Office of Research Oversight Reporting Who What When

















































- Slides: 67

Office of Research Oversight Reporting: Who, What, When, and Why February, 2012

CASE #1 - A Serious Adverse Event The Background: • A PI became aware of a local unanticipated serious adverse event on January 1, 2012. It was not reported to the IRB by the PI until January 25, 2012, as part of a continuing review submission. • The IRB noted the delay in reporting the SAE by the PI at the continuing review, and reminded the PI of the responsibility to report timely in the future. • The IRB made no documented determinations about the SAE, or whether serious noncompliance had occurred. VETERANS HEALTH ADMINISTRATION 1

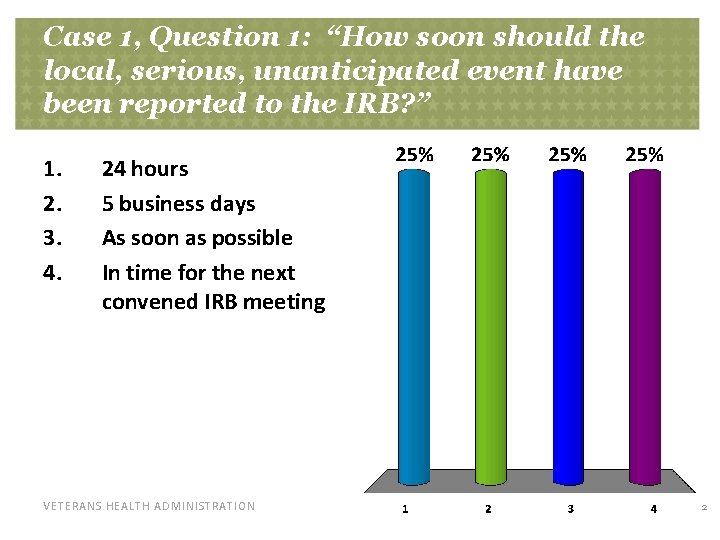
Case 1, Question 1: “How soon should the local, serious, unanticipated event have been reported to the IRB? ” 1. 2. 3. 4. 24 hours 5 business days As soon as possible In time for the next convened IRB meeting VETERANS HEALTH ADMINISTRATION 2

ORO Resources for Research Compliance Requirements: Tech Assist Share. Point Site VETERANS HEALTH ADMINISTRATION 3

ORO Resources for Research Compliance Requirements: Tech Assist Share. Point Site VETERANS HEALTH ADMINISTRATION 4

ORO Resources for Research Compliance Requirements: Tech Assist Share. Point Site VETERANS HEALTH ADMINISTRATION 5

ORO Resources for Research Compliance Requirements: Tech Assist Share. Point Site VETERANS HEALTH ADMINISTRATION 6

ORO Resources for Research Compliance Requirements: Tech Assist Share. Point Site VETERANS HEALTH ADMINISTRATION 7

ORO Resources for Research Compliance Requirements: Tech Assist Share. Point Site VETERANS HEALTH ADMINISTRATION 8

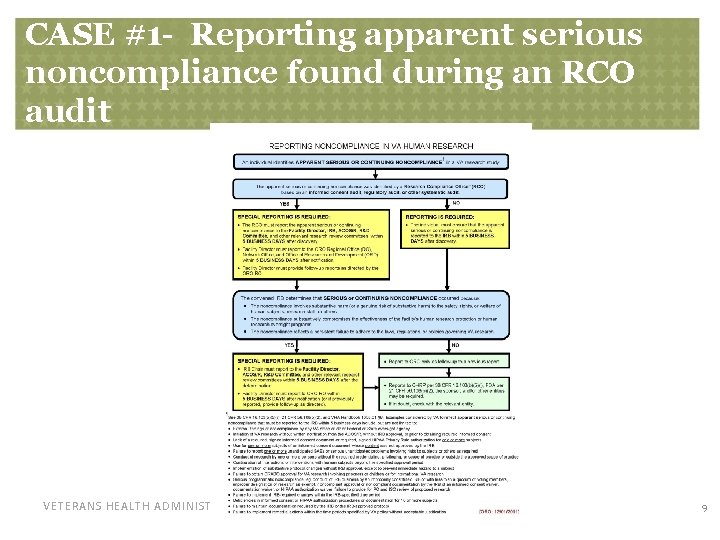
CASE #1 - Reporting apparent serious noncompliance found during an RCO audit VETERANS HEALTH ADMINISTRATION 9

CASE #1 - A Serious Adverse Event How Soon must an SAE be reported? VETERANS HEALTH ADMINISTRATION 10

CASE #1 - A Serious Adverse Event The Background: A PI became aware of a local unanticipated serious adverse event on January 1, 2012. It was not reported to the IRB by the PI until January 25, 2012, as part of a continuing review submission. VETERANS HEALTH ADMINISTRATION 11

CASE #1 - A Serious Adverse Event How Soon must an SAE be reported? VHA Handbook 1058. 01: 7. REQUIREMENTS RELATED TO HUMAN RESEARCH – a. Unanticipated Problems Involving Risks to Subjects or Others. Members of the VA research community are required to ensure that unanticipated problems involving risks to subjects or others in research are reported promptly to the IRB in accordance with the time periods established under local SOPs. – b. Serious Unanticipated Problems Involving Risks to Subjects or Others. Within 5 business days of becoming aware of any serious unanticipated problem involving risks to subjects or others in VA research, members of the VA research community are required to ensure that the problem has been reported in writing to the IRB. VETERANS HEALTH ADMINISTRATION 12

CASE #1 - A Serious Adverse Event The Background: • The IRB noted the delay in reporting the SAE by the PI at the continuing review, and reminded the PI of the responsibility to report timely in the future. • The IRB made no documented determinations about the SAE, or whether serious noncompliance had occurred. VETERANS HEALTH ADMINISTRATION 13

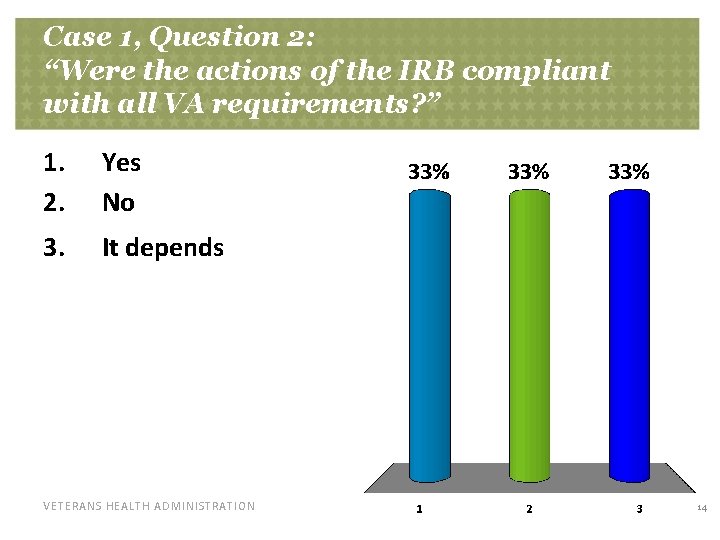
Case 1, Question 2: “Were the actions of the IRB compliant with all VA requirements? ” 1. 2. Yes No 3. It depends VETERANS HEALTH ADMINISTRATION 14

CASE #1 - A Serious Adverse Event The THIRD QUESTION: “What determinations should the IRB have made and documented? ” (pause for discussion) VETERANS HEALTH ADMINISTRATION 15

Case 1, Question 4: “What determinations should the IRB have made and documented? ” 1. 2. 3. 4. 5. Whether the event was serious, unanticipated, and related to research Whether the delay in reporting the SAE rose to the level of serious or continuing noncompliance Whether the subject should be compensated financially 1+2 All of the above VETERANS HEALTH ADMINISTRATION 16

CASE #1 - A Serious Adverse Event VHA HANDBOOK 1058. 01: 7 (d). IRB Review of Serious Unanticipated Problems and Unanticipated SAEs. – Within 5 business days after a report of a serious unanticipated problem involving risks to subjects or others, or of a local unanticipated SAE, the convened IRB or a qualified IRB member-reviewer must determine and document whether or not the reported incident was serious, unanticipated, and related to the research. NOTE: Per subparagraph 4 p, related means the event or problem may reasonably be regarded as caused by, or probably caused by, the research. VETERANS HEALTH ADMINISTRATION 17

CASE #1 - A Serious Adverse Event Determining whether non-compliance is serious or continuing • Therefore, failure by the PI to report an unanticipated SAE within the required time MUST BE REPORTED TO THE IRB and considered as APPARENT serious non-compliance. • However, it is extremely important to note that only the IRB has the authority (and the duty) to determine whether the specific facts of a case rise to the level of actual serious or continuing noncompliance. • Apparent serious non-compliance may or may not be actual serious non-compliance. The IRB has to decide. VETERANS HEALTH ADMINISTRATION 18

CASE #1 - A Serious Adverse Event VHA HANDBOOK 1058. 01: • 7(i). IRB Review of Apparent Serious or Continuing Noncompliance. – (4) An initial report of an IRB determination that serious noncompliance or continuing noncompliance occurred is required, even where the determination is preliminary or disposition of the matter has not been resolved at the time of the report. – NOTE: The IRB must reach a determination that serious or continuing noncompliance did or did not occur within 30 -45 days after receiving a report of apparent noncompliance. VETERANS HEALTH ADMINISTRATION 19

CASE #1 - A Serious Adverse Event The Next Step in the Story: • On June 15, 2012, during a regulatory audit, the RCO identified the late report of the local, unanticipated SAE as a compliance concern. • The RCO could not find any documentation in the IRB minutes regarding: – Whether the IRB determined that the SAE was serious, unanticipated, and related to research – Whether the IRB determined that the late report by the PI rose to the level of serious non-compliance VETERANS HEALTH ADMINISTRATION 20

CASE #1 - A Serious Adverse Event The Question Now: “What action(s) does the RCO need to take? ” (pause for discussion) VETERANS HEALTH ADMINISTRATION 21

Case 1, Question 5: “What action(s) does the RCO need to take? ” 1. 2. 3. 4. 5. Report to ACOS/R within 5 days Report to IRB within 5 days Report to Director within 5 days 1+2 All of the above VETERANS HEALTH ADMINISTRATION 22

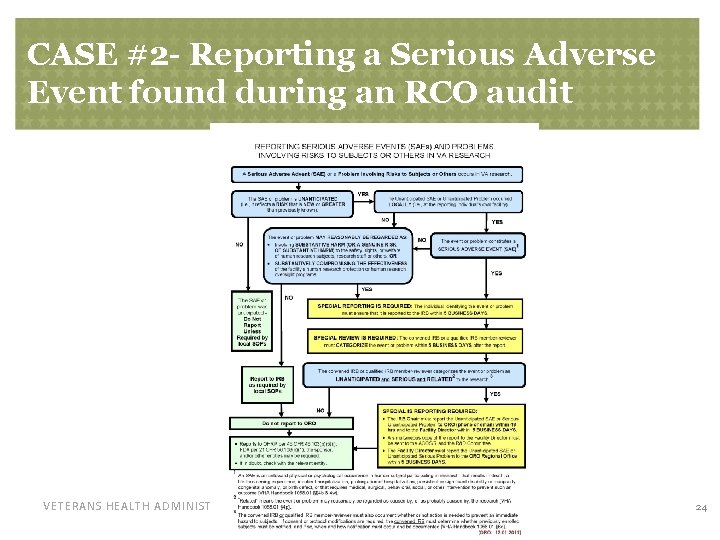
CASE #2 - A Serious Adverse Event • The RCO found during a regulatory audit that a local, unanticipated SAE was not evaluated by the IRB as required by VHA Handbook 1058. 01 • No determination was made whether the SAE was serious, unanticipated, and related to research. • The RCO was required to report the SAE back to the IRB within 5 days, this time for the required determination within 5 days by the IRB or designated, qualified member VETERANS HEALTH ADMINISTRATION 23

CASE #2 - Reporting a Serious Adverse Event found during an RCO audit VETERANS HEALTH ADMINISTRATION 24

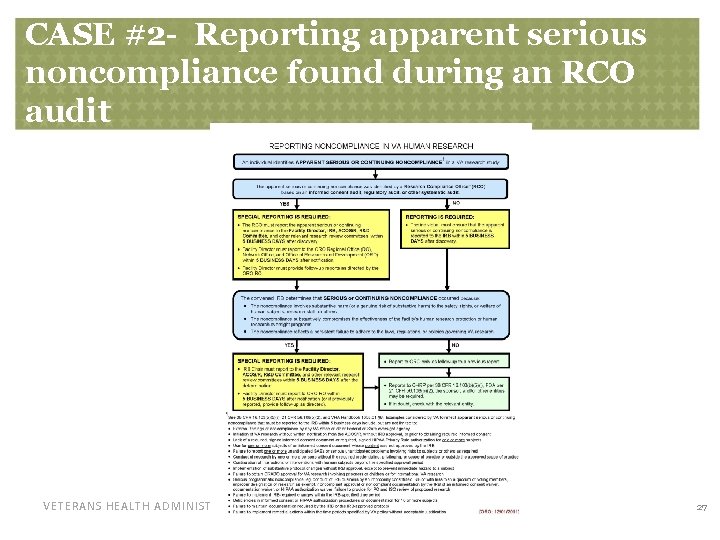
CASE #2 - A Serious Adverse Event – noncompliance in reporting • The RCO found 2 different noncompliance concerns: – The RCO found during a regulatory audit that a local, unanticipated SAE was not reported to the IRB as required by VHA Handbook 1058. 01 – In addition, the RCO found during a regulatory audit that the IRB had not reviewed a local SAE and documented their determination as required by VHA Handbook 1058. 01 VETERANS HEALTH ADMINISTRATION 25

CASE #2 - A Serious Adverse Eventnoncompliance in reporting • “ Failure to report one or more unanticipated SAEs or unanticipated serious problems involving risks to subjects or others as required by this (VHA) Handbook (1058. 01). ” • Therefore, the RCO was required to report the finding of late reporting of the local SAE as apparent serious noncompliance found during an audit. • Because this was found during an RCO audit, it triggers a special, fast, within-5 -day reporting to ORO even while the IRB is still considering the facts VETERANS HEALTH ADMINISTRATION 26

CASE #2 - Reporting apparent serious noncompliance found during an RCO audit VETERANS HEALTH ADMINISTRATION 27

CASE #2 - Noncompliance in IRB review of an SAE • The IRB had not reviewed a local SAE and documented their determination as required • This may represent “serious programmatic noncompliance” – (e) Any programmatic noncompliance involving substantive harm, or a genuine risk of substantive harm, to the safety, rights, or welfare of human research subjects, research staff, or others; and – (f) Any programmatic noncompliance that substantively compromises the effectiveness of the facility’s human research protection or human research oversight programs. • If so, the RCO was required to report noncompliant review of the local SAE as apparent serious noncompliance found during an audit. VETERANS HEALTH ADMINISTRATION 28

CASE #2 - A Serious Adverse Event ORO Common Findings: IRB #7 7. “When reviewing local serious adverse events (SAEs) or unanticipated problems involving risks to subjects or others (UPRs), the IRB (or qualified IRB-member reviewer) did not make proper determinations in a timely manner as required by VHA Handbook 1058. 01” VETERANS HEALTH ADMINISTRATION 29

CASE #2 - A Serious Adverse Event ORO Common Findings: IRB #7 • VHA Handbook 1058. 01 § 7. d • “Within 5 business days after a report of a serious unanticipated problem involving risks to subjects or others, or of a local unanticipated SAE, the convened IRB or a qualified IRB memberreviewer must determine and document whether or not the reported incident was serious, unanticipated, and related to the research. ” • ORO found that some IRBs, or qualified IRB-member reviewers, did not complete the review of local SAEs or UPRs in a timely manner as required. ORO review of IRB minutes revealed that some IRBs reviewed hundreds of reports of AEs, SAEs, and UPRs during every meeting without separating the reports of local SAEs and UPRs from the reports of external events, or making determinations whether local SAEs and UPRs are serious, unanticipated and related to research. VETERANS HEALTH ADMINISTRATION 30

Overview of Handbook 1058. 01 “Research Compliance Reporting Requirements” PURPOSE. This Veterans Health Administration Handbook sets forth the requirements for reporting certain research events to facility officials, relevant research review committees, and the Office of Research Oversight (ORO). NOTE: This Handbook does not preempt or otherwise alter any other applicable research reporting requirements, whether within the Department of Veterans Affairs (VA) or involving other Federal or state agencies or commercial sponsors. VETERANS HEALTH ADMINISTRATION 31

4. Definitions 5. General Requirements • Serious Adverse Events (SAEs) - Term is limited to Human Subjects Research • Serious Non-Compliance: – (1) Involving substantive harm, or a genuine risk of substantive harm, to the safety, rights, or welfare of human research subjects, research staff, or others; or – (2) Substantively compromising the effectiveness of a facility’s human research protection or human research oversight programs. • Contents of initial and follow-up reports to ORO. VETERANS HEALTH ADMINISTRATION 32

Major Topic Sections in VHA Handbook 1058. 01 6. Responsibilities of the Facility Director 7. Requirements Related to Human Research 8. Requirements Related to Animal Research 9. Requirements Related to Research Safety 10. Requirements Related to Research Laboratory Security 11. Requirements Related to Research Information Protection 12. Requirements Related to Research Misconduct Appendix: Table Summarizing Reporting Requirements VETERANS HEALTH ADMINISTRATION 33

6. Responsibilities of the Facility Director a. Ensuring that detailed SOPs are developed and implemented to satisfy all requirements of this Handbook, including requirements affecting the facility’s academic affiliates. b. Ensuring that all persons working in research or performing any research activities have been officially appointed by Human Resources Management. e. Ensuring that the results of all RCO audits are reported to the R&D Committee and all other relevant research review committees f. Reporting to ORO VETERANS HEALTH ADMINISTRATION 34

6. Responsibilities of the Facility Director c. Appointing one or more RCOs to conduct annual research informed consent audits and triennial regulatory audits in accordance with a written audit plan or SOP, and to assist in facility assessments of regulatory compliance – (2) RCO must report directly to the facility Director. . – (3) may serve as a non-voting consultant to oversight committees – (4) may participate in education activities – (5) must report any change in status of the facility RCO VETERANS HEALTH ADMINISTRATION 35

6. Responsibilities of the Facility Director (c) • “NOTE: Procedures and materials related to RCO training requirements and RCO audit requirements are updated periodically and posted prominently on ORO’s Web site at: http: //www. va. gov/oro/. ” VETERANS HEALTH ADMINISTRATION 36

7. Requirements Related to Human Research • Review and Reporting local unanticipated SAEs and serious unanticipated problems related to research (UPRs) • Definition of apparent serious or continuing noncompliance (SCN), required review and reporting • Examples that must be so considered (“the list”) • “Fast Track” reporting requirements for apparent SCN found during audits by the RCO • Reference to ORO Decision Charts for above matters • Reporting to ORO of program and accreditation changes VETERANS HEALTH ADMINISTRATION 37

8, 9, 10: Requirements Related to Animal Research, Research Safety, and Research Laboratory Security • Reportable events described • Required review • Required reporting of oversight committee determinations and actions VETERANS HEALTH ADMINISTRATION 38

11. Requirements Related to Research Information Protection • Research Information Protection Incidents defined • Reporting requirements to ORO, Information Security Officer (ISO) and Privacy Officer (PO) • Immediate (1 hour) reporting to the ACOS for Research, the facility ISO, and the facility PO requirement for any unauthorized use, disclosure, transmission, removal, theft, loss, or destruction of VA research-related protected health information (PHI), individually identifiable private information, or confidential information, as defined by the HIPAA Privacy Rule, the Common Rule, the Privacy Act, or 38 U. S. C. §§ 5701, 5705, and 7332. VETERANS HEALTH ADMINISTRATION 39

12. Requirements Related to Research Misconduct • Research Misconduct is defined as fabrication, falsification, or plagiarism in proposing, performing, or reviewing research, or in reporting research results. • For any allegation of Research Misconduct, ORO CO (Peter Poon) must be notified as soon as possible, preferably by telephone or email. Formal written notification may come later. Appendix B of this Handbook summarizes reporting procedures for allegations of Research Misconduct. • Reference VHA Handbook 1058. 2 “Research Misconduct” (May 4, 2005) VETERANS HEALTH ADMINISTRATION 40

CASE #2 - Reporting a Local SAE The Background: • Foxtrot VAMC used their Medical Affiliate as their IRB of record. • The IRB reviewed a local, unanticipated serious adverse event, and determined that it might reasonably be regarded as caused by the research. VETERANS HEALTH ADMINISTRATION 41

CASE #2 - Reporting a Local SAE The Situation Now: The IRB Chair sent a letter the same day to the ACOS for Research and the Chair of the R&D Committee at the Foxtrot VAMC, notifying them of the IRB’s determination. VETERANS HEALTH ADMINISTRATION 42

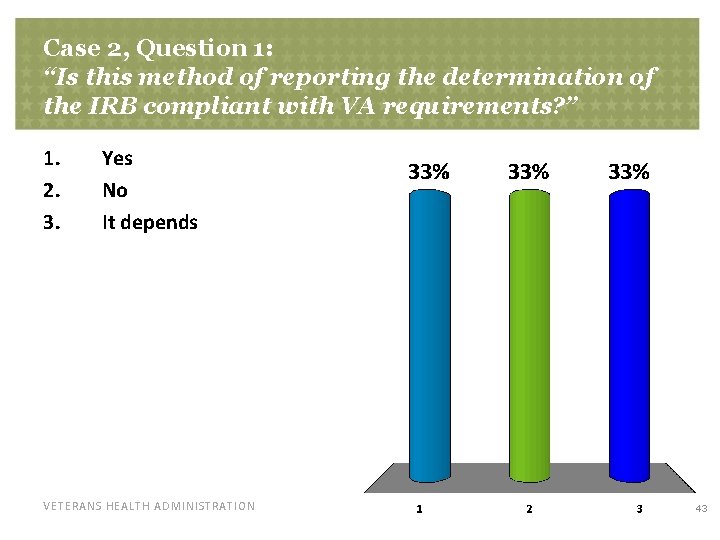
Case 2, Question 1: “Is this method of reporting the determination of the IRB compliant with VA requirements? ” 1. 2. 3. Yes No It depends VETERANS HEALTH ADMINISTRATION 43

CASE #2 - Reporting a Local SAE ORO Common Findings: IRB #12 12. “The IRB did not reportable unanticipated problems involving risks to subjects or others (UPRs), local serious adverse events (SAEs), or serious or continuing noncompliance to the Medical Center Director (only reported to ACOS/R&D and R&DC)” VETERANS HEALTH ADMINISTRATION 44

CASE #2 - Reporting a Local SAE ORO Common Findings: IRB #12 • VHA Handbook 1058. 01 § 7. d(1) • “If the convened IRB or the qualified IRB member-reviewer determines that the problem or event is serious and unanticipated and related to the research, the IRB Chair or designee must notify ORO via telephone or e-mail within 48 hours and report the problem or event directly (without intermediaries) to the facility Director within 5 business days after the determination. ” • ORO found that some IRBs, particularly IRBs at affiliate universities, reported these reportable research events to the ACOS/R&D and/or the R&DC, but not directly to the Medical Center Director as required. VETERANS HEALTH ADMINISTRATION 45

CASE #2 - Reporting a Local SAE Follow-Up QUESTION: “In addition to notification of ORO and the Facility Director, what further actions must the IRB take when a local adverse event is found to be serious, unanticipated, and related to research? ” (pause for discussion) VETERANS HEALTH ADMINISTRATION 46

CASE #2 - Reporting a Local SAE “Further IRB actions needed: ” 1. A simultaneous determination is required regarding the need for any action (e. g. , suspension of activities; notification of subjects) necessary to prevent an immediate hazard to subjects in accordance with VA regulations in 38 CFR 16. 103(b)(4)(iii). VETERANS HEALTH ADMINISTRATION 47

CASE #2 - Reporting a Local SAE “Further IRB actions needed: ” 2. The convened IRB must determine and document whether or not a protocol or informed consent modification is warranted. 3. If informed consent modification is warranted, the IRB must also determine and document the following: (a) Whether or not previously enrolled subjects must be notified of the modification and, if (b) When such notification must take place and how such notification must be documented. VETERANS HEALTH ADMINISTRATION 48

CASE #2 - Reporting a Local SAE “Further IRB actions needed: ” Are there other actions required? (pause for discussion) VETERANS HEALTH ADMINISTRATION 49

CASE #2 - Reporting a Local SAE “Further IRB actions needed: ” Who else may need to be notified when a local adverse event is found to be serious, unanticipated, and related to research? (pause for discussion) VETERANS HEALTH ADMINISTRATION 50

CASE #2 - Reporting a Local SAE “Further IRB actions needed: ” • The Common Rule 38 CFR 16. 103(b)(5) “Procedures for reporting” (OHRP) • FDA regs at 21 CFR 56. 108(b) • The PI may also have a responsibility to report to the study sponsor VETERANS HEALTH ADMINISTRATION 51

CASE #3 -Complex SAE Decisions The Background: • Alpha VAMC had many oncology research protocols, with frequent adverse events that may or may not have been related to research depending on complex fact patterns. • In their experience, the Alpha VAMC IRB found that often more than 5 days were needed to gather and evaluate all the facts, in order to best determine whether the adverse event was serious, unanticipated, and probably related to research VETERANS HEALTH ADMINISTRATION 52

CASE # 3 -Complex SAE Decisions The Situation Now: The Alpha VAMC IRB SOPs required the IRB Chair to forward the report to the convened IRB when more time was needed to reasonably make a determination whether a reported UPR or local SAE was serious, unanticipated, and related to research. VETERANS HEALTH ADMINISTRATION 53

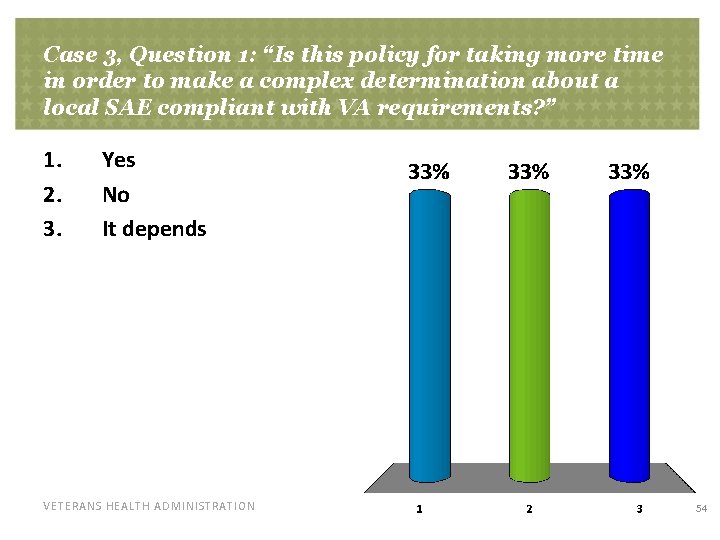
Case 3, Question 1: “Is this policy for taking more time in order to make a complex determination about a local SAE compliant with VA requirements? ” 1. 2. 3. Yes No It depends VETERANS HEALTH ADMINISTRATION 54

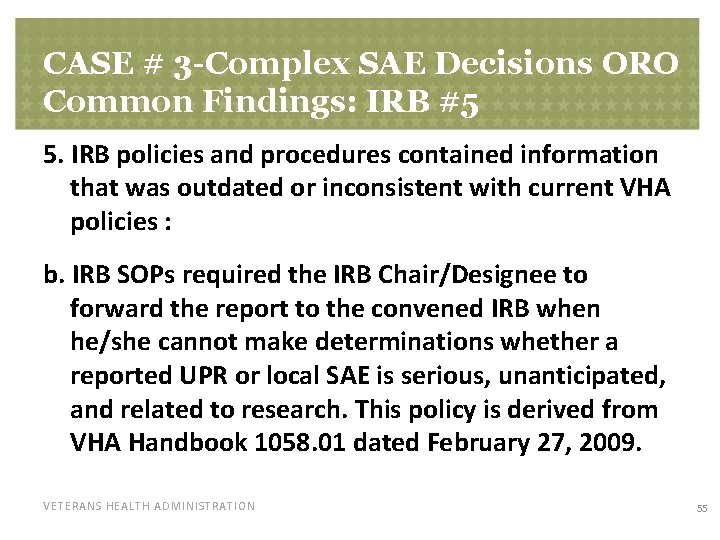
CASE # 3 -Complex SAE Decisions ORO Common Findings: IRB #5 5. IRB policies and procedures contained information that was outdated or inconsistent with current VHA policies : b. IRB SOPs required the IRB Chair/Designee to forward the report to the convened IRB when he/she cannot make determinations whether a reported UPR or local SAE is serious, unanticipated, and related to research. This policy is derived from VHA Handbook 1058. 01 dated February 27, 2009. VETERANS HEALTH ADMINISTRATION 55

CASE # 3 -Complex SAE Decisions ORO Common Findings: IRB #5 The current effective VHA Handbook 1058. 01 dated November 15, 2011, stipulates that: “Within 5 business days after a report of a serious unanticipated problem involving risks to subjects or others, or of a local unanticipated SAE, the convened IRB or a qualified IRB member-reviewer must determine and document whether or not the reported incident was serious, unanticipated, and related to the research. ” (§ 7. d) VETERANS HEALTH ADMINISTRATION 56

CASE # 3 -Complex SAE Decisions What is best compliant practice? • An initial determination MUST be made within 5 days, based on the best facts available. • If that determination is that YES, the local SAE was serious, unanticipated, and probably related to research, then ORO must be notified within 48 hours and formally through the Medical Center Director within 5 days plus 5 days VETERANS HEALTH ADMINISTRATION 57

CASE # 3 -Complex SAE Decisions What is best compliant practice? • Regardless of the initial determination of the designated qualified member, if more time is needed for fact-finding and analysis, that time may be taken by the IRB. • A final determination can be made later based on a more complete analysis. This final determination may uphold or reverse the initial determination. VETERANS HEALTH ADMINISTRATION 58

CASE #4 -Reporting Anticipated Adverse Events-The Background: • Foxtrot VAMC IRB is reviewing a protocol that is an oncology trial for Stage 4 cancer. • The primary outcome measure for the trial is death, and death is listed in the protocol as the expected outcome. VETERANS HEALTH ADMINISTRATION 59

CASE # 4 -Anticipated SAEs The QUESTION: “Does every death that occurs in subjects during this study have to be reported within 5 days to the IRB and a determination made within 5 days whether the SAE was unanticipated and related to research? ” (pause for discussion) VETERANS HEALTH ADMINISTRATION 60

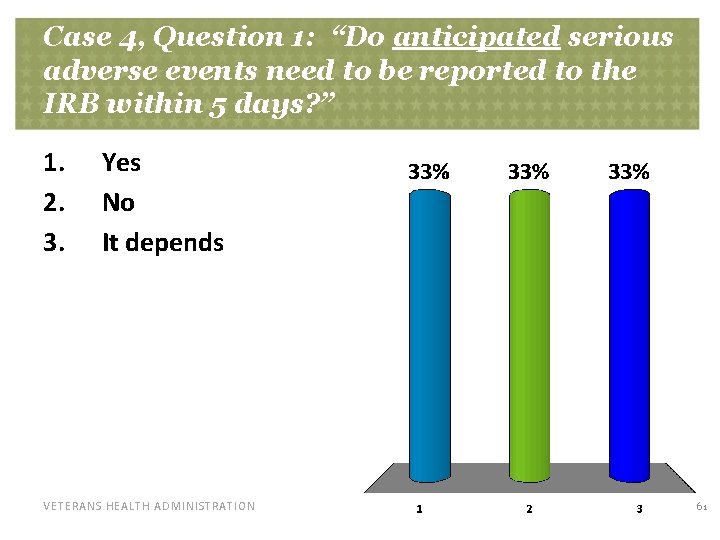
Case 4, Question 1: “Do anticipated serious adverse events need to be reported to the IRB within 5 days? ” 1. 2. 3. Yes No It depends VETERANS HEALTH ADMINISTRATION 61

CASE # 4 Reporting Anticipated SAEs: • Only SAEs judged by the PI to be unanticipated require reporting to within 5 days to the IRB for a determination. • Anticipated SAEs are reported to the IRB as described in the protocol and/or local SOPs VETERANS HEALTH ADMINISTRATION 62

CASE # 4 Reporting Anticipated SAEs: • In the example given, death from cancer would be an anticipated SAE • However, if the PI suspected death occurred for some other reason, for example allergic reaction, that death might be unanticipated and require reporting VETERANS HEALTH ADMINISTRATION 63

CASE # 5: Anticipating SAEs in protocol design and description: • A study is proposed to measure hearing acuity over time in human subjects who are on renal dialysis and IV gentamycin therapy • Every subject will be followed with repeated hearing tests for at least 12 months • Hospitalizations are anticipated to occur in this patient population during the follow-up period, unrelated to the study or the hearing tests. VETERANS HEALTH ADMINISTRATION 64

CASE # 5: Anticipating SAEs in protocol design and description: • Hospitalizations are defined in local SOPs as serious adverse events. • How can the PI avoid reporting every hospitalization to the IRB for a determination of whether the hospitalization is related to the research study? VETERANS HEALTH ADMINISTRATION 65

VHA Handbook 1058. 01 “Research Compliance Reporting Requirements” WHAT ARE YOUR MOST COMMON ISSUES RELATED TO THE REQUIREMENTS OF THIS HANDBOOK? (pause for discussion) VETERANS HEALTH ADMINISTRATION 66
 Audit oversight board
Audit oversight board Jcids instruction
Jcids instruction Aob sanction
Aob sanction Joint requirements oversight council
Joint requirements oversight council Provincial medical oversight
Provincial medical oversight Board of directors risk oversight responsibilities
Board of directors risk oversight responsibilities Management oversight and risk tree
Management oversight and risk tree Public procurement oversight advisory board
Public procurement oversight advisory board Ohrps
Ohrps Delivery oversight
Delivery oversight Oversight
Oversight Administrative oversight definition
Administrative oversight definition Risk based oversight
Risk based oversight Regulatory oversight definition
Regulatory oversight definition Low income oversight board
Low income oversight board The idea that judges ought to freely strike down
The idea that judges ought to freely strike down Reporting and evaluating research
Reporting and evaluating research Reporting and evaluating research
Reporting and evaluating research Reporting research findings
Reporting research findings Office and factory
Office and factory Wvu research office
Wvu research office Research report vs research proposal
Research report vs research proposal Methodology vs method
Methodology vs method Example of appendices in research paper
Example of appendices in research paper Conclusive descriptive research
Conclusive descriptive research Applied vs fundamental research
Applied vs fundamental research Theoretical background example
Theoretical background example Contrast applied research and basic research
Contrast applied research and basic research Identification of the problem in research
Identification of the problem in research Research instrument in experimental research
Research instrument in experimental research Operational thought
Operational thought Causal-comparative research
Causal-comparative research Chapter 3 research qualitative
Chapter 3 research qualitative Examples of applied research
Examples of applied research Basic research vs applied research
Basic research vs applied research Objectives of reseach
Objectives of reseach Qualitative research methods
Qualitative research methods Practical research 2 inquiry and research
Practical research 2 inquiry and research Exploratory research vs conclusive research
Exploratory research vs conclusive research Research design for exploratory research
Research design for exploratory research General objective in research example
General objective in research example Research instrument in experimental research
Research instrument in experimental research Nature of qualitative research
Nature of qualitative research Frx report manager
Frx report manager Actuate reporting tool tutorial
Actuate reporting tool tutorial F10 curriculum
F10 curriculum Atibulon
Atibulon Urs version 2
Urs version 2 Programme reporting dashboard
Programme reporting dashboard Standard accounting budgeting and reporting system
Standard accounting budgeting and reporting system Truesight smart reporting
Truesight smart reporting Tattling vs reporting
Tattling vs reporting What wind is forecast for stl at 12 000 feet
What wind is forecast for stl at 12 000 feet Aabe ethiopia
Aabe ethiopia Tceq spill reporting
Tceq spill reporting Mandatory reporting arizona
Mandatory reporting arizona Spla reporting tool
Spla reporting tool Louisiana special education reporting system
Louisiana special education reporting system What are the steps in performance management process
What are the steps in performance management process What does sentinel event mean
What does sentinel event mean Lds seminary attendance requirements
Lds seminary attendance requirements Segment reporting example
Segment reporting example 8th grade science staar review reporting category 4
8th grade science staar review reporting category 4 Sbu reporting
Sbu reporting Competent person's reporting
Competent person's reporting 12vac35-105-160
12vac35-105-160 Riskman incident reporting system
Riskman incident reporting system Query tools in data mining
Query tools in data mining