Mantle Cell Lymphoma MCL Approach to Upfront Treatment














- Slides: 28

Mantle Cell Lymphoma (MCL): Approach to Upfront Treatment Lauren C Pinter-Brown, MD Director, UCLA Lymphoma Program Clinical Professor of Medicine Geffen School of Medicine at UCLA

Interest in Topics Related to the Treatment of Patients with CTCL or PTCL (Percent Responding 9 or 10) Copyright © 2011 Research To Practice. All rights reserved.

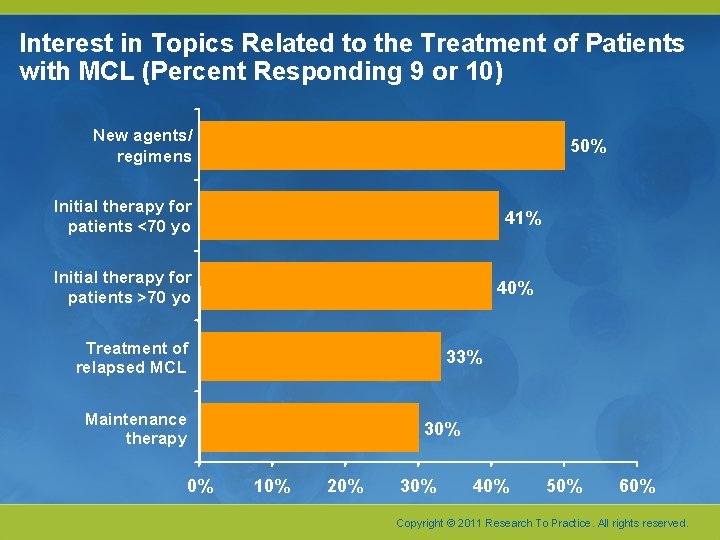
Interest in Topics Related to the Treatment of Patients with MCL (Percent Responding 9 or 10) New agents/ regimens 50% Initial therapy for patients <70 yo 41% Initial therapy for patients >70 yo 40% Treatment of relapsed MCL 33% Maintenance therapy 0% 30% 10% 20% 30% 40% 50% 60% Copyright © 2011 Research To Practice. All rights reserved.

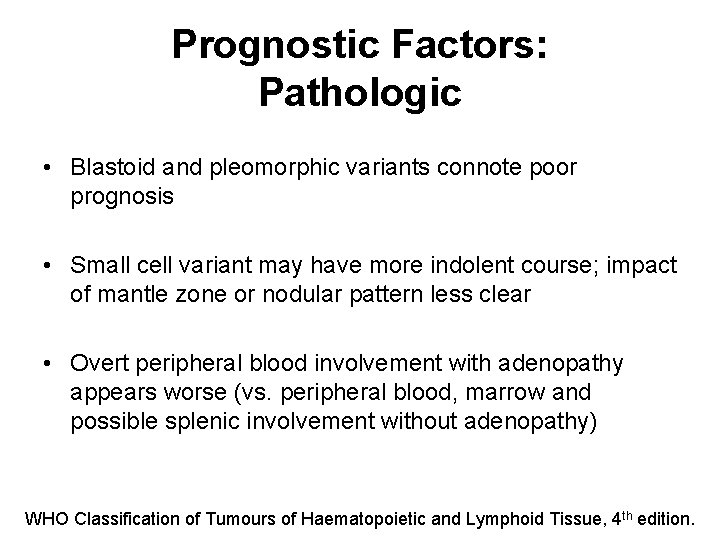
Prognostic Factors: Pathologic • Blastoid and pleomorphic variants connote poor prognosis • Small cell variant may have more indolent course; impact of mantle zone or nodular pattern less clear • Overt peripheral blood involvement with adenopathy appears worse (vs. peripheral blood, marrow and possible splenic involvement without adenopathy) WHO Classification of Tumours of Haematopoietic and Lymphoid Tissue, 4 th edition.

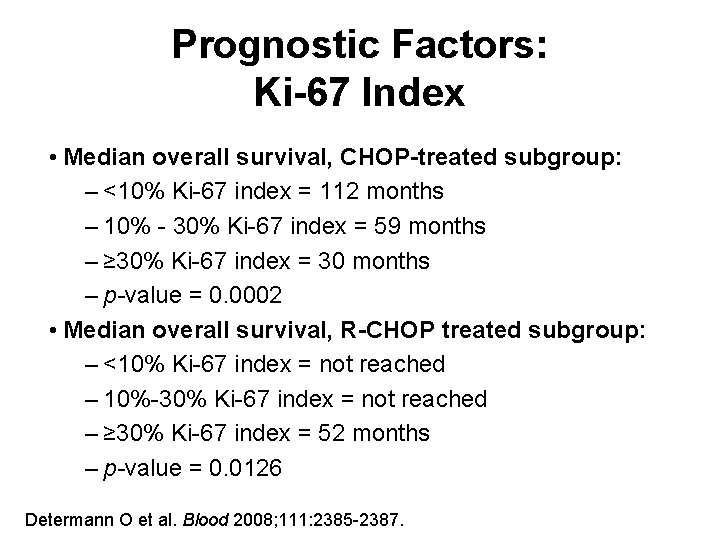
Prognostic Factors: Ki-67 Index • Median overall survival, CHOP-treated subgroup: – <10% Ki-67 index = 112 months – 10% - 30% Ki-67 index = 59 months – ≥ 30% Ki-67 index = 30 months – p-value = 0. 0002 • Median overall survival, R-CHOP treated subgroup: – <10% Ki-67 index = not reached – 10%-30% Ki-67 index = not reached – ≥ 30% Ki-67 index = 52 months – p-value = 0. 0126 Determann O et al. Blood 2008; 111: 2385 -2387.

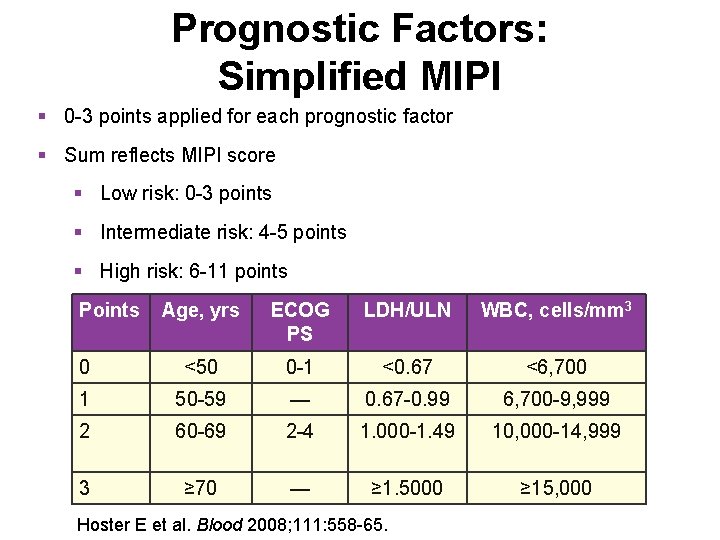
Prognostic Factors: Simplified MIPI § 0 -3 points applied for each prognostic factor § Sum reflects MIPI score § Low risk: 0 -3 points § Intermediate risk: 4 -5 points § High risk: 6 -11 points Points Age, yrs ECOG PS LDH/ULN WBC, cells/mm 3 0 <50 0 -1 <0. 67 <6, 700 1 50 -59 — 0. 67 -0. 99 6, 700 -9, 999 2 60 -69 2 -4 1. 000 -1. 49 10, 000 -14, 999 3 ≥ 70 — ≥ 1. 5000 ≥ 15, 000 Hoster E et al. Blood 2008; 111: 558 -65.

Frontline Treatment Considerations • Optimal frontline standard of care not yet defined • MCL not yet shown to be curable with currently available frontline treatments • Prolongation of survival by frontline treatment has been definitely shown • May consider treatment options separately for: – Patients for whom a dose intensive approach is an option (65 and under without comorbidities) – Patients for whom a dose intensive approach is not an option (older than 65 or with significant comorbidities) – Median age is around 60

Patients for Whom a Dose-Intensive Approach is Not an Option • In truth… – Will likely need additional treatment earlier than if the patient had an aggressive initial therapy (NCCN database) – ? Similar OS than if the patient had aggressive therapy (Cornell retrospective data, N=97…in selected asymptomatic patients “watch and wait” is acceptable management 1) – Many choices that include: • R-bendamustine vs R-CHOP (Rummel data: BR has stat sig increased PFS, 33 vs 23 mo, and better tox profile than R-CHOP in MCL, N=932) • Modified hyper. CVAD (Vc. R-CVAD) with R maintenance (ECOG-E 1405 shows high CR rate 3) • Rituximab alone, R-CVP, R-fludarabine/CDA 1. 2. 3. Martin P, JCO 2009; 27: 1209 -1213. Rummel MJ, ASH 2009, abs 405. Kahl B, ASH 2009, abs 1661.

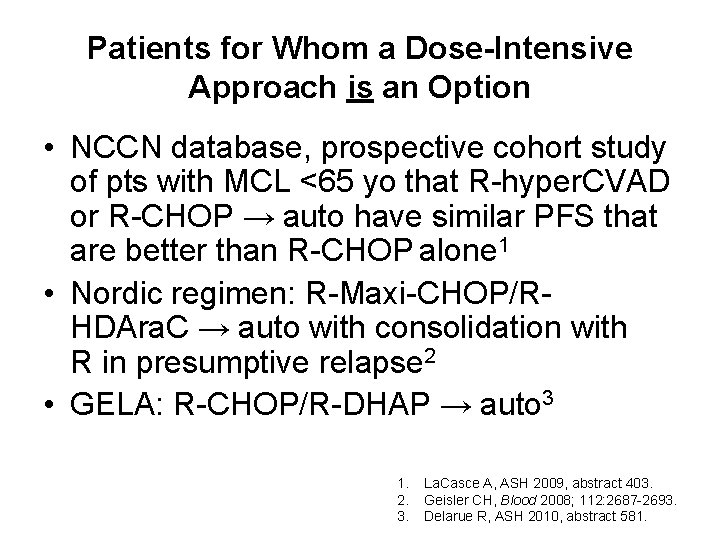
Patients for Whom a Dose-Intensive Approach is an Option • NCCN database, prospective cohort study of pts with MCL <65 yo that R-hyper. CVAD or R-CHOP → auto have similar PFS that are better than R-CHOP alone 1 • Nordic regimen: R-Maxi-CHOP/RHDAra. C → auto with consolidation with R in presumptive relapse 2 • GELA: R-CHOP/R-DHAP → auto 3 1. 2. 3. La. Casce A, ASH 2009, abstract 403. Geisler CH, Blood 2008; 112: 2687 -2693. Delarue R, ASH 2010, abstract 581.

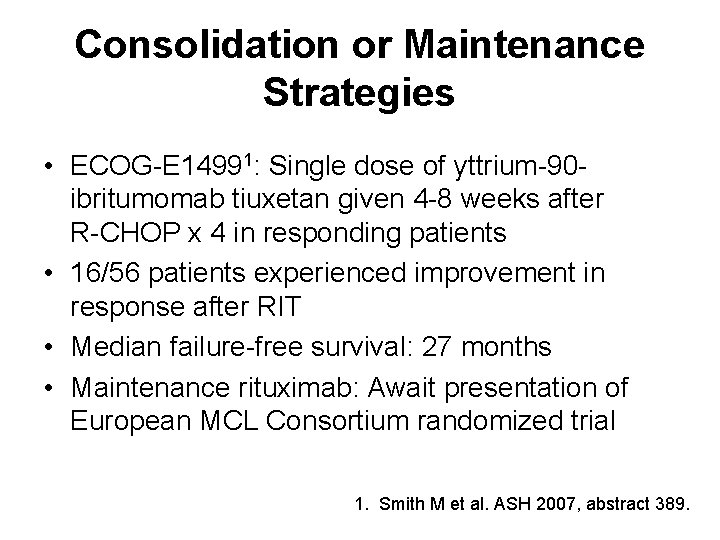
Consolidation or Maintenance Strategies • ECOG-E 14991: Single dose of yttrium-90 ibritumomab tiuxetan given 4 -8 weeks after R-CHOP x 4 in responding patients • 16/56 patients experienced improvement in response after RIT • Median failure-free survival: 27 months • Maintenance rituximab: Await presentation of European MCL Consortium randomized trial 1. Smith M et al. ASH 2007, abstract 389.

PTCL/CTCL: Approaches to Relapsed/Refractory Disease Lauren C Pinter-Brown, MD Director, UCLA Lymphoma Program Clinical Professor of Medicine Geffen School of Medicine at UCLA

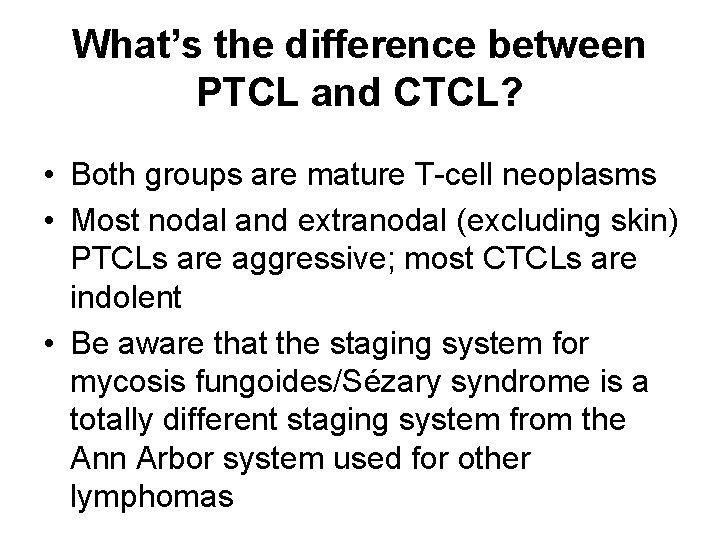
What’s the difference between PTCL and CTCL? • Both groups are mature T-cell neoplasms • Most nodal and extranodal (excluding skin) PTCLs are aggressive; most CTCLs are indolent • Be aware that the staging system for mycosis fungoides/Sézary syndrome is a totally different staging system from the Ann Arbor system used for other lymphomas

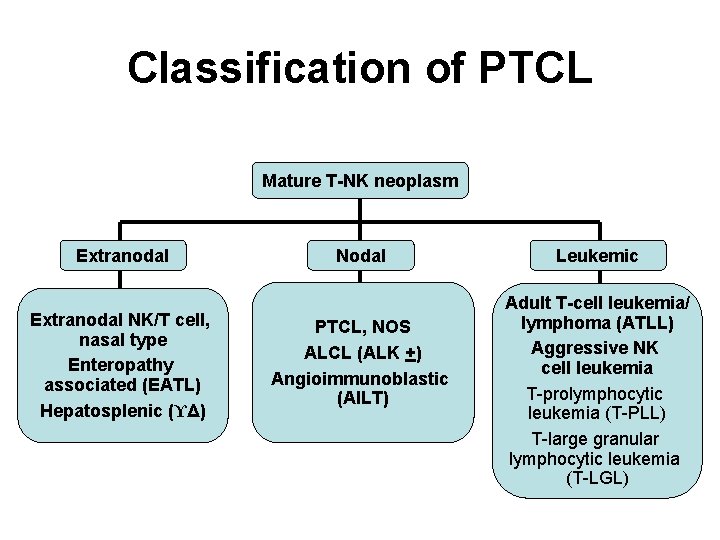
Classification of PTCL Mature T-NK neoplasm Extranodal NK/T cell, nasal type Enteropathy associated (EATL) Hepatosplenic (ϒΔ) Nodal PTCL, NOS ALCL (ALK +) Angioimmunoblastic (AILT) Leukemic Adult T-cell leukemia/ lymphoma (ATLL) Aggressive NK cell leukemia T-prolymphocytic leukemia (T-PLL) T-large granular lymphocytic leukemia (T-LGL)

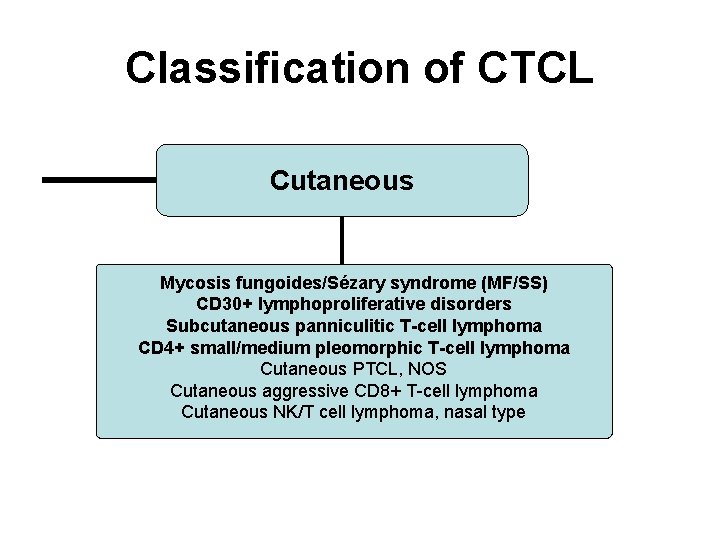
Classification of CTCL Cutaneous Mycosis fungoides/Sézary syndrome (MF/SS) CD 30+ lymphoproliferative disorders Subcutaneous panniculitic T-cell lymphoma CD 4+ small/medium pleomorphic T-cell lymphoma Cutaneous PTCL, NOS Cutaneous aggressive CD 8+ T-cell lymphoma Cutaneous NK/T cell lymphoma, nasal type

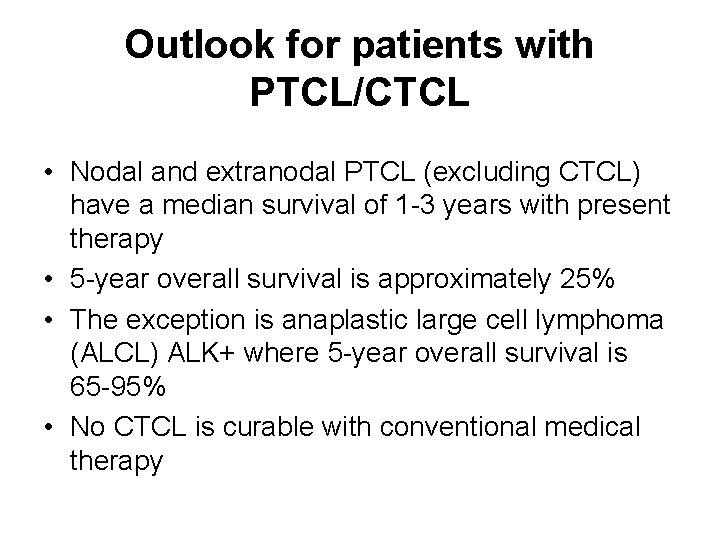
Outlook for patients with PTCL/CTCL • Nodal and extranodal PTCL (excluding CTCL) have a median survival of 1 -3 years with present therapy • 5 -year overall survival is approximately 25% • The exception is anaplastic large cell lymphoma (ALCL) ALK+ where 5 -year overall survival is 65 -95% • No CTCL is curable with conventional medical therapy

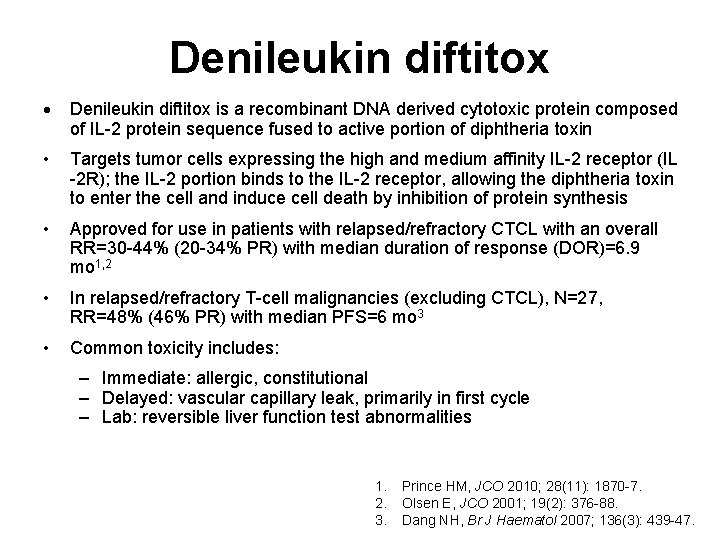
Denileukin diftitox is a recombinant DNA derived cytotoxic protein composed of IL-2 protein sequence fused to active portion of diphtheria toxin • Targets tumor cells expressing the high and medium affinity IL-2 receptor (IL -2 R); the IL-2 portion binds to the IL-2 receptor, allowing the diphtheria toxin to enter the cell and induce cell death by inhibition of protein synthesis • Approved for use in patients with relapsed/refractory CTCL with an overall RR=30 -44% (20 -34% PR) with median duration of response (DOR)=6. 9 mo 1, 2 • In relapsed/refractory T-cell malignancies (excluding CTCL), N=27, RR=48% (46% PR) with median PFS=6 mo 3 • Common toxicity includes: – Immediate: allergic, constitutional – Delayed: vascular capillary leak, primarily in first cycle – Lab: reversible liver function test abnormalities 1. 2. 3. Prince HM, JCO 2010; 28(11): 1870 -7. Olsen E, JCO 2001; 19(2): 376 -88. Dang NH, Br J Haematol 2007; 136(3): 439 -47.

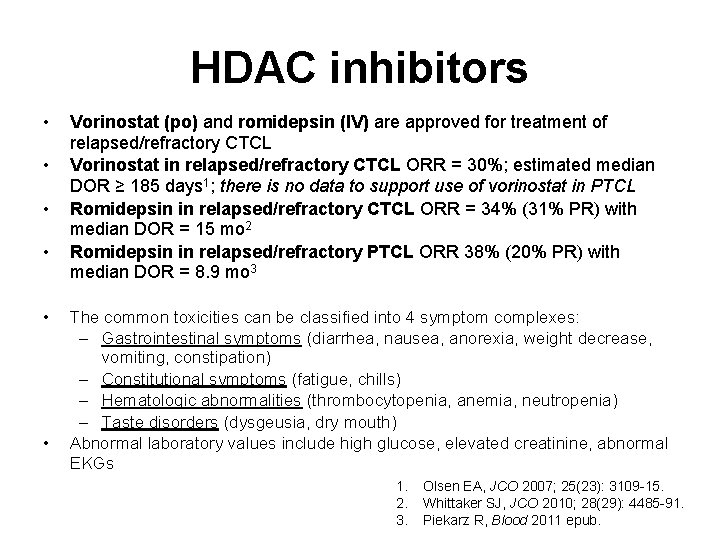
HDAC inhibitors • • • Vorinostat (po) and romidepsin (IV) are approved for treatment of relapsed/refractory CTCL Vorinostat in relapsed/refractory CTCL ORR = 30%; estimated median DOR ≥ 185 days 1; there is no data to support use of vorinostat in PTCL Romidepsin in relapsed/refractory CTCL ORR = 34% (31% PR) with median DOR = 15 mo 2 Romidepsin in relapsed/refractory PTCL ORR 38% (20% PR) with median DOR = 8. 9 mo 3 The common toxicities can be classified into 4 symptom complexes: – Gastrointestinal symptoms (diarrhea, nausea, anorexia, weight decrease, vomiting, constipation) – Constitutional symptoms (fatigue, chills) – Hematologic abnormalities (thrombocytopenia, anemia, neutropenia) – Taste disorders (dysgeusia, dry mouth) Abnormal laboratory values include high glucose, elevated creatinine, abnormal EKGs 1. 2. 3. Olsen EA, JCO 2007; 25(23): 3109 -15. Whittaker SJ, JCO 2010; 28(29): 4485 -91. Piekarz R, Blood 2011 epub.

Pralatrexate • Novel folate antagonist approved for treatment of relapsed or refractory PTCL • In patients with relapsed/refractory PTCL at dose of 30 mg/m 2 six out of seven weeks, ORR = 29% (18% PR) with median DOR = 10. 1 mo 1 • In patients with relapsed/refractory CTCL, the optimal dose was 15 mg/m 2 three out of four weeks with an ORR = 43%2 • Common toxicities include: mucositis, thrombocytopenia, neutropenia, anemia 1. 2. O’Connor OA JCO 2011; 29(9): 1182 -9. Horwitz SM, ASH 2010, abstract 2800.

How would I sequence these drugs? • In relapsed/refractory PTCL (if not using typical salvage chemotherapy): • (1) Pralatrexate • (2) Romidepsin • (3) Denileukin diftitox • In relapsed/refractory CTCL (if inappropriate for topical or other FDA-approved therapies such as bexarotene): • (1) Romidepsin (or vorinostat) • (2) Denileukin diftitox • (3) Pralatrexate

What is your usual preferred initial systemic treatment for MCL in patients older than age 75? Copyright © 2011 Research To Practice. All rights reserved.

Do you generally use rituximab maintenance in MCL? Copyright © 2011 Research To Practice. All rights reserved.

What is your usual preferred initial systemic treatment for MCL in patients older than age 75? Copyright © 2011 Research To Practice. All rights reserved.

Do you generally use rituximab maintenance in MCL? Copyright © 2011 Research To Practice. All rights reserved.

What Clinicians Want to Know A Live CME Event Addressing the Most Common Questions and Controversies in the Current Clinical Management of Select Hematologic Cancers Sunday, June 5, 2011 7: 00 PM – 9: 30 PM Chicago, Illinois Moderator Neil Love, MD Faculty Sergio Giralt, MD John P Leonard, MD Lauren C Pinter-Brown, MD Antonio Palumbo, MD Susan M O’Brien, MD Professor Michael Hallek Copyright © 2011 Research To Practice. All rights reserved.

What is your perception and experience concerning the side effects and tolerability of pralatrexate in patients with TCL? Copyright © 2011 Research To Practice. All rights reserved.

What is your perception and experience concerning the side effects and tolerability of romidepsin in patients with TCL? Copyright © 2011 Research To Practice. All rights reserved.

What is your perception and experience concerning the side effects and tolerability of pralatrexate in patients with TCL? Copyright © 2011 Research To Practice. All rights reserved.

What Clinicians Want to Know A Live CME Event Addressing the Most Common Questions and Controversies in the Current Clinical Management of Select Hematologic Cancers Sunday, June 5, 2011 7: 00 PM – 9: 30 PM Chicago, Illinois Moderator Neil Love, MD Faculty Sergio Giralt, MD John P Leonard, MD Lauren C Pinter-Brown, MD Antonio Palumbo, MD Susan M O’Brien, MD Professor Michael Hallek Copyright © 2011 Research To Practice. All rights reserved.
 Copanlisib package insert
Copanlisib package insert Maintenance rituximab mantle cell lymphoma
Maintenance rituximab mantle cell lymphoma Hodgkin's lymphoma classification
Hodgkin's lymphoma classification New york times upfront
New york times upfront Upfront scholastic
Upfront scholastic Hepatosplenic t-cell lymphoma
Hepatosplenic t-cell lymphoma Diff between hodgkin and non hodgkin
Diff between hodgkin and non hodgkin Count the dots audiogram
Count the dots audiogram Mcl technologies
Mcl technologies Marine corps league red jacket
Marine corps league red jacket Mcl designer
Mcl designer Ortho mcl
Ortho mcl Mcl e procurement
Mcl e procurement Half reaction
Half reaction Garam terhidrolisis sebagian
Garam terhidrolisis sebagian Contoh soal hidrolisis
Contoh soal hidrolisis Hiv roga lakshana
Hiv roga lakshana Indolent non-hodgkin lymphoma quizlet
Indolent non-hodgkin lymphoma quizlet Difference between hodgkin and non hodgkin lymphoma
Difference between hodgkin and non hodgkin lymphoma What does lymphoma lump look like
What does lymphoma lump look like Is lymphoma curable
Is lymphoma curable Pancreazin
Pancreazin Mark juckett
Mark juckett Leukemia vs lymphoma
Leukemia vs lymphoma Chylous ascites lymphoma
Chylous ascites lymphoma Classification of hodgkin lymphoma
Classification of hodgkin lymphoma Neoplasia
Neoplasia Hodjkins lymphoma
Hodjkins lymphoma Burkitt lymphoma
Burkitt lymphoma