Novel Agents for Indolent Lymphoma and Mantle Cell




- Slides: 24

Novel Agents for Indolent Lymphoma and Mantle Cell Lymphoma Stephen Ansell, MD, Ph. D Mayo Clinic

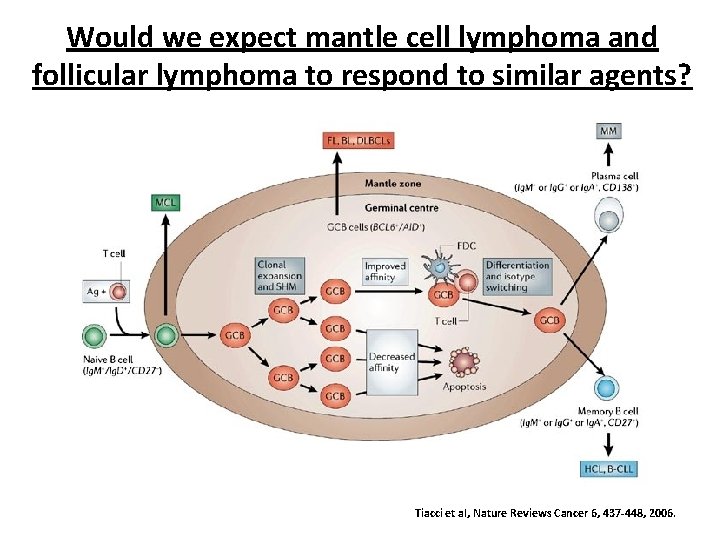
Would we expect mantle cell lymphoma and follicular lymphoma to respond to similar agents? Tiacci et al, Nature Reviews Cancer 6, 437 -448, 2006.

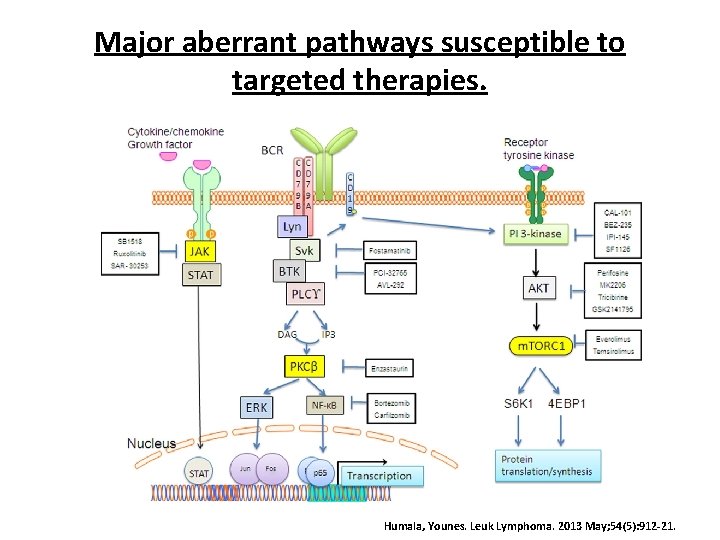
Major aberrant pathways susceptible to targeted therapies. Humala, Younes. Leuk Lymphoma. 2013 May; 54(5): 912 -21.

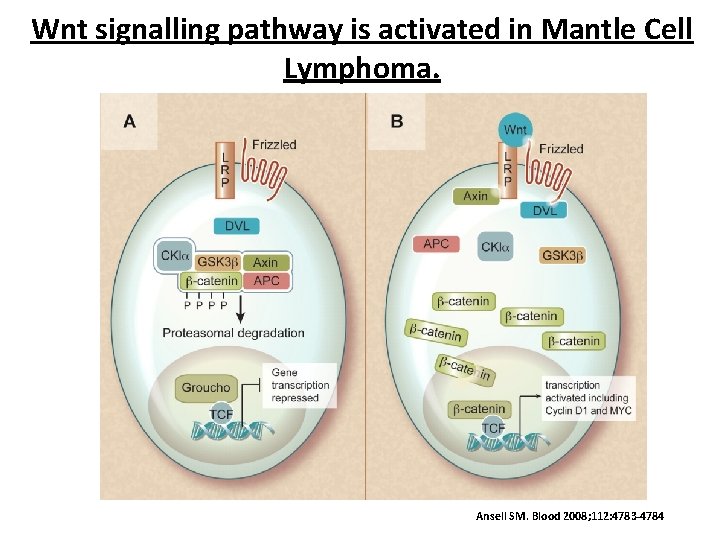
Wnt signalling pathway is activated in Mantle Cell Lymphoma. Ansell SM. Blood 2008; 112: 4783 -4784

Novel Agents for Mantle Cell Lymphoma

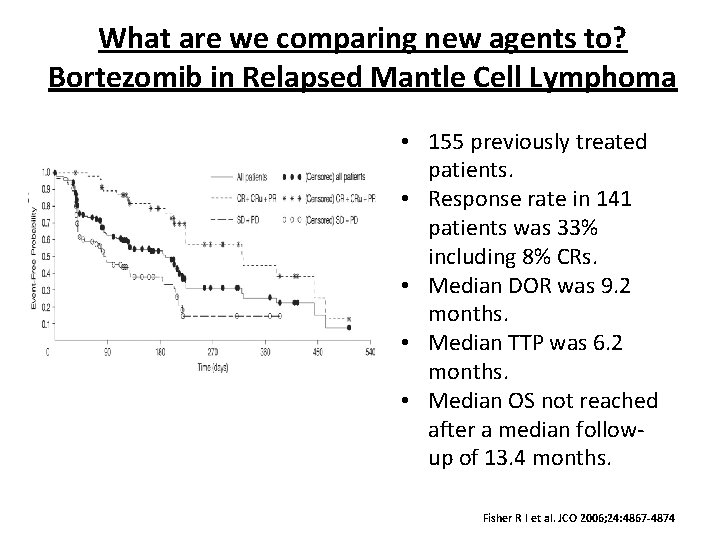
What are we comparing new agents to? Bortezomib in Relapsed Mantle Cell Lymphoma • 155 previously treated patients. • Response rate in 141 patients was 33% including 8% CRs. • Median DOR was 9. 2 months. • Median TTP was 6. 2 months. • Median OS not reached after a median followup of 13. 4 months. Fisher R I et al. JCO 2006; 24: 4867 -4874

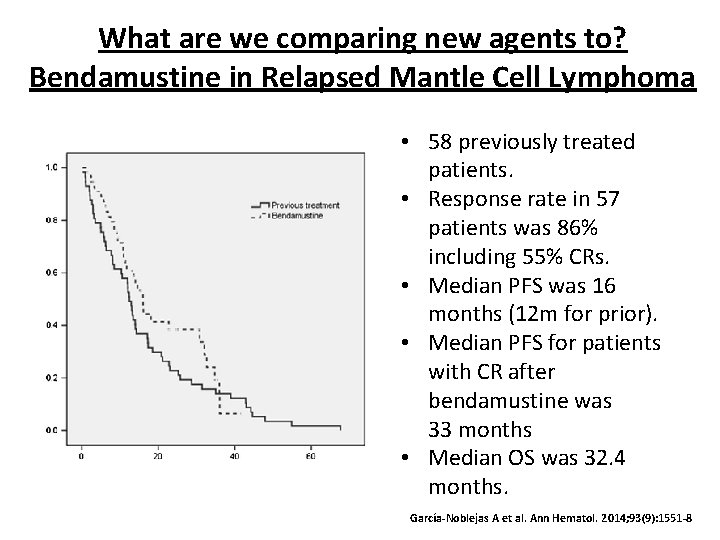
What are we comparing new agents to? Bendamustine in Relapsed Mantle Cell Lymphoma • 58 previously treated patients. • Response rate in 57 patients was 86% including 55% CRs. • Median PFS was 16 months (12 m for prior). • Median PFS for patients with CR after bendamustine was 33 months • Median OS was 32. 4 months. García-Noblejas A et al. Ann Hematol. 2014; 93(9): 1551 -8

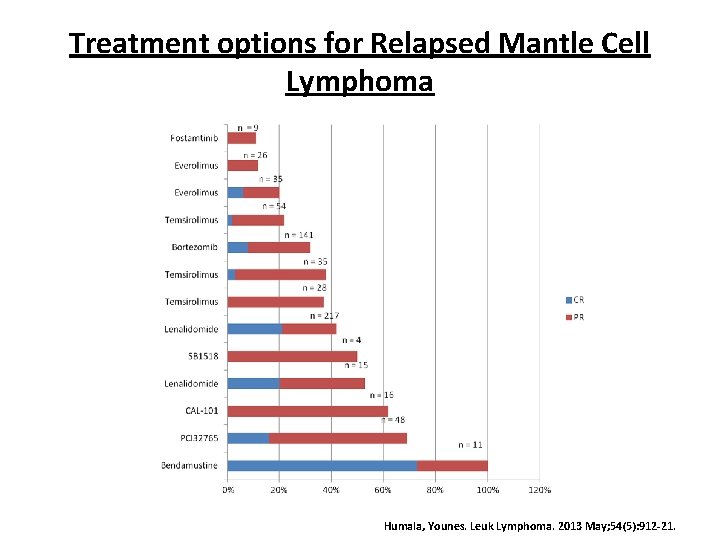
Treatment options for Relapsed Mantle Cell Lymphoma Humala, Younes. Leuk Lymphoma. 2013 May; 54(5): 912 -21.

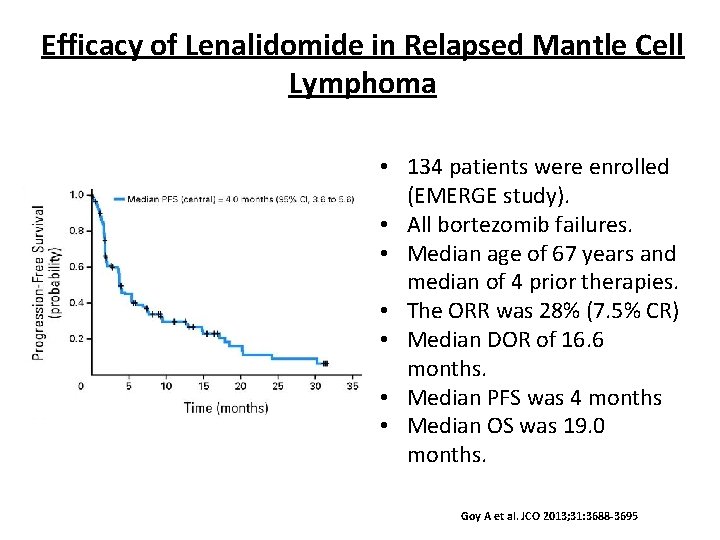
Efficacy of Lenalidomide in Relapsed Mantle Cell Lymphoma • 134 patients were enrolled (EMERGE study). • All bortezomib failures. • Median age of 67 years and median of 4 prior therapies. • The ORR was 28% (7. 5% CR) • Median DOR of 16. 6 months. • Median PFS was 4 months • Median OS was 19. 0 months. Goy A et al. JCO 2013; 31: 3688 -3695

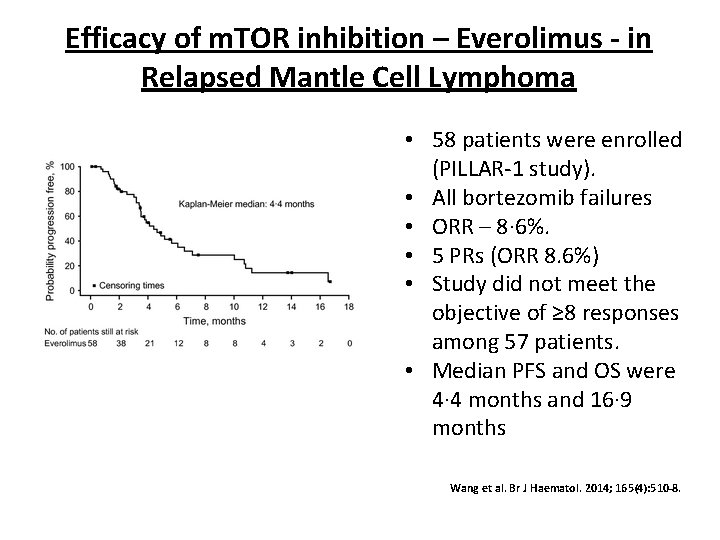
Efficacy of m. TOR inhibition – Everolimus - in Relapsed Mantle Cell Lymphoma • 58 patients were enrolled (PILLAR-1 study). • All bortezomib failures • ORR – 8· 6%. • 5 PRs (ORR 8. 6%) • Study did not meet the objective of ≥ 8 responses among 57 patients. • Median PFS and OS were 4· 4 months and 16· 9 months Wang et al. Br J Haematol. 2014; 165(4): 510 -8.

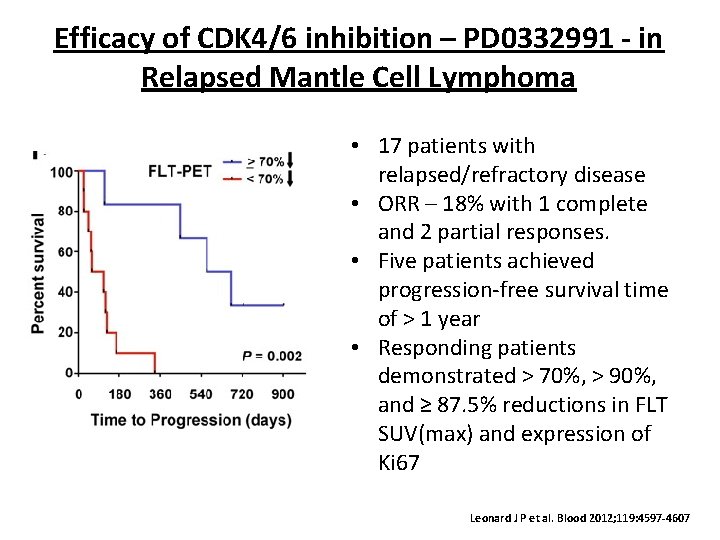
Efficacy of CDK 4/6 inhibition – PD 0332991 - in Relapsed Mantle Cell Lymphoma • 17 patients with relapsed/refractory disease • ORR – 18% with 1 complete and 2 partial responses. • Five patients achieved progression-free survival time of > 1 year • Responding patients demonstrated > 70%, > 90%, and ≥ 87. 5% reductions in FLT SUV(max) and expression of Ki 67 Leonard J P et al. Blood 2012; 119: 4597 -4607

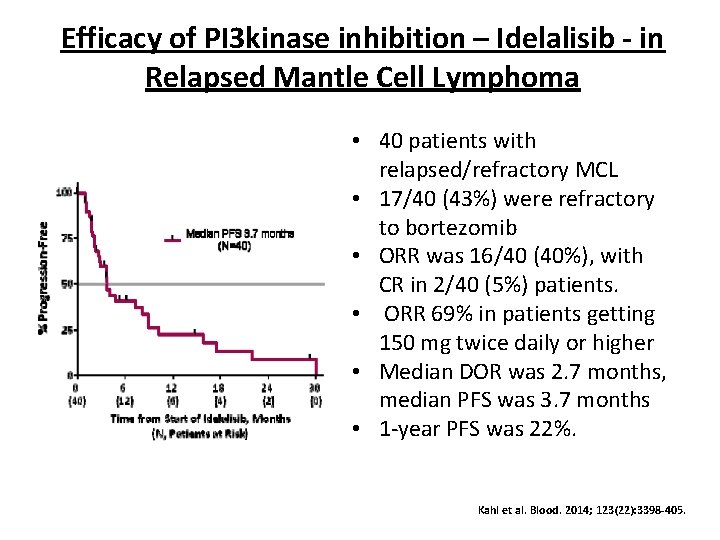
Efficacy of PI 3 kinase inhibition – Idelalisib - in Relapsed Mantle Cell Lymphoma • 40 patients with relapsed/refractory MCL • 17/40 (43%) were refractory to bortezomib • ORR was 16/40 (40%), with CR in 2/40 (5%) patients. • ORR 69% in patients getting 150 mg twice daily or higher • Median DOR was 2. 7 months, median PFS was 3. 7 months • 1 -year PFS was 22%. Kahl et al. Blood. 2014; 123(22): 3398 -405.

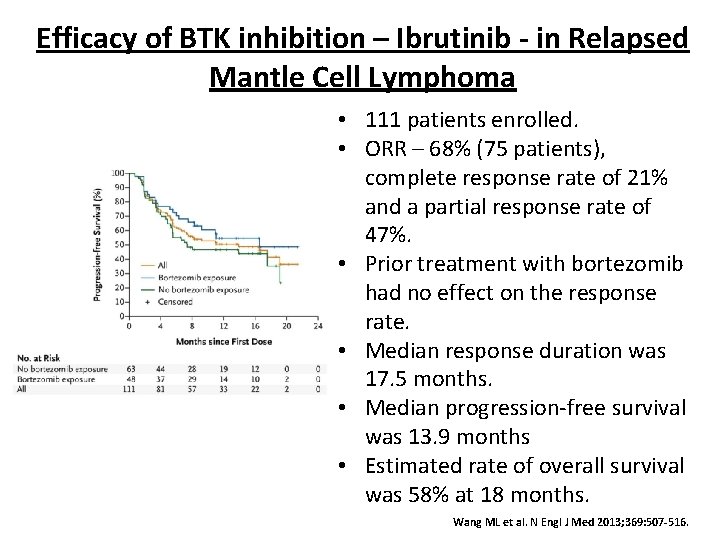
Efficacy of BTK inhibition – Ibrutinib - in Relapsed Mantle Cell Lymphoma • 111 patients enrolled. • ORR – 68% (75 patients), complete response rate of 21% and a partial response rate of 47%. • Prior treatment with bortezomib had no effect on the response rate. • Median response duration was 17. 5 months. • Median progression-free survival was 13. 9 months • Estimated rate of overall survival was 58% at 18 months. Wang ML et al. N Engl J Med 2013; 369: 507 -516.

Novel Agents for Follicular Lymphoma

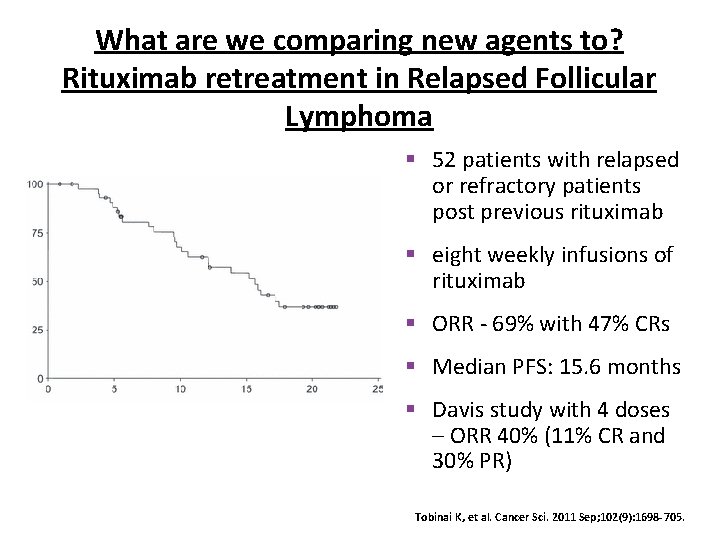
What are we comparing new agents to? Rituximab retreatment in Relapsed Follicular Lymphoma § 52 patients with relapsed or refractory patients post previous rituximab § eight weekly infusions of rituximab § ORR - 69% with 47% CRs § Median PFS: 15. 6 months § Davis study with 4 doses – ORR 40% (11% CR and 30% PR) Tobinai K, et al. Cancer Sci. 2011 Sep; 102(9): 1698 -705.

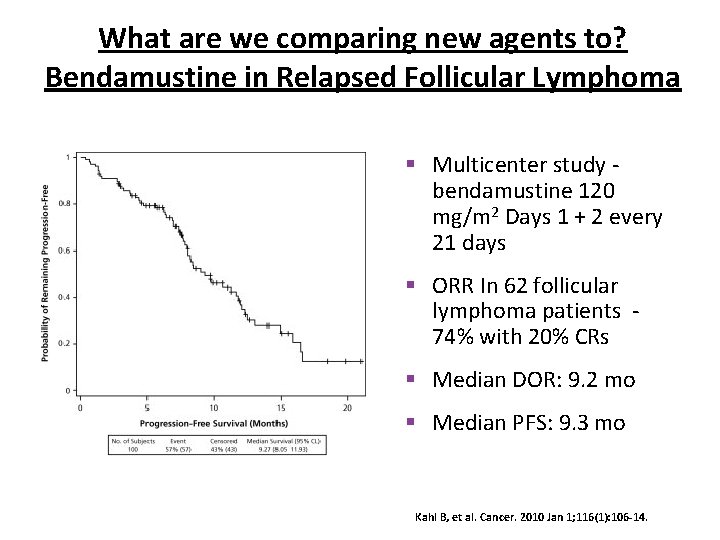
What are we comparing new agents to? Bendamustine in Relapsed Follicular Lymphoma § Multicenter study - bendamustine 120 mg/m 2 Days 1 + 2 every 21 days § ORR In 62 follicular lymphoma patients - 74% with 20% CRs § Median DOR: 9. 2 mo § Median PFS: 9. 3 mo Kahl B, et al. Cancer. 2010 Jan 1; 116(1): 106 -14.

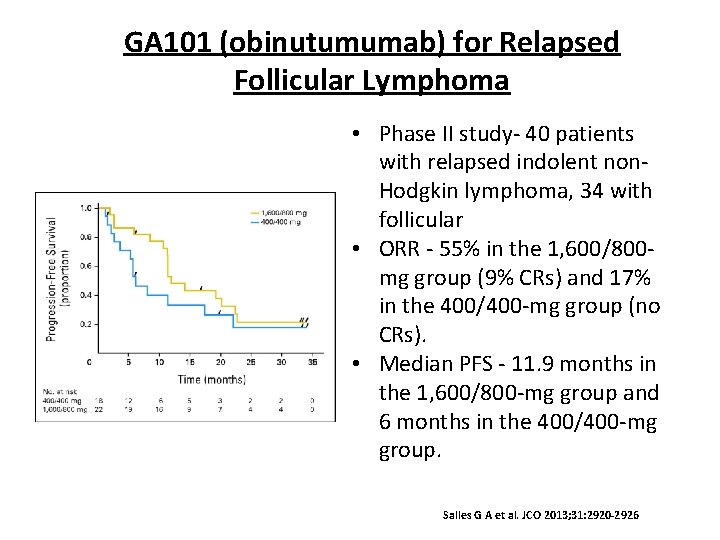
GA 101 (obinutumumab) for Relapsed Follicular Lymphoma • Phase II study- 40 patients with relapsed indolent non. Hodgkin lymphoma, 34 with follicular • ORR - 55% in the 1, 600/800 mg group (9% CRs) and 17% in the 400/400 -mg group (no CRs). • Median PFS - 11. 9 months in the 1, 600/800 -mg group and 6 months in the 400/400 -mg group. Salles G A et al. JCO 2013; 31: 2920 -2926

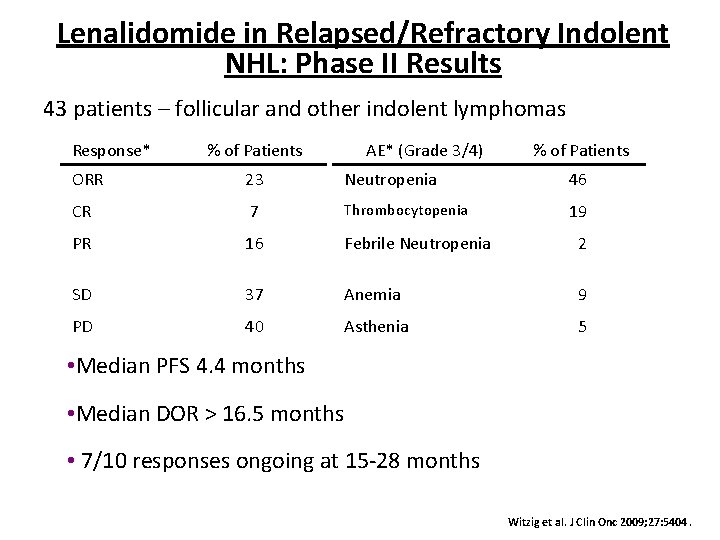
Lenalidomide in Relapsed/Refractory Indolent NHL: Phase II Results 43 patients – follicular and other indolent lymphomas Response* % of Patients AE* (Grade 3/4) % of Patients ORR 23 Neutropenia 46 CR 7 Thrombocytopenia 19 PR 16 Febrile Neutropenia 2 SD 37 Anemia 9 PD 40 Asthenia 5 • Median PFS 4. 4 months • Median DOR > 16. 5 months • 7/10 responses ongoing at 15 -28 months Witzig et al. J Clin Onc 2009; 27: 5404.

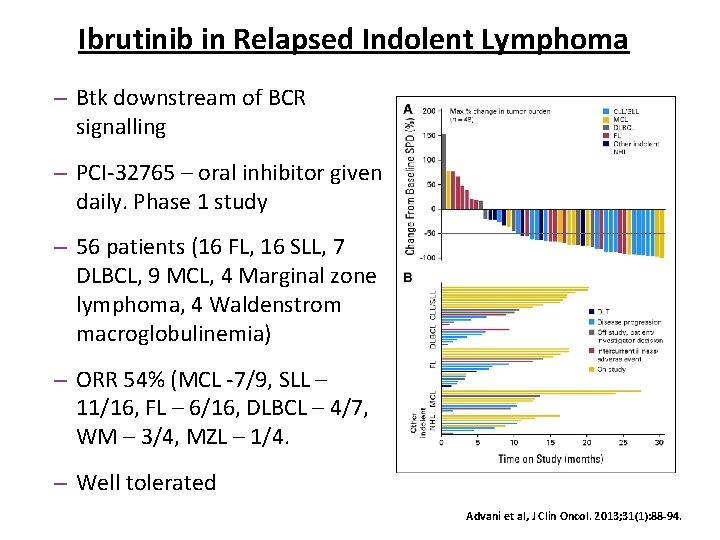
Ibrutinib in Relapsed Indolent Lymphoma – Btk downstream of BCR signalling – PCI-32765 – oral inhibitor given daily. Phase 1 study – 56 patients (16 FL, 16 SLL, 7 DLBCL, 9 MCL, 4 Marginal zone lymphoma, 4 Waldenstrom macroglobulinemia) – ORR 54% (MCL -7/9, SLL – 11/16, FL – 6/16, DLBCL – 4/7, WM – 3/4, MZL – 1/4. – Well tolerated Advani et al, J Clin Oncol. 2013; 31(1): 88 -94.

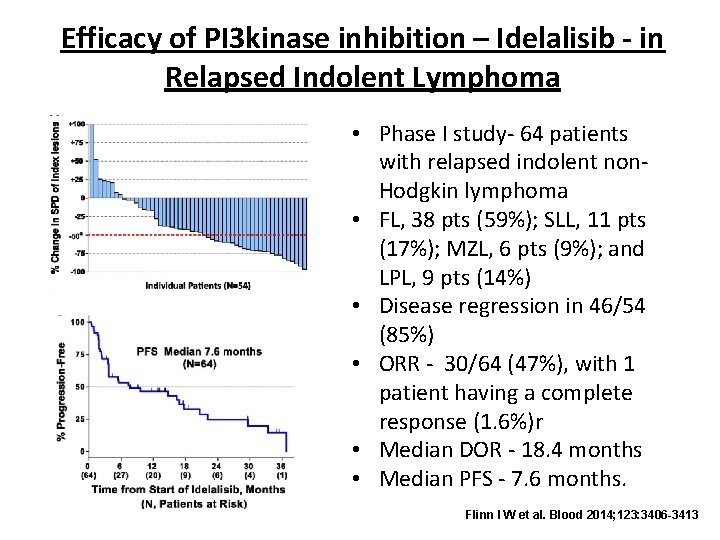
Efficacy of PI 3 kinase inhibition – Idelalisib - in Relapsed Indolent Lymphoma • Phase I study- 64 patients with relapsed indolent non. Hodgkin lymphoma • FL, 38 pts (59%); SLL, 11 pts (17%); MZL, 6 pts (9%); and LPL, 9 pts (14%) • Disease regression in 46/54 (85%) • ORR - 30/64 (47%), with 1 patient having a complete response (1. 6%)r • Median DOR - 18. 4 months • Median PFS - 7. 6 months. Flinn I W et al. Blood 2014; 123: 3406 -3413

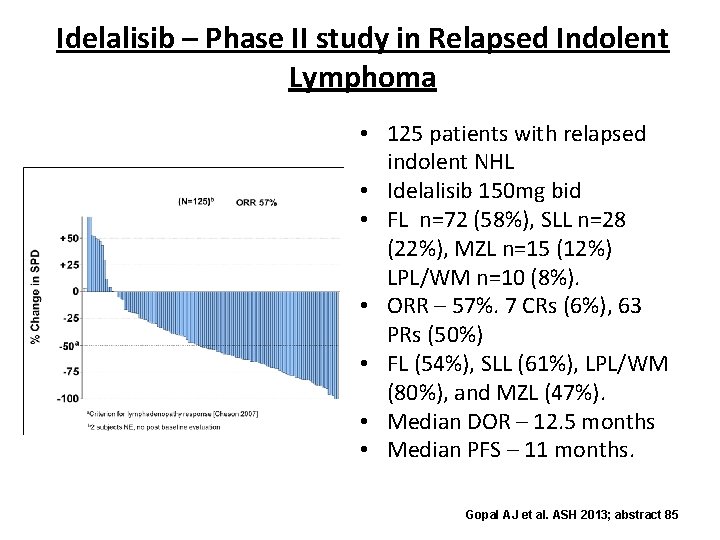
Idelalisib – Phase II study in Relapsed Indolent Lymphoma • 125 patients with relapsed indolent NHL • Idelalisib 150 mg bid • FL n=72 (58%), SLL n=28 (22%), MZL n=15 (12%) LPL/WM n=10 (8%). • ORR – 57%. 7 CRs (6%), 63 PRs (50%) • FL (54%), SLL (61%), LPL/WM (80%), and MZL (47%). • Median DOR – 12. 5 months • Median PFS – 11 months. Gopal AJ et al. ASH 2013; abstract 85

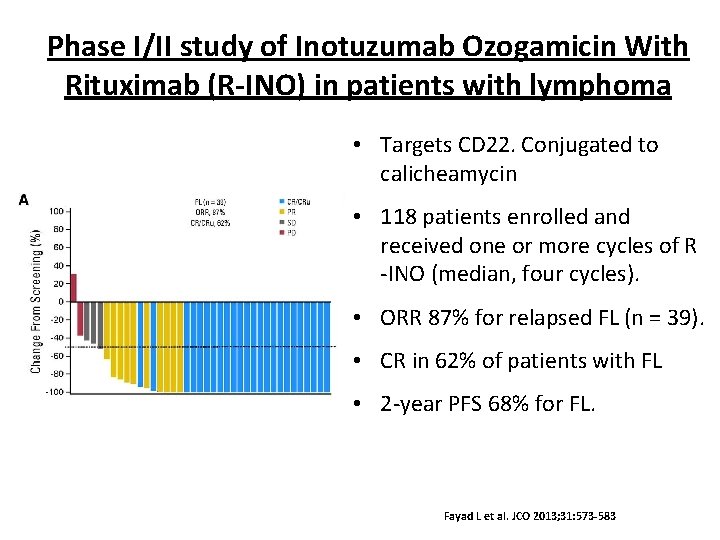
Phase I/II study of Inotuzumab Ozogamicin With Rituximab (R-INO) in patients with lymphoma • Targets CD 22. Conjugated to calicheamycin • 118 patients enrolled and received one or more cycles of R -INO (median, four cycles). • ORR 87% for relapsed FL (n = 39). • CR in 62% of patients with FL • 2 -year PFS 68% for FL. Fayad L et al. JCO 2013; 31: 573 -583

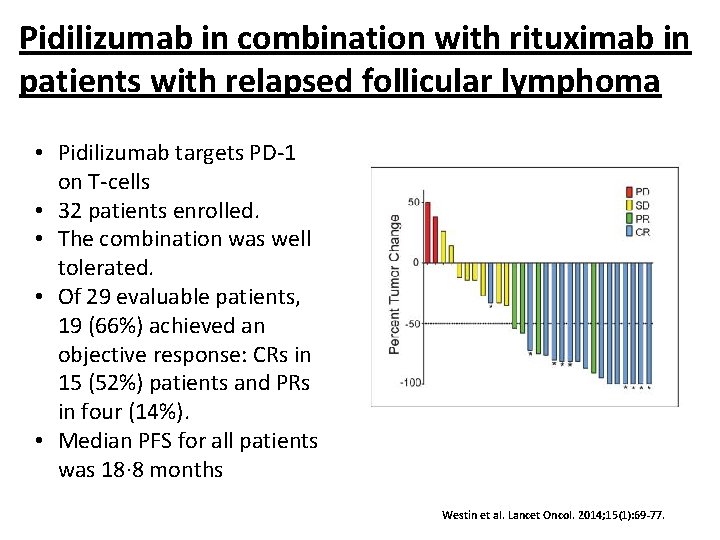
Pidilizumab in combination with rituximab in patients with relapsed follicular lymphoma • Pidilizumab targets PD-1 on T-cells • 32 patients enrolled. • The combination was well tolerated. • Of 29 evaluable patients, 19 (66%) achieved an objective response: CRs in 15 (52%) patients and PRs in four (14%). • Median PFS for all patients was 18· 8 months Westin et al. Lancet Oncol. 2014; 15(1): 69 -77.

Conclusions • Similar classes of agents have promising activity in relapsed and refractory mantle cell lymphoma as well as indolent lymphomas. • Many current trials are combining these agents to improve results. • Future studies may need a rational approach to decide which drugs to combine. • A standard regimen and patient population will be needed to allow for comparisons between studies.
 Indolent non-hodgkin lymphoma quizlet
Indolent non-hodgkin lymphoma quizlet Rbac mantle cell lymphoma
Rbac mantle cell lymphoma Maintenance rituximab mantle cell lymphoma
Maintenance rituximab mantle cell lymphoma Futile in the most dangerous game
Futile in the most dangerous game Indolent carditis
Indolent carditis The indolent judge
The indolent judge Hepatosplenic t-cell lymphoma
Hepatosplenic t-cell lymphoma Difference between hodgkin and non hodgkin lymphoma
Difference between hodgkin and non hodgkin lymphoma Difference hodgkin and non hodgkin lymphoma
Difference hodgkin and non hodgkin lymphoma Leukemia vs lymphoma
Leukemia vs lymphoma The strong lower part of the mantle
The strong lower part of the mantle Hiv family name
Hiv family name Lymphoma
Lymphoma Nhl classification
Nhl classification Alcohol-induced pain in hodgkin lymphoma
Alcohol-induced pain in hodgkin lymphoma Clinical presentation of hodgkin's lymphoma
Clinical presentation of hodgkin's lymphoma Lymphoma
Lymphoma Chylothorax
Chylothorax Classification of hodgkin lymphoma
Classification of hodgkin lymphoma Burkitt lymphoma
Burkitt lymphoma Hodjkins disease
Hodjkins disease Burkitt lymphoma
Burkitt lymphoma Szóbajön
Szóbajön Non-hodgkin lymphoma
Non-hodgkin lymphoma Hematological malignancies
Hematological malignancies