Pharmacy 325 Infrared IR Spectroscopy Dr David Wishart













- Slides: 39

Pharmacy 325 Infrared (IR) Spectroscopy Dr. David Wishart Rm. 2123 Ph. 492 -0383 david. wishart@ualberta. ca Hours: anytime after 4 pm

Lecture Notes Available At: • http: //redpoll. pharmacy. ualberta. ca • http: //www. pharmacy. ualberta. ca/pharm 325/

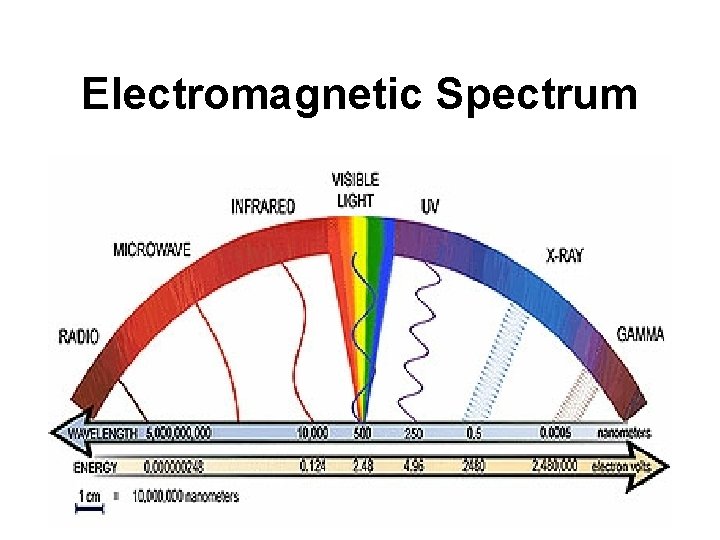
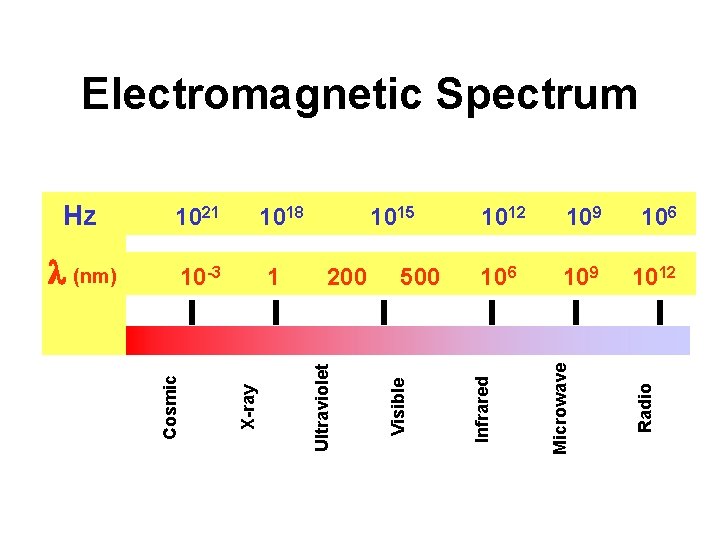
Electromagnetic Spectrum

Electromagnetic Spectrum 106 109 1012 Radio 500 109 Microwave 200 1012 Infrared 1 Visible 10 -3 1015 Ultraviolet (nm) 1018 X-ray 1021 Cosmic Hz

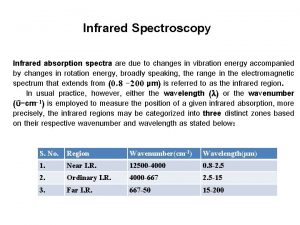
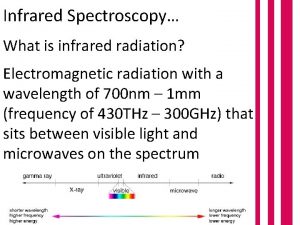
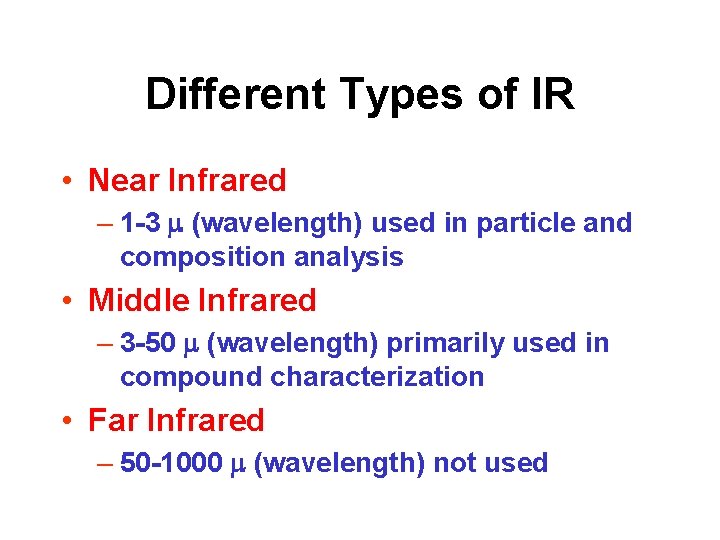
Different Types of IR • Near Infrared – 1 -3 m (wavelength) used in particle and composition analysis • Middle Infrared – 3 -50 m (wavelength) primarily used in compound characterization • Far Infrared – 50 -1000 m (wavelength) not used

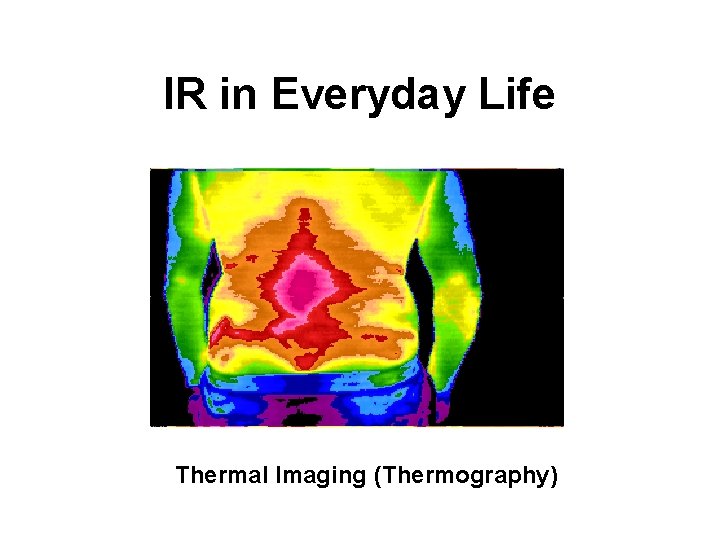
IR in Everyday Life Thermal Imaging (Thermography)

IR in Everyday Life Night Vision Goggles

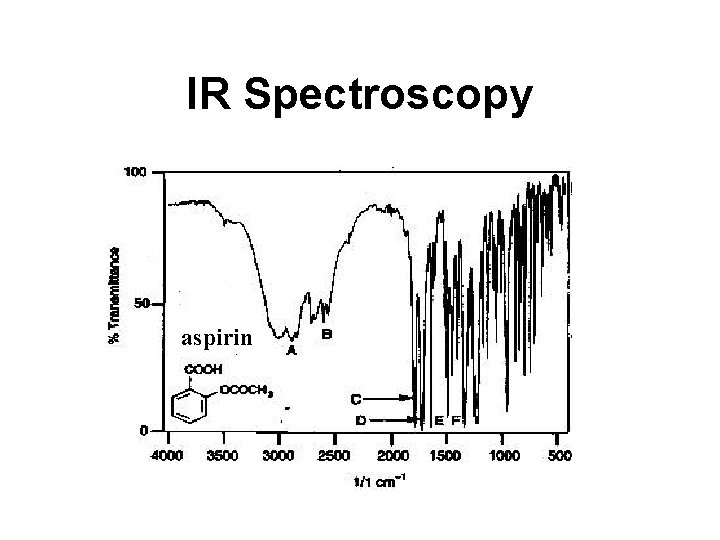
IR Spectroscopy aspirin

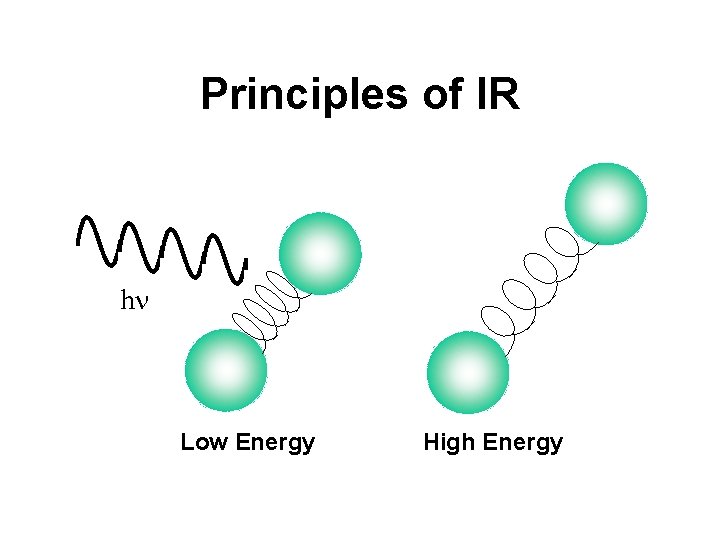
Principles of IR • Measures heat or thermal energy in a molecule or a system • IR spectroscopy measures the absorption of light due to bond stretching or bending • IR energies correspond to the energies of bond stretching in most molecules • Different types of bonds absorb at different energies (frequencies)

Principles of IR hn Low Energy High Energy

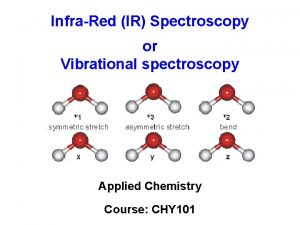
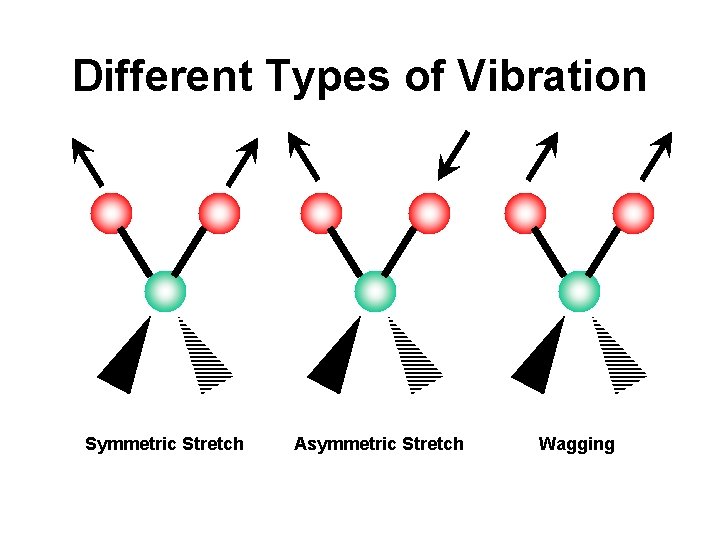
Different Types of Vibration Symmetric Stretch Asymmetric Stretch Wagging

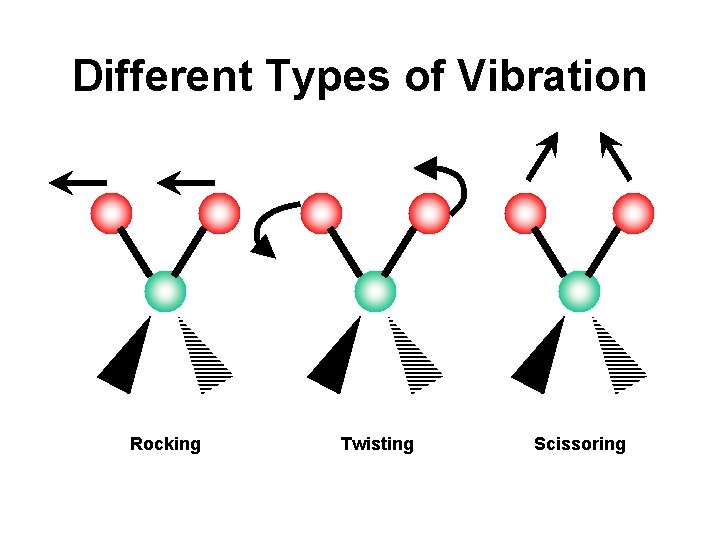
Different Types of Vibration Rocking Twisting Scissoring

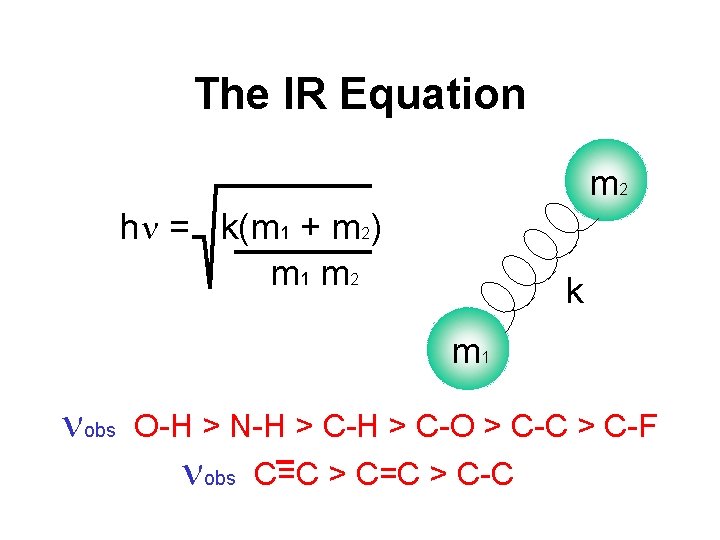
The IR Equation m 2 hn = k(m 1 + m 2) m 1 m 2 k m 1 nobs O-H > N-H > C-O > C-C > C-F nobs C=C > C-C

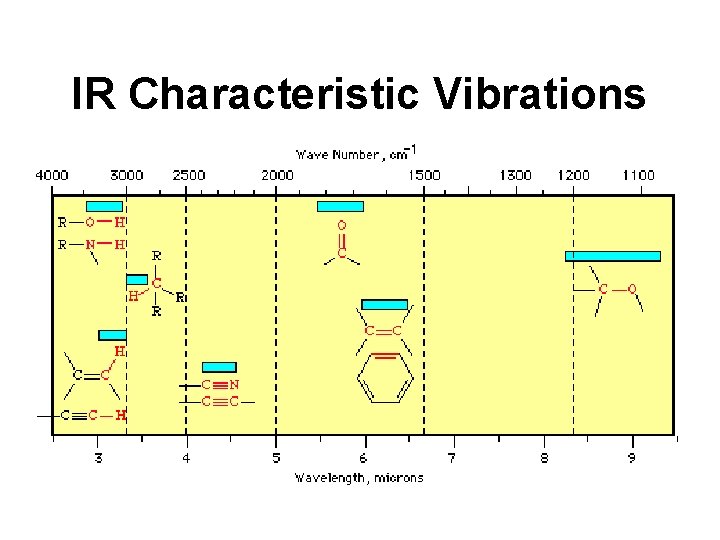
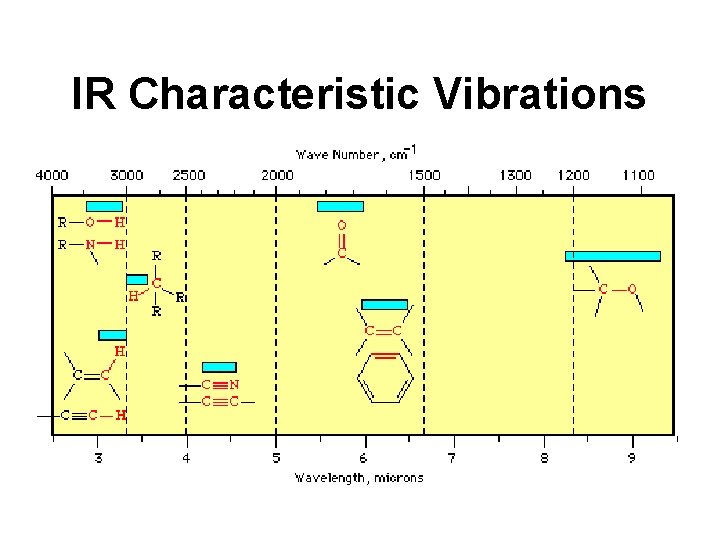
IR Characteristic Vibrations

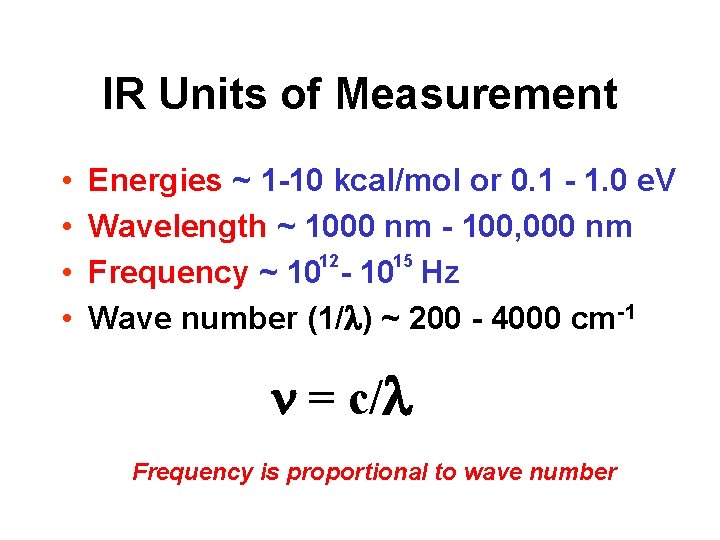
IR Units of Measurement • • Energies ~ 1 -10 kcal/mol or 0. 1 - 1. 0 e. V Wavelength ~ 1000 nm - 100, 000 nm 12 15 Frequency ~ 10 - 10 Hz Wave number (1/ ) ~ 200 - 4000 cm-1 n = c/ Frequency is proportional to wave number

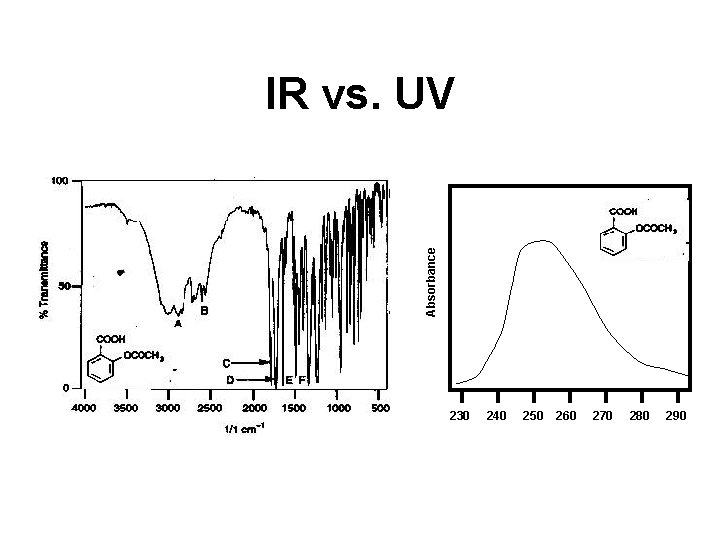
UV vs. IR • IR has narrower peaks relative to UV • IR yields more information than UV • IR allows you to collect data on solids, liquids and gases • UV is more quantitative than IR • UV spectra are easier/faster to collect • UV samples are easier to prepare • UV spectrometers are cheaper

Absorbance IR vs. UV 230 240 250 260 270 280 290

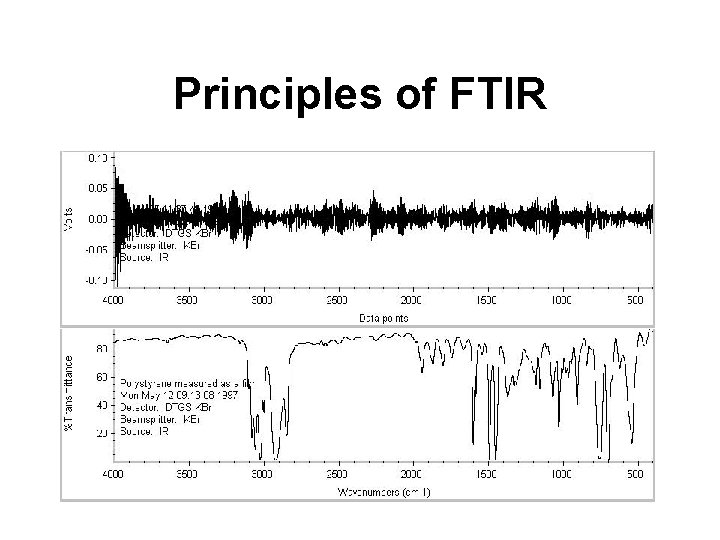
A Modern FTIR instrument FT = Fourier Transfrom

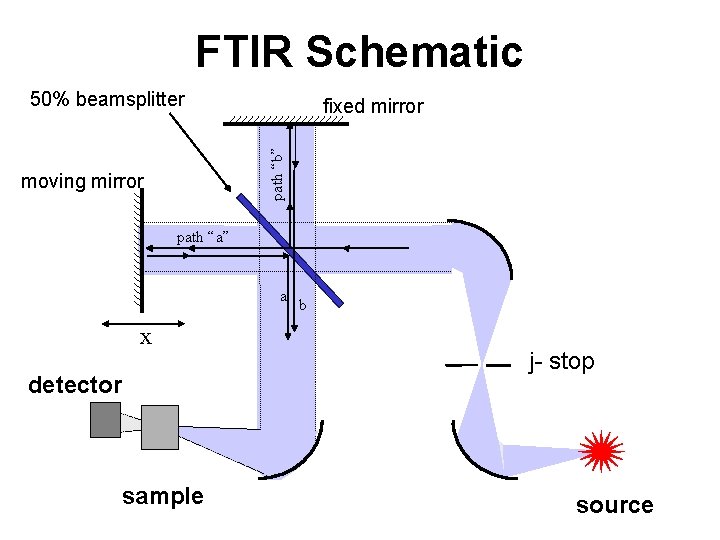
FTIR Schematic 50% beamsplitter path “b” fixed mirror moving mirror path “a” a x detector sample b j- stop source

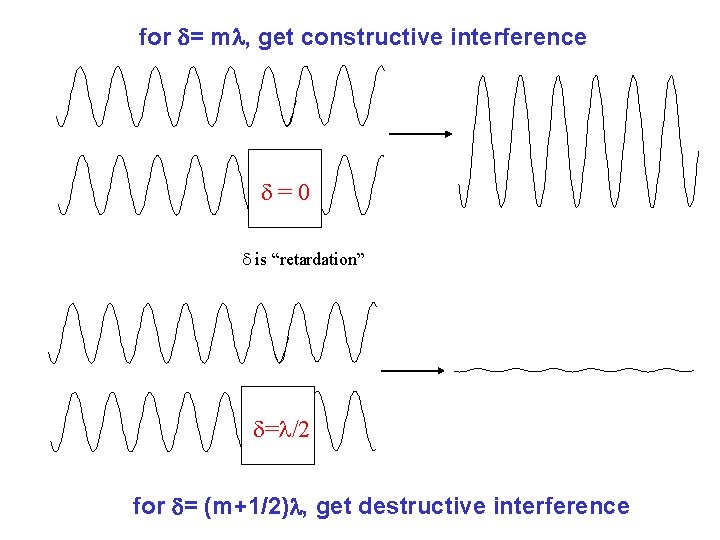
for = m , get constructive interference =0 is “retardation” = /2 for = (m+1/2) , get destructive interference

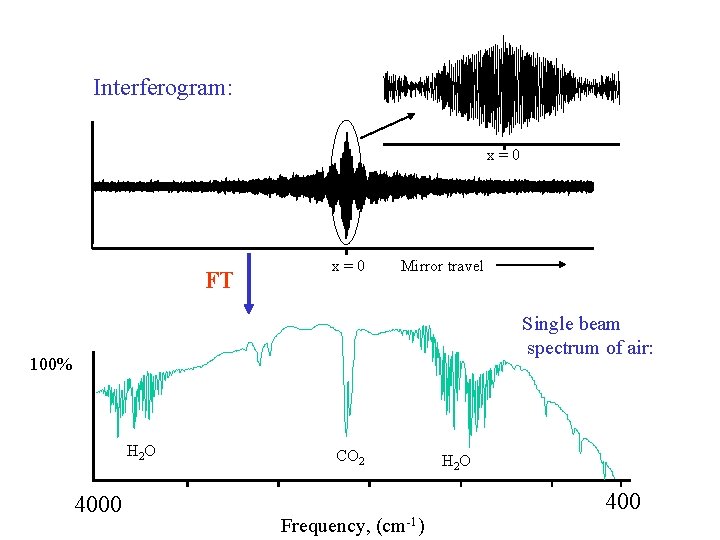
Interferogram: x=0 FT x=0 Mirror travel Single beam spectrum of air: 100% H 2 O 4000 CO 2 Frequency, (cm-1) H 2 O 400

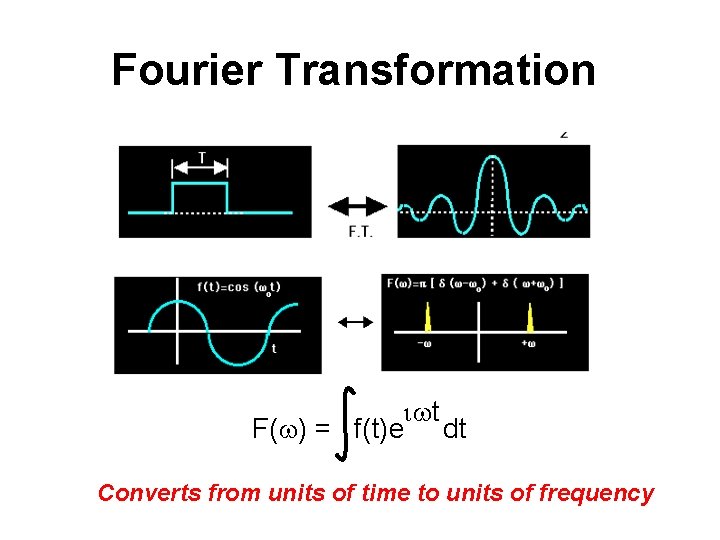
Fourier Transformation iwt F(w) = f(t)e dt Converts from units of time to units of frequency

Principles of FTIR

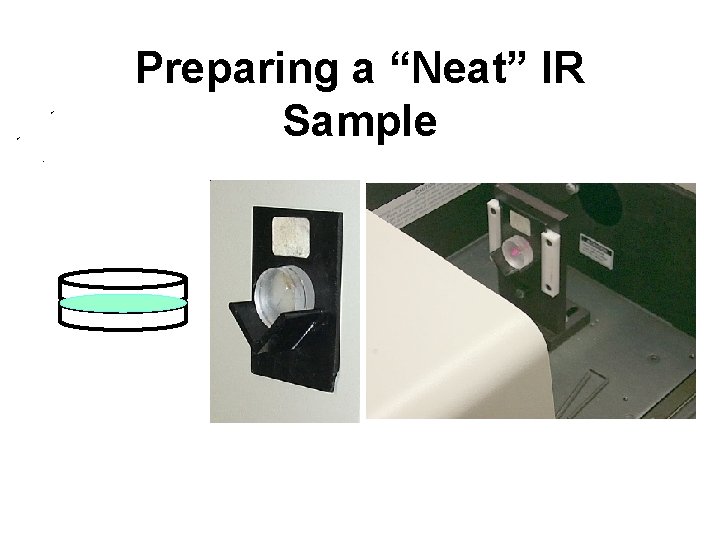
IR Sample Preparation • Most flexible system for analyzing all 3 states of matter (solid, liquid, gas) • “Neat” (analysis of liquids/oils) • Pellet (analysis of solids) • Thin Cell (analysis of dissolved solid samples - solutions) • Long Cell (analysis of gases)

Preparing a “Neat” IR Sample

Preparing a KBr Disk

Apparatus for KBr Disk

Pressed Disk Preparation • Use powdered, dry KBr, KI, Cs. I • Mix reagent with KBr in 1: 10 ratio • Grind material to 2 m diameter using agate mortar or vibrating ball mill (Wig -L-Bug amalgamator) • Place into die and press to 30 tons/in 2 using hand press or wrench + nut • Remove carefully, handle with gloves

IR Liquid Sample Cell

IR Gas Sample “Cell”

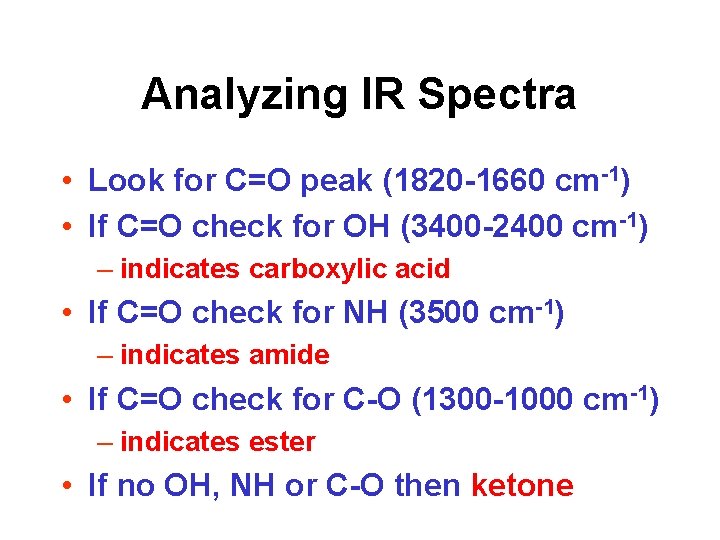
Analyzing IR Spectra • Look for C=O peak (1820 -1660 cm-1) • If C=O check for OH (3400 -2400 cm-1) – indicates carboxylic acid • If C=O check for NH (3500 cm-1) – indicates amide • If C=O check for C-O (1300 -1000 cm-1) – indicates ester • If no OH, NH or C-O then ketone

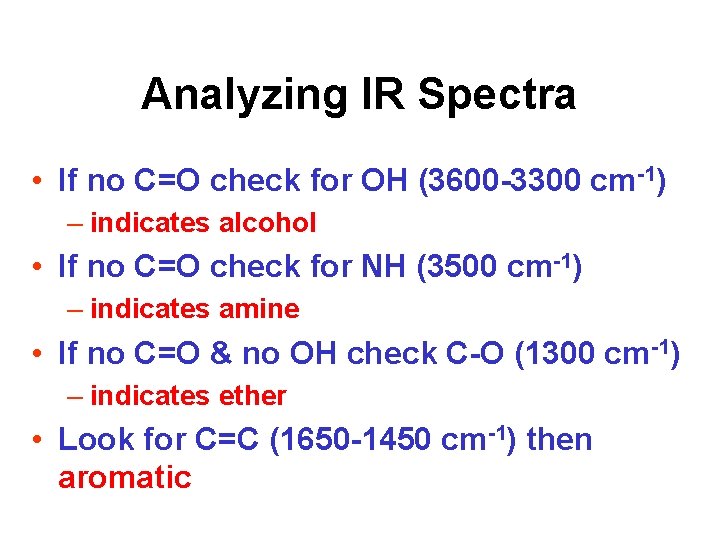
Analyzing IR Spectra • If no C=O check for OH (3600 -3300 cm-1) – indicates alcohol • If no C=O check for NH (3500 cm-1) – indicates amine • If no C=O & no OH check C-O (1300 cm-1) – indicates ether • Look for C=C (1650 -1450 cm-1) then aromatic

IR Characteristic Vibrations

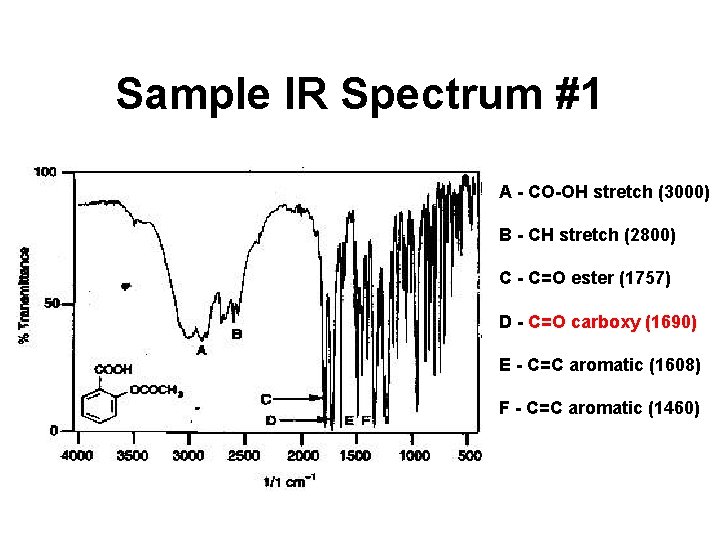
Sample IR Spectrum #1 A - CO-OH stretch (3000) B - CH stretch (2800) C - C=O ester (1757) D - C=O carboxy (1690) E - C=C aromatic (1608) F - C=C aromatic (1460)

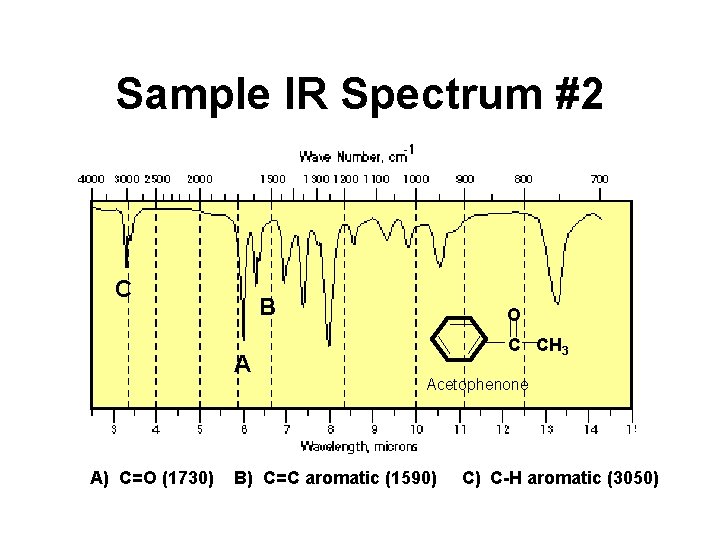
Sample IR Spectrum #2 C B A A) C=O (1730) O C CH 3 Acetophenone B) C=C aromatic (1590) C) C-H aromatic (3050)

Applications • Qualitative “fingerprint” check for identification of drugs • Used for screening compounds and rapid identification of C=O groups • Can be used to characterize samples in solid states (creams and tablets) • Can detect different crystal isoforms (polymorphs) • Water content measurement

Applications • Analysis of urine and other biofluids (urea, creatinine, protein)

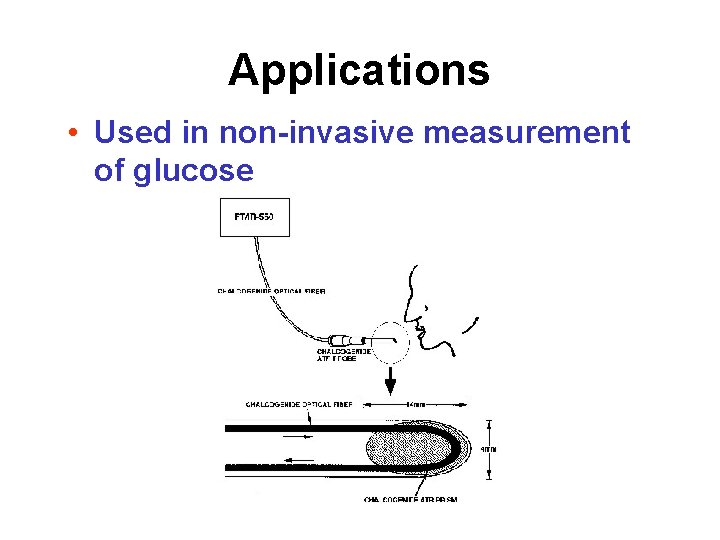
Applications • Used in non-invasive measurement of glucose

Applications of Near IR (NIR) • Quality control of pharmaceutical formulations • Determination of particle size • Determination of blend uniformity • Determination or identification of polymorphic drugs
 David wishart alberta
David wishart alberta Infrared spectroscopy ppt
Infrared spectroscopy ppt Nir spectroscopy instrumentation
Nir spectroscopy instrumentation Infrared spectroscopy
Infrared spectroscopy Ir spectrum of ethyl acetate
Ir spectrum of ethyl acetate Infrared spectroscopy theory
Infrared spectroscopy theory Catherine wishart
Catherine wishart Catherine wishart
Catherine wishart Cpsc 325
Cpsc 325 What is the angle of inclination
What is the angle of inclination Ece 325
Ece 325 Cpsc 325
Cpsc 325 Asteroide 327
Asteroide 327 Star 325
Star 325 Infs 325
Infs 325 A 325
A 325 Article 325
Article 325 325 ad
325 ad Apes 325 template
Apes 325 template Cpsc 325
Cpsc 325 A box of books weighing 325 n
A box of books weighing 325 n Cse 325
Cse 325 325/100 simplificado
325/100 simplificado Dcma form 1797
Dcma form 1797 Cse 325
Cse 325 Infs 325
Infs 325 Theotokos vs christotokos
Theotokos vs christotokos Cse 325
Cse 325 Pague 87 por un libro un traje y un sombrero
Pague 87 por un libro un traje y un sombrero Council of nicea 325
Council of nicea 325 A 325
A 325 Methodology of econometrics
Methodology of econometrics Semdr communication model
Semdr communication model Catalytic heater oil and gas
Catalytic heater oil and gas Thermal infrared
Thermal infrared National infrared operations
National infrared operations Characteristics of infrared
Characteristics of infrared Infrared sensor principle
Infrared sensor principle Bluetooth vs infrared
Bluetooth vs infrared Infrared vs bluetooth
Infrared vs bluetooth