Phenols Dr Md Ashraful Alam ArOH Phenols are





















- Slides: 38

Phenols Dr Md Ashraful Alam

Ar-OH Phenols are compounds with an –OH group attached to an aromatic carbon. Although they share the same functional group with alcohols, where the –OH group is attached to an aliphatic carbon, the chemistry of phenols is very different from that of alcohols.

Nomenclature. Phenols are usually named as substituted phenols. The methylphenols are given the special name, cresols. Some other phenols are named as hydroxy compounds.

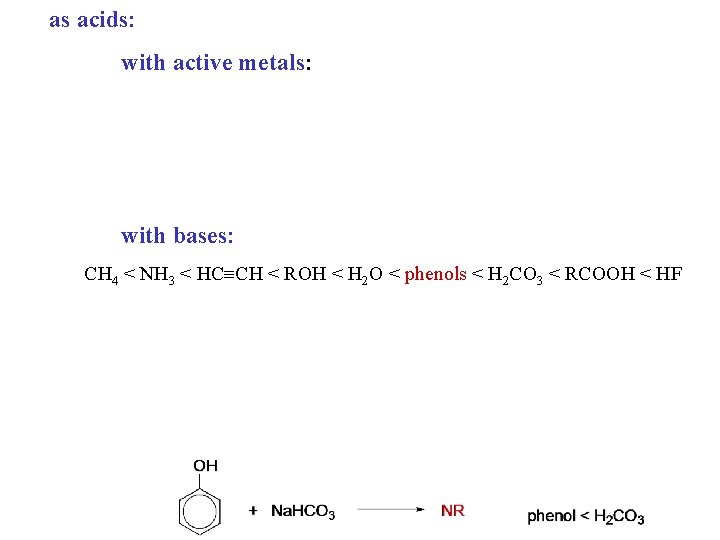
Physical properties üThe crystals are hygroscopic and turn pink to red in air üphenols are polar and can hydrogen bond üphenols are water insoluble üphenols are stronger acids than water and will dissolve in 5% Na. OH üphenols are weaker acids than carbonic acid and (do not dissolve in 5% Na. HCO 3 ) Phenol: ◦ is poisonous, corrosive, and flammable. ◦ affects the central nervous system and targets the liver and kidneys. ◦ is mutagenic and possibly teratogenic.

Intramolecular hydrogen bonding is possible in some ortho-substituted phenols. This intramolecular hydrogen bonding reduces water solubility and increases volatility. Thus, o-nitrophenol is steam distillable while the isomeric p-nitrophenol is not.

Routes of Exposure: Absorption All forms of phenol cause irritation, and acute toxic effects of phenol most often occur by skin contact. Even dilute solutions (1 to 2%) may cause severe burns if contact is prolonged. Due to its local anesthetizing properties, skin burns may be painless. Phenol vapor and liquid penetrate the skin readily. Systemic poisoning effects follow skin absorption. • Discoloration and severe burns may occur, but may be disguised by a loss of pain sensation. 6

Routes of Exposure: Products Containing Phenol, in low doses, can be found in some consumer products. It is used as a disinfectant, antiseptic and pain reliever Mostly used in the manufacture of resins and plastics, but it is also found in explosives, fertilizers, paints rubber, textiles, adhesives, drugs, paper, soap, wood preservatives and photographic developers. 7

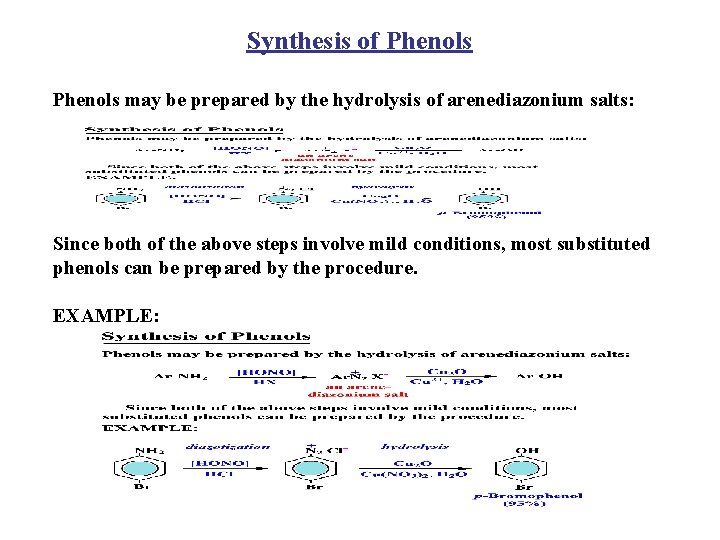
Synthesis of Phenols may be prepared by the hydrolysis of arenediazonium salts: Since both of the above steps involve mild conditions, most substituted phenols can be prepared by the procedure. EXAMPLE:

Industrial Syntheses of Phenol is important in the production of soaps, aspirin and plastics. Annual production in the United States is more than 4 billion pounds. There are several commercial syntheses for phenol. Alkali fusion of sodium benzenesulfonate This first commercial synthesis was introduced in Germany in 1890 and later in the United States as demand grew for phenol because of the success of Bakelite, a polymer of phenol and formaldehyde.

The Dow Process (1924) An improved electrochemical synthesis provided a relatively cheap source of chlorine (Cl 2) early in the 20 th century, and this permitted a variation of the original commercial synthesis of phenol to be developed.

Oxidation of Cumene (Isopropylbenzene) This process, originally developed in Germany in 1944, is the preferred way to produce phenol commercially. For each pound of phenol produced, 0. 6 pound of acetone (another important industrial chemical) is produced. The overall industrial process begins with two petrochemicals: benzene and propene.

Synthesis of Cumene: Acid-Catalyzed Alkylation of Benzene This synthesis is carried out under conditions that minimize the polyalkylation of benzene:

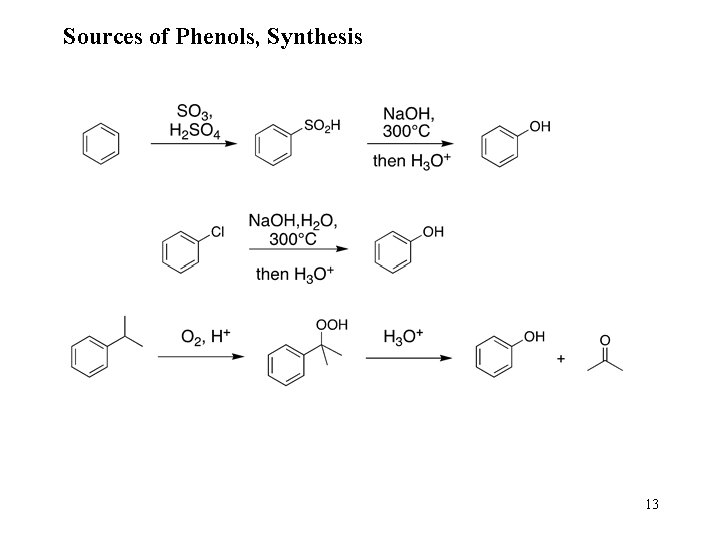
Sources of Phenols, Synthesis 13

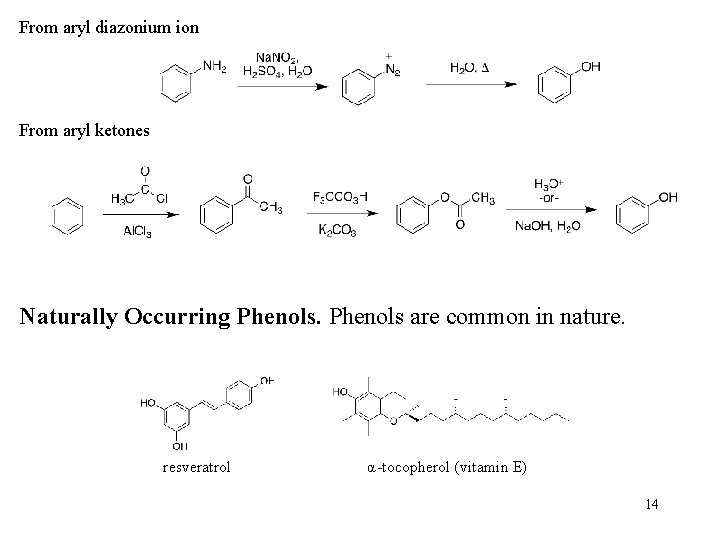
From aryl diazonium ion From aryl ketones Naturally Occurring Phenols are common in nature. resveratrol α-tocopherol (vitamin E) 14

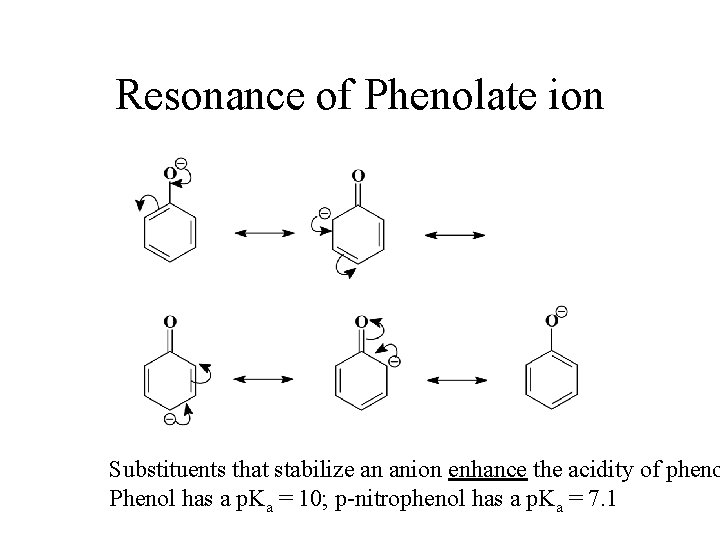
Resonance of Phenolate ion Substituents that stabilize an anion enhance the acidity of pheno Phenol has a p. Ka = 10; p-nitrophenol has a p. Ka = 7. 1

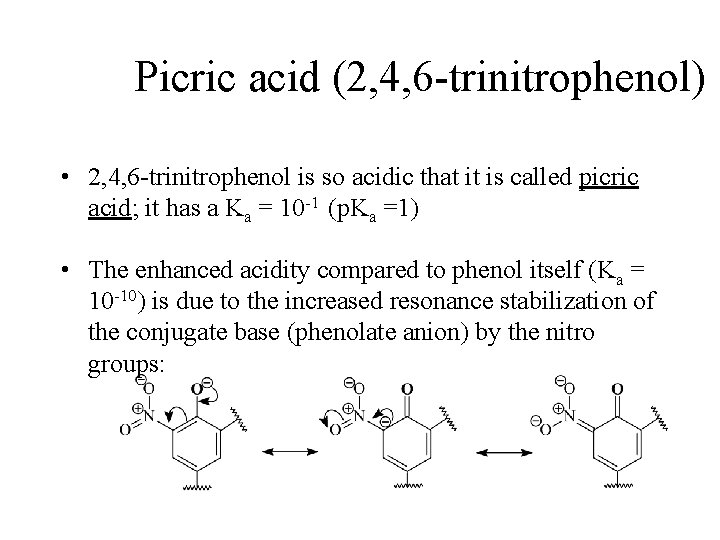
Picric acid (2, 4, 6 -trinitrophenol) • 2, 4, 6 -trinitrophenol is so acidic that it is called picric acid; it has a Ka = 10 -1 (p. Ka =1) • The enhanced acidity compared to phenol itself (Ka = 10 -10) is due to the increased resonance stabilization of the conjugate base (phenolate anion) by the nitro groups:

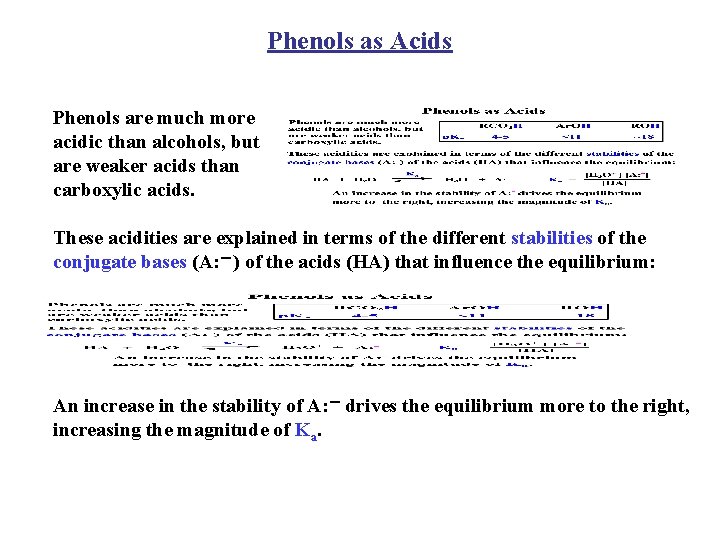
Phenols as Acids Phenols are much more acidic than alcohols, but are weaker acids than carboxylic acids. These acidities are explained in terms of the different stabilities of the conjugate bases (A: -) of the acids (HA) that influence the equilibrium: An increase in the stability of A: - drives the equilibrium more to the right, increasing the magnitude of Ka.

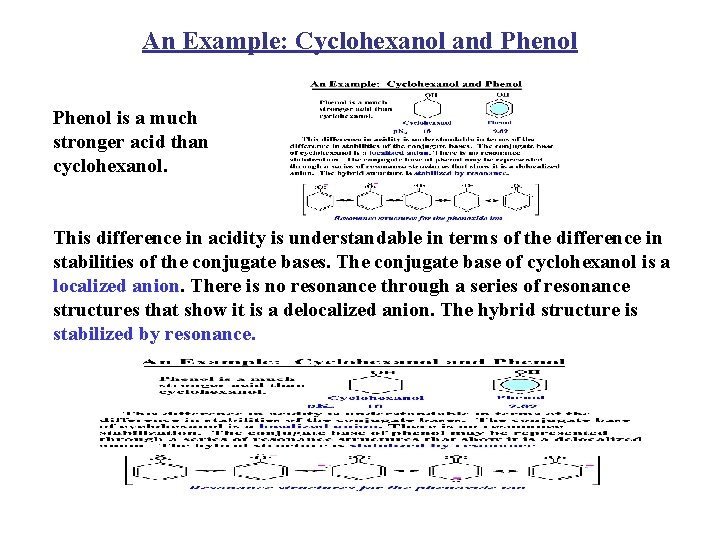
An Example: Cyclohexanol and Phenol is a much stronger acid than cyclohexanol. This difference in acidity is understandable in terms of the difference in stabilities of the conjugate bases. The conjugate base of cyclohexanol is a localized anion. There is no resonance through a series of resonance structures that show it is a delocalized anion. The hybrid structure is stabilized by resonance.

Carboxylic Acids and Phenols: Solubilities A standard way to separate water-insoluble carboxylic acids and phenols is by extraction with an aqueous solution of sodium bicarbonate. Carboxylic acids are soluble in the aqueous phase through their salts, while the less acidic phenols remain in the organic phase. Relative acidities: Consider the following equilibria: Bicarbonate ion will selectively deprotonate carboxylic acids in the presence of phenols because of the above equilibria.

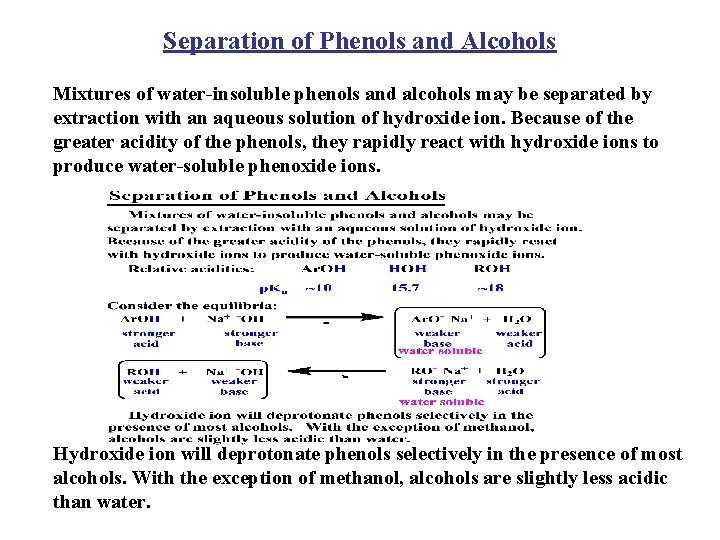
Separation of Phenols and Alcohols Mixtures of water-insoluble phenols and alcohols may be separated by extraction with an aqueous solution of hydroxide ion. Because of the greater acidity of the phenols, they rapidly react with hydroxide ions to produce water-soluble phenoxide ions. Hydroxide ion will deprotonate phenols selectively in the presence of most alcohols. With the exception of methanol, alcohols are slightly less acidic than water.

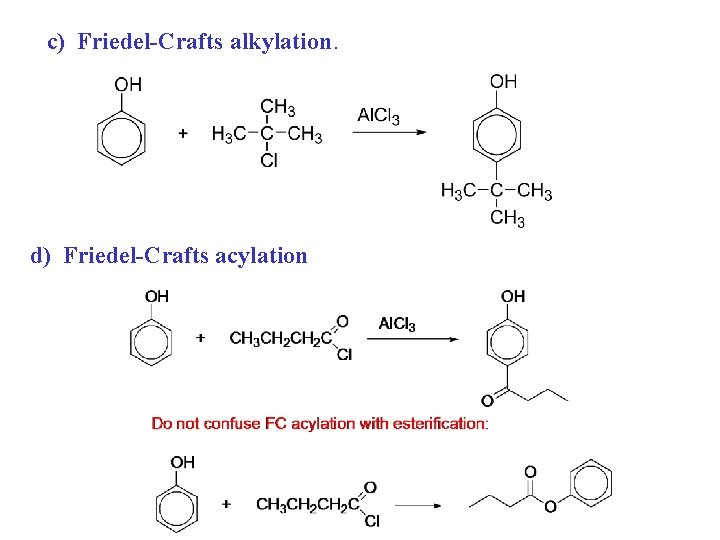
Phenols, reactions 1. as acids 2. ester formation 3. ether formation 4. EAS a) nitration b) coupling with diaz salts c) halogenation d) Friedel-Crafts alkylation

as acids: with active metals: with bases: CH 4 < NH 3 < HC CH < ROH < H 2 O < phenols < H 2 CO 3 < RCOOH < HF

2. ester formation (similar to alcohols)

Reactions of Phenols Acylation of the Hydroxyl Group Acylation is the introduction of the acyl group, The hydroxyl group in phenols may be acylated by either of two general procedures:

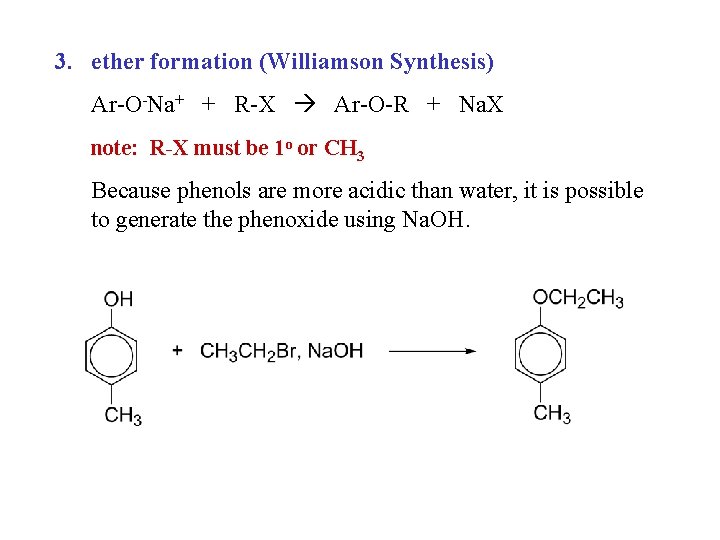
3. ether formation (Williamson Synthesis) Ar-O-Na+ + R-X Ar-O-R + Na. X note: R-X must be 1 o or CH 3 Because phenols are more acidic than water, it is possible to generate the phenoxide using Na. OH.

Phenols in the Williamson Synthesis of Ethers Because of their acidity (p. Ka~10), phenols are easily converted into their phenoxide ions with sodium or potassium hydroxide. The nucleophilic phenoxide ions react with alkyl halides (or equivalent compounds) by an SN 2 mechanism to yield aryl alkyl ethers.

Cleavage of Aryl Alkyl Ethers Reaction with HI or HBr cleaves these ethers in a specific direction: Nucleophilic attack does not occur on the aromatic ring.

4. Electrophilic Aromatic Substitution The –OH group is a powerful activating group in EAS and an ortho/para director. a) nitration

b) halogenation

c) Friedel-Crafts alkylation. d) Friedel-Crafts acylation

f) coupling with diazonium salts (EAS with the weak electrophile diazonium)

g) Kolbe reaction (carbonation)

h) Reimer-Tiemann reaction

Quiz 1 The order of acidity (strongest to weakest) of the oxygen acids below is

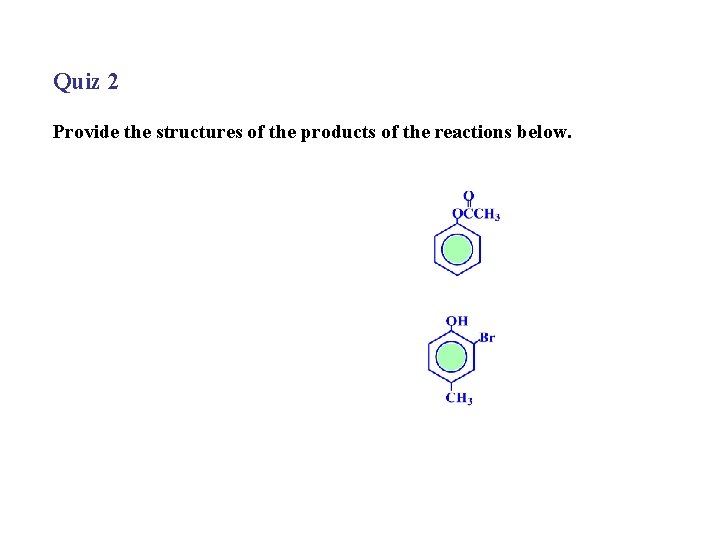
Quiz 2 Provide the structures of the products of the reactions below.

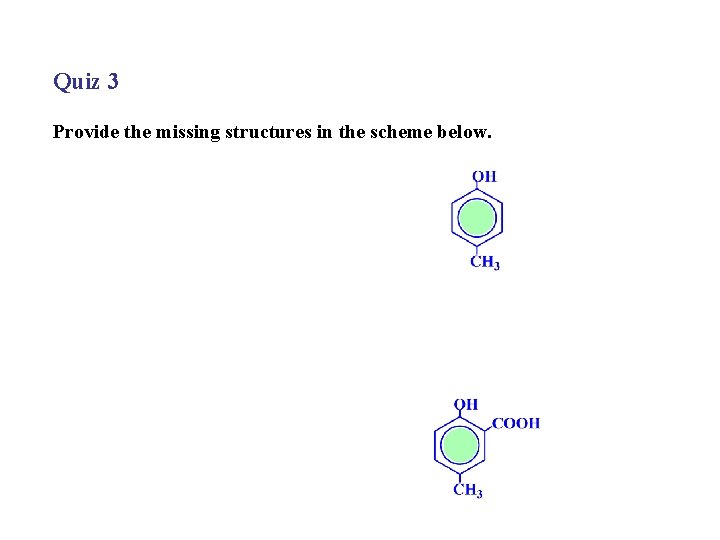
Quiz 3 Provide the missing structures in the scheme below.

Quiz 4 In a nucleophilic aromatic substitution reaction like that above, which of the aryl chlorides below would be the most reactive?

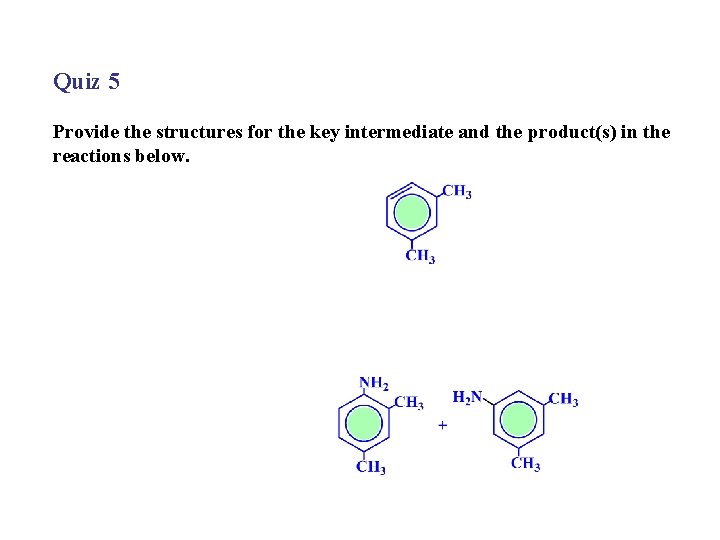
Quiz 5 Provide the structures for the key intermediate and the product(s) in the reactions below.
 Insidan region jh
Insidan region jh Naming ethers
Naming ethers Simple phenols
Simple phenols Alcohols phenols thiols and ethers
Alcohols phenols thiols and ethers Pt anugrah alam jaya perkasa
Pt anugrah alam jaya perkasa He works in a primary school in narail
He works in a primary school in narail Kondisi alam benua australia
Kondisi alam benua australia Apakah bukti persuratan masyarakat kerajaan alam melayu
Apakah bukti persuratan masyarakat kerajaan alam melayu Daftar fomema
Daftar fomema Alam sebagai sistem
Alam sebagai sistem Alam awatif
Alam awatif Apa itu kamaloka
Apa itu kamaloka Manfaat karya rekayasa teknologi tepat guna adalah...
Manfaat karya rekayasa teknologi tepat guna adalah... Ahmad rafay alam
Ahmad rafay alam Butil parabenzoat
Butil parabenzoat Dalam ilmu pengetahuan istilah gaya bisa diartikan sebagai
Dalam ilmu pengetahuan istilah gaya bisa diartikan sebagai Divergen
Divergen Materi kondisi alam negara jepang
Materi kondisi alam negara jepang Falsafah alam tabi'i
Falsafah alam tabi'i Teknik sumberdaya alam dan lingkungan
Teknik sumberdaya alam dan lingkungan Hikmah kebersihan
Hikmah kebersihan Alam musyahadah
Alam musyahadah Kenampakan alam benua australia
Kenampakan alam benua australia Bahan alam dalam berkarya
Bahan alam dalam berkarya Kerajaan chih tu
Kerajaan chih tu Demand concept map tagalog
Demand concept map tagalog Pengurusan alam pembelajaran
Pengurusan alam pembelajaran Matlamat sains islam
Matlamat sains islam Keterampilan mengenal alam
Keterampilan mengenal alam Iugs classification of igneous rocks
Iugs classification of igneous rocks Lingkungan alam terdiri atas
Lingkungan alam terdiri atas Materi gejala alam biotik dan abiotik kelas 10 smk pdf
Materi gejala alam biotik dan abiotik kelas 10 smk pdf Phonetic alphabet usa
Phonetic alphabet usa Keadaan alam vietnam
Keadaan alam vietnam Dinas pendidikan kota pagar alam
Dinas pendidikan kota pagar alam Pengambilan keputusan lawan alam
Pengambilan keputusan lawan alam Nurul alam school and college
Nurul alam school and college Keadaan alam benua amerika
Keadaan alam benua amerika Ekonomi kerajaan alam melayu
Ekonomi kerajaan alam melayu