Periodic Trends Cont Shielding Ion Size Ionization Energy








- Slides: 13

Periodic Trends, Cont. Shielding Ion Size Ionization Energy Electronegativity

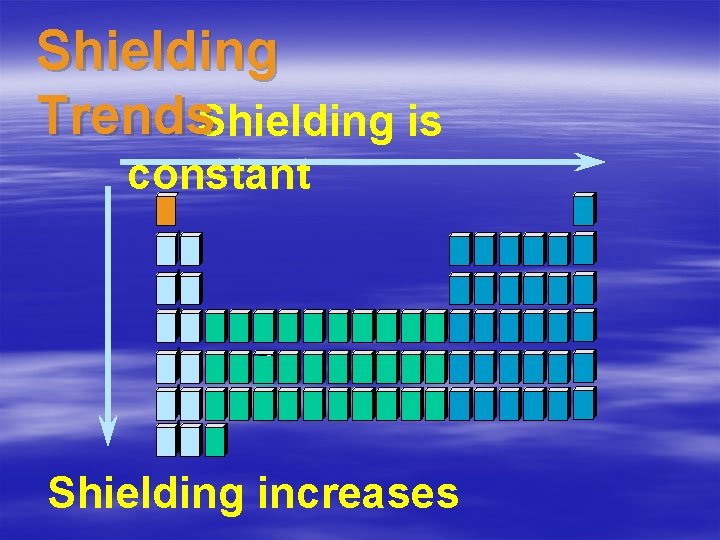
Shielding • The electrons in the outside energy level are blocked from the pull of the nucleus by all the inner electrons. • The shielding does not change as you add electrons to the same energy level.

Shielding Trends. Shielding is constant Shielding increases

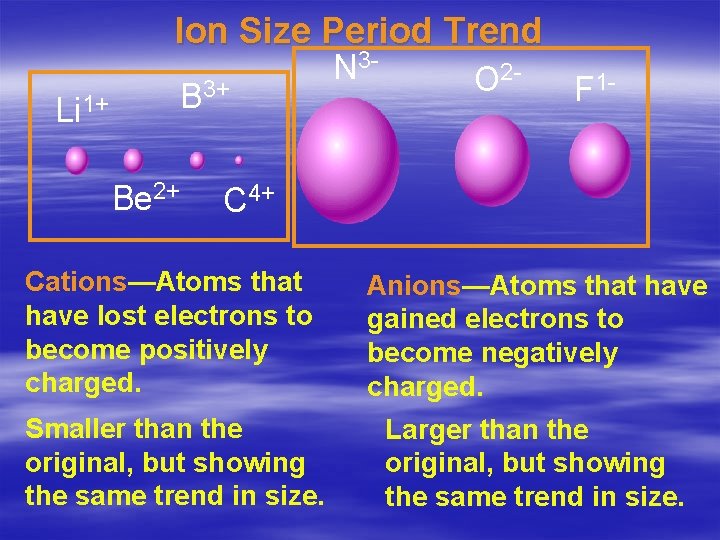
Ion Size § To form ions, atoms may gain or lose electrons. § If an atom loses electrons, a positive ion is formed called a cation. –Metals tend to lose electrons. § This cation is smaller than the atom from which it formed. –It now has fewer occupied

Ion Size § When an atom gains electrons, a negative ion is formed called an anion. –Nonmetals tend to gain electrons. § This anion is larger than the atom from which it was formed. § The period and group trends for ions are the same as atomic radius…. except in the period trend when you cross the metal-

Ion Size Period Trend B 3+ Li 1+ Be 2+ N 3 - O 2 - F 1 - C 4+ Cations—Atoms that have lost electrons to become positively charged. Anions—Atoms that have gained electrons to become negatively charged. Smaller than the original, but showing the same trend in size. Larger than the original, but showing the same trend in size.

Ionization Energy • The amount of energy required to completely remove an electron from an atom. • Removing one electron makes a +1 ion • The energy required to remove the outermost electron is called the first ionization energy. • Also known as…IE

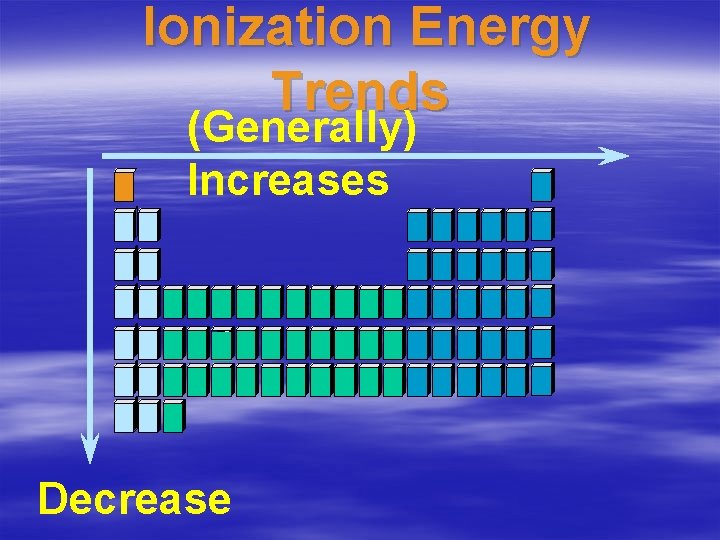
Trends • As you go across a period… • The electrons are closer to the positive nucleus and therefore harder to pull off. • The harder it is to pull off the electron, the higher the ionization energy.

Trends • As you go down a group… • The electrons are farther away from the positive nucleus and therefore easier to pull off. • There are more electrons shielding the outer electrons from the positive nucleus, making them easier to pull off. • The easier it is to pull off the

Ionization Energy Trends (Generally) Increases Decrease

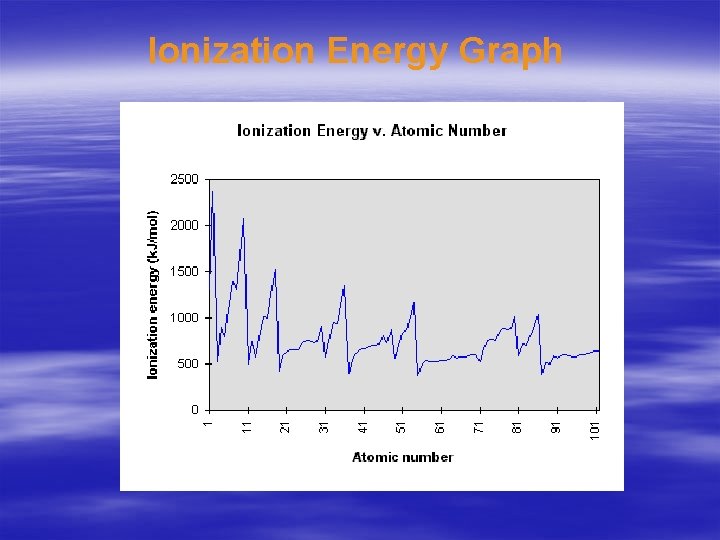
Ionization Energy Graph

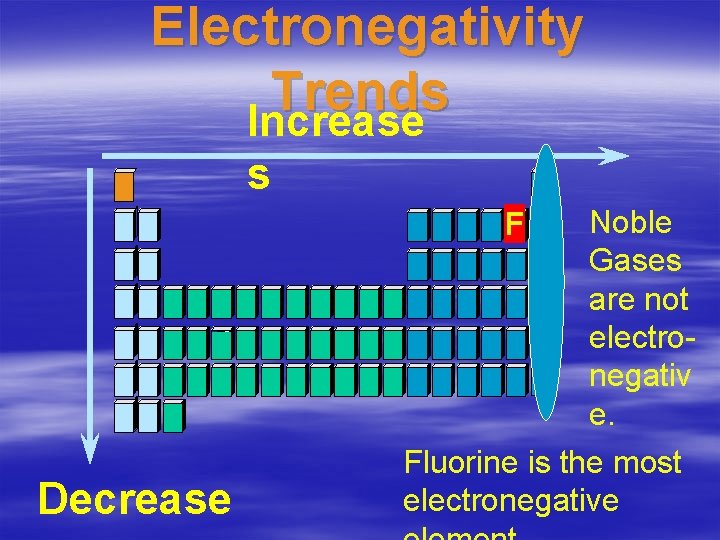
Electronegativity • The tendency for an atom to attract electrons to itself when it is chemically combined with another element. • Measures how fair an atom shares electrons. • Large electronegativity means it pulls the electron toward it strongly. • Fluorine is the most electronegative

Electronegativity Trends Increase s Noble Gases are not electronegativ e. Fluorine is the most electronegative F Decrease