Opioid Narcotic Analgesics Opioid Antagonists and Nonopioid Centrally






































![Nonopioid Centrally Acting Analgesics Tramadol (Ultram]) Ø Suicide risk Clonidine (Duraclon) Ziconotide (Prialt) Dexmedetomidine Nonopioid Centrally Acting Analgesics Tramadol (Ultram]) Ø Suicide risk Clonidine (Duraclon) Ziconotide (Prialt) Dexmedetomidine](https://slidetodoc.com/presentation_image_h2/c049b830bb748bf46aad2b707f75316c/image-47.jpg)



- Slides: 51

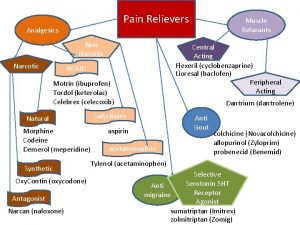
Opioid (Narcotic) Analgesics, Opioid Antagonists, and Nonopioid Centrally Acting Analgesics

Analgesics and Opioids Analgesics are drugs that relieve pain without causing loss of consciousness. Opioids are the most effective pain relievers available.

Opioid Analgesics, Opioid Antagonists, and Nonopioid Centrally Acting Analgesics Introduction to the opioids Basic pharmacology of the opioids Clinical use of opioids Opioid antagonists Nonopioid centrally acting analgesics

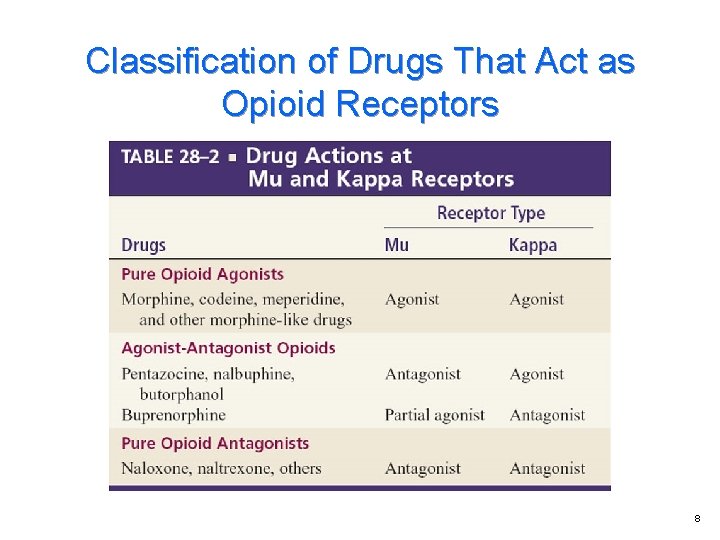
Introduction to the Opioids Terminology Endogenous opioid peptides Opioid receptors Classification of drugs that act as opioid receptors

Terminology Opioid Ø A general term defined as any drug, natural or synthetic, that has actions similar to those of morphine Opiate Ø Applies only to compounds present in opium

Endogenous Opioid Peptides Three families of peptides Ø Ø Ø Enkephalins Endorphins Dynorphins

Opioid Receptors Three main classes of opioid receptors Ø Ø Ø Mu receptors Kappa receptors Delta receptors

Classification of Drugs That Act as Opioid Receptors 8

Basic Pharmacology of the Opioids Strong opioid agonists Ø Ø Morphine Other strong opioid agonists Moderate to strong opioid agonists Agonist-antagonist opioids

Morphine Source Ø Seedpod of the poppy plant Overview of pharmacologic actions Ø Ø Ø Receptors involved Pain relief Drowsiness Mental clouding Anxiety reduction Sense of well-being

Morphine Therapeutic use: relief of pain Ø Ø Mechanism of analgesic action Moderate to severe pain Constant dull pain vs. sharp intermittent pain Preoperative treatment of anxiety

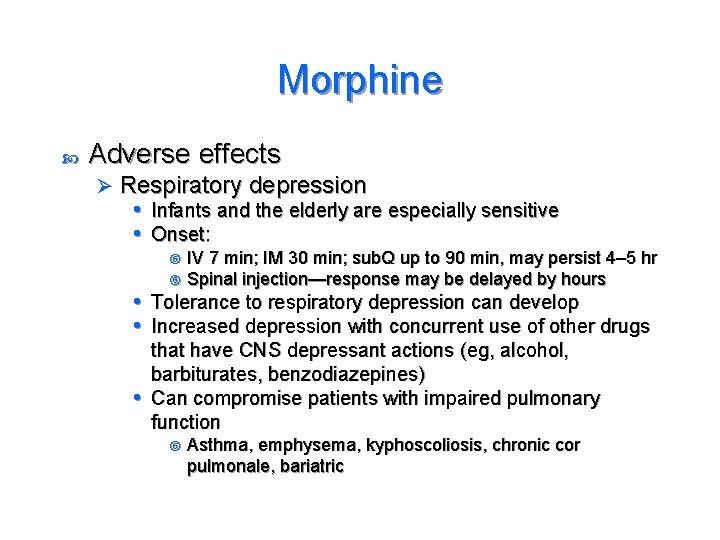
Morphine Adverse effects Ø Respiratory depression • Infants and the elderly are especially sensitive • Onset: IV 7 min; IM 30 min; sub. Q up to 90 min, may persist 4– 5 hr Spinal injection—response may be delayed by hours • Tolerance to respiratory depression can develop • Increased depression with concurrent use of other drugs that have CNS depressant actions (eg, alcohol, barbiturates, benzodiazepines) • Can compromise patients with impaired pulmonary function Asthma, emphysema, kyphoscoliosis, chronic cor pulmonale, bariatric

Morphine Adverse effects (cont’d) Ø Ø Ø Ø Constipation Orthostatic hypotension Cough suppression Biliary colic Emesis Urinary retention Euphoria/dysphoria Sedation

Morphine Adverse effects (cont’d) Ø Ø Ø Miosis Neurotoxicity Intracranial pressure (ICP) Birth defects Adverse effects from prolonged use

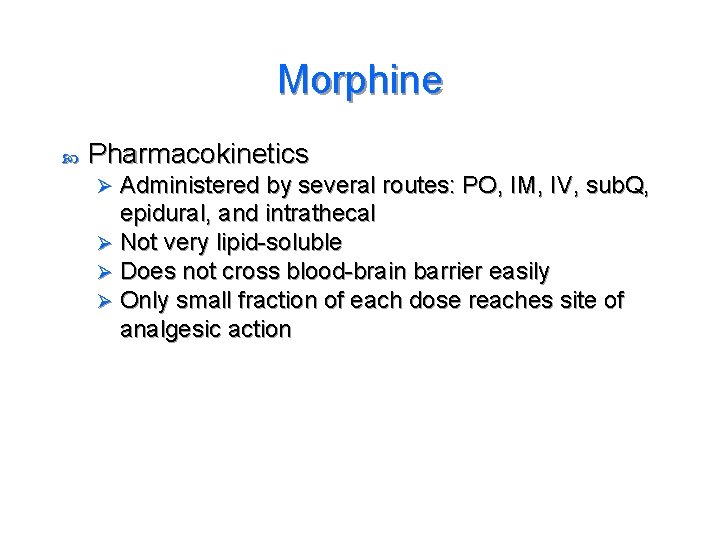
Morphine Pharmacokinetics Administered by several routes: PO, IM, IV, sub. Q, epidural, and intrathecal Ø Not very lipid-soluble Ø Does not cross blood-brain barrier easily Ø Only small fraction of each dose reaches site of analgesic action Ø

Morphine Tolerance and physical dependence Ø Tolerance • Increased doses needed to obtain same response • Develops with analgesia, euphoria, sedation, respiratory depression • Cross-tolerance to other opioid agonists • No tolerance to miosis or constipation develops

Morphine Tolerance and physical dependence Ø Physical dependence • Abstinence syndrome with abrupt discontinuation • About 10 hours after last dose: Initial reaction (yawning, rhinorrhea, sweating) Violent sneezing, weakness, nausea, vomiting, diarrhea, abdominal cramps, bone and muscle pain, muscle spasm, kicking movements • Progresses to: • • Lasts 7– 10 days if untreated Withdrawal unpleasant but not lethal, as is possible with CNS depressants

Morphine Abuse liability Precautions Ø Ø Ø Decreased respiratory reserve Pregnancy Labor and delivery Head injury Other precautions

Morphine Drug interactions Ø Ø Ø Ø CNS depressants Anticholinergic drugs Hypotensive drugs Monoamine oxidase inhibitors Agonist-antagonist opioids Opioid antagonists Other interactions

Morphine Toxicity Ø Clinical manifestations • Classic triad Coma Respiratory depression Pinpoint pupils Treatment • Ventilatory support • Antagonist: naloxone (Narcan) Ø General guidelines • Monitor full vitals before giving • Give on a fixed schedule Ø

Morphine Preparations, dosage, and administration Ø General guidelines on dosage and administration • Preparations • Morphine/Naltrexone (Embeda): designed to discourage morphine abuse • Dosage and routes of administration Oral Intramuscular and subcutaneous Intravenous Epidural and intrathecal Extended-release liposomal formulation (Depo. Dur)

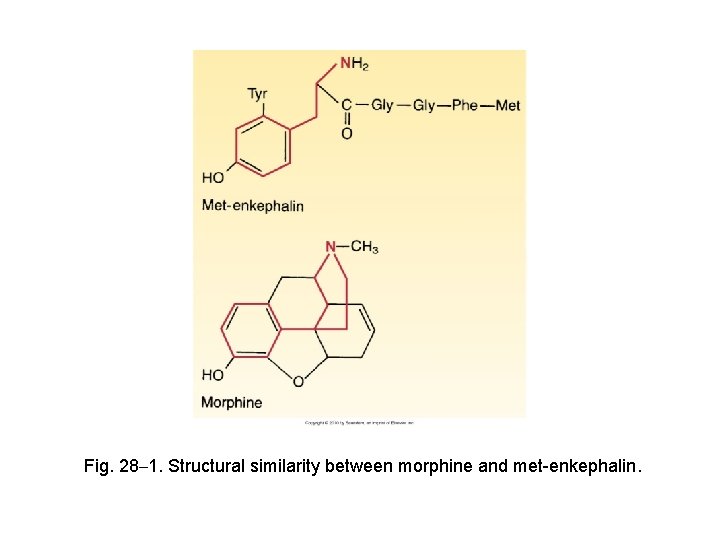
Fig. 28– 1. Structural similarity between morphine and met-enkephalin.

Other Strong Opioid Agonists Fentanyl (Sublimaze, Duragesic, Abstral, Actiq, Fentora, Onsolis) Ø Ø 100 times the potency of morphine Five formulations in three routes • Parenteral (Sublimaze) Surgical anesthesia • Transdermal (Duragesic) Patch—heat acceleration Iontophoretic system—needle-free • Transmucosal Lozenge on a stick (Actiq) Buccal film (Onsolis) Buccal tablets (Fentora) Sublingual tablets (Abstral)

Other Strong Opioid Agonists Alfentanil and sufentanil Remifentanil Meperidine Ø Ø Ø Short half-life Interacts adversely with several other drugs Toxic metabolite accumulation Methadone Ø Treatment for pain and opioid addicts

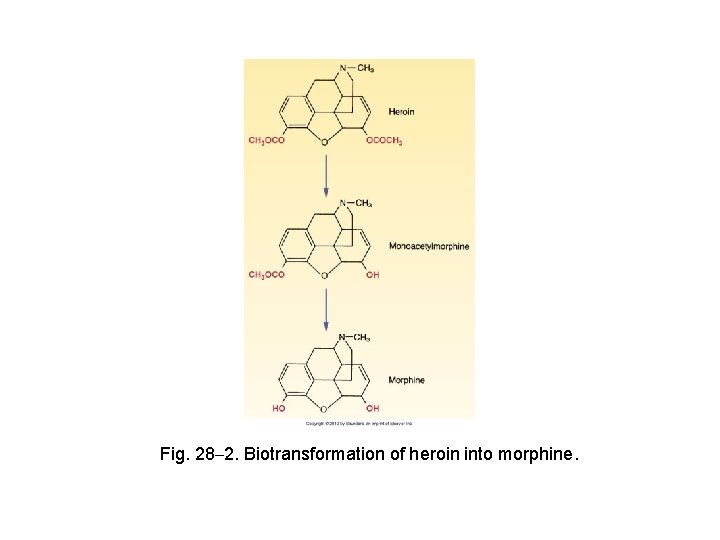
Other Strong Opioid Agonists Heroin Ø Ø Used legally in Europe to relieve pain High abuse liability Not more effective than other opioids See Figure 28 -2 Hydromorphone, oxymorphone, and levorphanol Ø Ø Basic pharmacology Preparations, dosage, and administration

Fig. 28– 2. Biotransformation of heroin into morphine.

Moderate to Strong Opioid Agonists Similar to morphine in most respects Ø Ø Produce analgesia, sedation, euphoria Can cause: • Respiratory depression, constipation, urinary retention, cough suppression, and miosis Ø Can be reversed with naloxone Different from morphine Produce less analgesia and respiratory depression than morphine Ø Somewhat lower potential for abuse Ø

Moderate to Strong Opioid Agonists Codeine Actions and uses • 10% converts to morphine in liver • Pain and cough suppression Ø Preparations, dosage, and administration • Usually oral (formulated alone or with aspirin or Ø acetaminophen) • 30 mg produces same effect as 325 mg acetaminophen

Moderate to Strong Opioid Agonists Oxycodone Ø Ø Analgesic actions equivalent to those of codeine Long-acting analgesic • Immediate-release • Controlled-release (Oxy. Contin) Abuse: crushes and snorts or injects medication 2010 OP formulation much harder to crush and does not dissolve into an injectable solution to decrease risk of abuse

Moderate to Strong Opioid Agonists Hydrocodone Ø Ø Tapentadol Ø Ø Most widely prescribed drug in the United States Combined with aspirin, acetaminophen, or ibuprofen Analgesic effects equivalent to oxycodone Causes less constipation than traditional medications Propoxyphene Ø Ø Ø Analgesic effect equal to that of aspirin Combined with aspirin or acetaminophen Propoxyphene products, such as Darvon and Darvocet, have been withdrawn owing to limited efficacy and the risk of potentially fatal dysrhythmias

Agonist-Antagonist Opioids Pentazocine Ø Ø Actions and uses Preparations, dosage, and administration Nalbuphine Butorphanol Buprenorphine Ø Ø 7 -day patch: Butrans Sublingual film: Suboxone

Clinical Use of Opioids Pain assessment Ø Ø Ø Essential component of management Based on patient’s description Evaluate: • Pain location, characteristics, and duration; things that improve/worsen pain • Status before drug and 1 hour after

Dosing Guidelines Assessment of pain Ø Dosage determination Ø Opioid analgesics must be adjusted to accommodate individual variation Dosing schedule Ø Pain status should be evaluated before opioid administration and about 1 hour after As a rule, opioids should be administered on a fixed schedule Avoiding withdrawal

Clinical Use of Opioids Physical dependence Ø Abuse Ø State in which an abstinence syndrome will occur if the dependence-producing drug is abruptly withdrawn; it is NOT equated with addiction Drug use that is inconsistent with medical or social norms Addiction Ø Behavior pattern characterized by continued use of a psychoactive substance despite physical, psychologic, or social harm

Clinical Use of Opioids Balance the need to provide pain relief with the desire to minimize abuse Minimize fears about: Ø Ø Physical dependence Addiction

Clinical Use of Opioids Patient-controlled analgesia PCA devices Drug selection and dosage regulations Comparison of PCA with traditional intramuscular therapy Ø Patient education Ø Ø Ø

Clinical Use of Opioids Using opioids in specific settings Ø Ø Ø Postoperative pain Obstetric analgesia Myocardial infarction Head injury Cancer-related pain Chronic noncancer pain

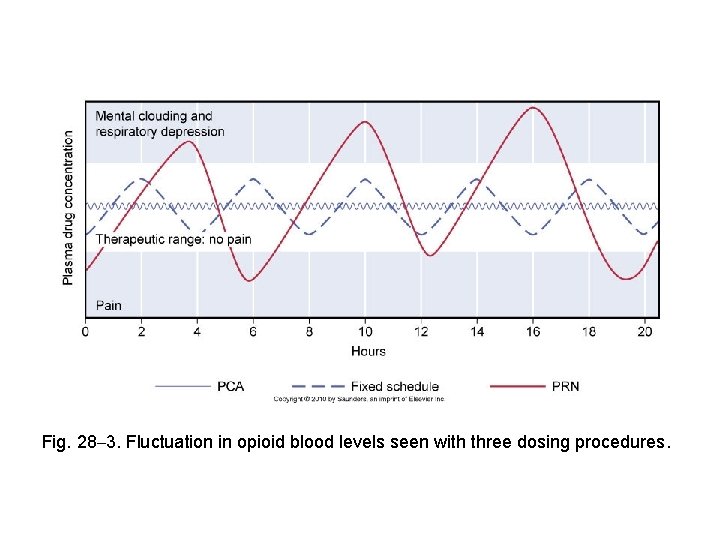
Fig. 28– 3. Fluctuation in opioid blood levels seen with three dosing procedures.

REMS Risk evaluation and mitigation strategy (REMS) Developed by the FDA Designed to reduce opioid-related injuries and deaths

Opioid Antagonists Drugs that block the effects of opioid agonists Principal uses: Treatment of opioid overdose, relief of opioidinduced constipation Ø Reversal of postoperative opioid effects Ø Management of opioid addiction Ø

Pure Opioid Antagonists Naloxone (Narcan) Other pure opioid antagonists Ø Ø Ø Methylnaltrexone (Relistor) Alvimopan (Entereg) Naltrexone (Re. Via, Vivitrol)

Naloxone Mechanism of action Ø Competitive antagonist Pharmacologic effects Pharmacokinetics

Naloxone Therapeutic uses Reversal of opioid overdose • Drug of choice with pure opioid agonist overdose • Titrated cautiously with physical dependence Ø Reversal of postoperative opioid effects • Titrated to achieve adequate ventilation and to maintain Ø pain relief Ø Reversal of neonatal respiratory depression • Opioids given during labor and delivery may cause respiratory depression in neonate

Naloxone Preparations, dosage, and administration Ø Ø Preparations and routes Opioid overdose Postoperative opioid effects Neonatal respiratory depression

Other Opioid Antagonists Methylnaltrexone Ø Ø Selective opioid antagonist Treatment of opioid-induced constipation in latestage disease for patients on constant opioids

Nonopioid Centrally Acting Analgesics Relieve pain by mechanisms largely or completely unrelated to opioid receptors Do not cause respiratory depression, physical dependence, or abuse Not regulated under the Controlled Substances Act
![Nonopioid Centrally Acting Analgesics Tramadol Ultram Ø Suicide risk Clonidine Duraclon Ziconotide Prialt Dexmedetomidine Nonopioid Centrally Acting Analgesics Tramadol (Ultram]) Ø Suicide risk Clonidine (Duraclon) Ziconotide (Prialt) Dexmedetomidine](https://slidetodoc.com/presentation_image_h2/c049b830bb748bf46aad2b707f75316c/image-47.jpg)
Nonopioid Centrally Acting Analgesics Tramadol (Ultram]) Ø Suicide risk Clonidine (Duraclon) Ziconotide (Prialt) Dexmedetomidine (Precedex)

Tramadol Mechanism of action Ø Therapeutic use Pharmacokinetics Adverse effects and interactions Drug interactions Ø Combination of opioid and nonopioid mechanisms CNS depressants Abuse liability Preparations, dosage, and administration Ø Immediate-release and extended-release

Clonidine Mechanism of pain relief Ø Analgesic use Ø Used in combination with opioid analgesic Pharmacokinetics Adverse effects Ø Alpha 2 -adrenergic agonist Cardiovascular: severe hypotension, rebound hypertension, bradycardia Contraindications Preparations, dosage, and administration

Ziconotide Mechanism of action Selective antagonist at N-type voltage-sensitive calcium channels on neurons Ø Blocks calcium channels on primary nociceptive afferent neurons in dorsal horn of the spinal cord Ø Clinical trials Ø Three randomized clinical trials

Ziconotide Pharmacokinetics Ø Adverse effects Ø CNS and muscle injury Drug interactions Ø Distributed through cerebral spinal fluid (CSF) and then transported to systemic circulation Formal studies not done Preparations, dosage, and administration Ø Intrathecal administration
 Mechanism of action of opioid analgesics
Mechanism of action of opioid analgesics Analgesic classification
Analgesic classification Liquid gabapentin for cats dosage chart
Liquid gabapentin for cats dosage chart Protagonist antagonist and agonist
Protagonist antagonist and agonist Types of characters
Types of characters Simile in romeo and juliet act 1
Simile in romeo and juliet act 1 Types of antagonists
Types of antagonists What is an agonist
What is an agonist Auxiliary muscle
Auxiliary muscle Centrally acting skeletal muscle relaxants
Centrally acting skeletal muscle relaxants Chapter 2 section 3 centrally planned economies answer key
Chapter 2 section 3 centrally planned economies answer key Describe how a centrally planned economy is organized
Describe how a centrally planned economy is organized Centrally planned economy
Centrally planned economy Antihypertensive drug classification
Antihypertensive drug classification Miosis
Miosis Pasero opioid sedation scale
Pasero opioid sedation scale Opiyatlar grubu
Opiyatlar grubu Opioid umrechnungstabelle
Opioid umrechnungstabelle Opioid overdose
Opioid overdose Opioid overdose
Opioid overdose Opioid overdose
Opioid overdose Types of pain
Types of pain Children's pain scale
Children's pain scale Opioid receptors location
Opioid receptors location Non-opioid
Non-opioid Opioid settlement calculator
Opioid settlement calculator Non-opioid
Non-opioid Kentucky opioid response effort
Kentucky opioid response effort Red orange yellow green blue purple pink brown black
Red orange yellow green blue purple pink brown black Willaim blake
Willaim blake West side story romeo and juliet worksheet
West side story romeo and juliet worksheet Shorter and older taller and younger
Shorter and older taller and younger Reading and writing money in symbols and in words
Reading and writing money in symbols and in words Red orange yellow green blue purple pink indigo
Red orange yellow green blue purple pink indigo Gabby and sydney bought some pens
Gabby and sydney bought some pens Young and dyslexic summary
Young and dyslexic summary Albany movement
Albany movement West yorkshire and harrogate health and care partnership
West yorkshire and harrogate health and care partnership Draw and label transverse wave
Draw and label transverse wave Compare and contrast spring and neap tides.
Compare and contrast spring and neap tides. Independent vs dependent variable
Independent vs dependent variable Transitive and intransitive and linking verbs
Transitive and intransitive and linking verbs A life role is
A life role is Role of nurse in maintaining records and reports
Role of nurse in maintaining records and reports Transportation and assignment problems
Transportation and assignment problems Compare and contrast cold wave and wind chill factor
Compare and contrast cold wave and wind chill factor Purchases in profit and loss account
Purchases in profit and loss account Lesson preparation siop
Lesson preparation siop Tomorrow and tomorrow and tomorrow kurt vonnegut
Tomorrow and tomorrow and tomorrow kurt vonnegut Mr biv ritz carlton
Mr biv ritz carlton Ron offutt house
Ron offutt house Muscles name
Muscles name