybörjare Boverket ka
Boverket ka