NEW PHARMACOLOGICAL APPROACHES IN SUPPORTIVE CARE Gaetano Lanzetta


















- Slides: 52

NEW PHARMACOLOGICAL APPROACHES IN SUPPORTIVE CARE Gaetano Lanzetta Oncologia Medica INI- Grottaferrata (RM )



OBIETTIVI TERAPEUTICI BONE METASTASES Supportive care PREVENT MANAGE COMPLICATIONS PATIENTS

“ What is not measured is not managed “ The "measurement" of the symptom is indispensable for setting up a proper treatment to evaluate its effectiveness, to vary it depending on the individual response. TIME LISTENING RELATIONSHIP Caraceni A. Evaluation and assessment of cancer pain and cancer pain treatment. Acta Anaesthesiol Scand. 2001; 45: 1067 -75


MEASUREMENT PATIENT REPORTED OUTCOME AND PHYSICIAN ASSESSED TOXICITIES

PATIENT REPORTED OUTCOME AND PHYSICIAN ASSESSED TOXICITIES MEASUREMENT PROs offer opportunity for labeling claims and are tools for comparative effectiveness Towards the development of a PRO version of the CTCAE

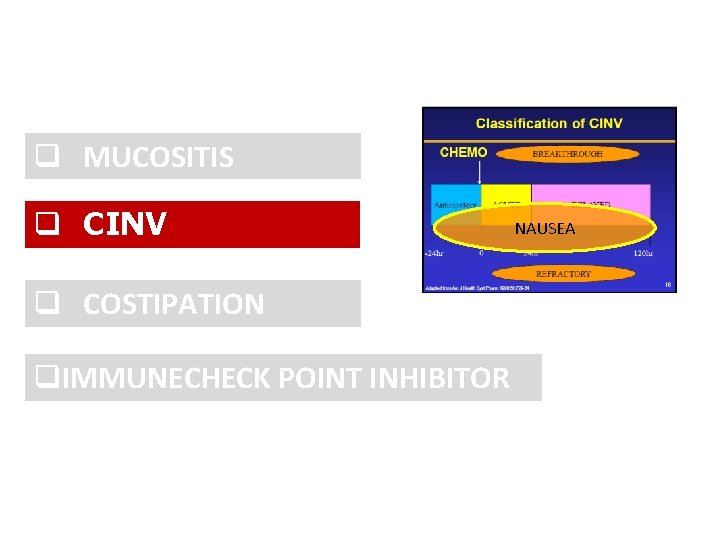
q MUCOSITIS q CINV q OPIOID INDUCED COSTIPATION q. IMMUNE CHECK POINT INHIBITOR

q MUCOSITIS q CINV q COSTIPATION q. IMMUNECHECK POINT INHIBITOR q. END OF LIFE

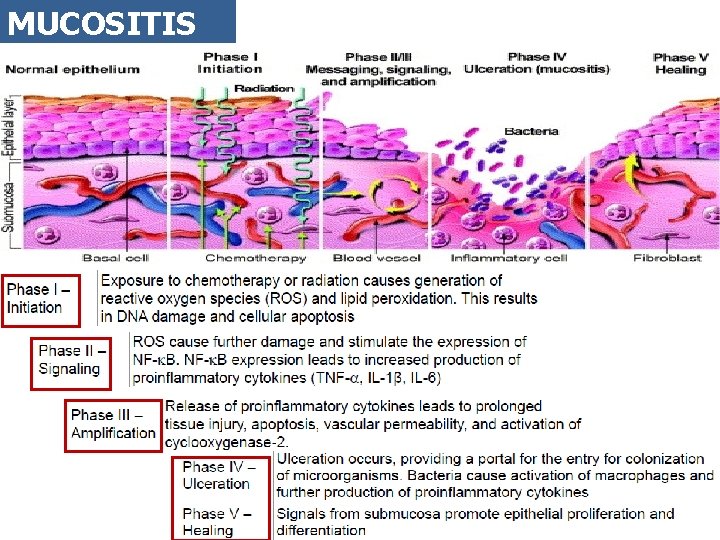
MUCOSITIS


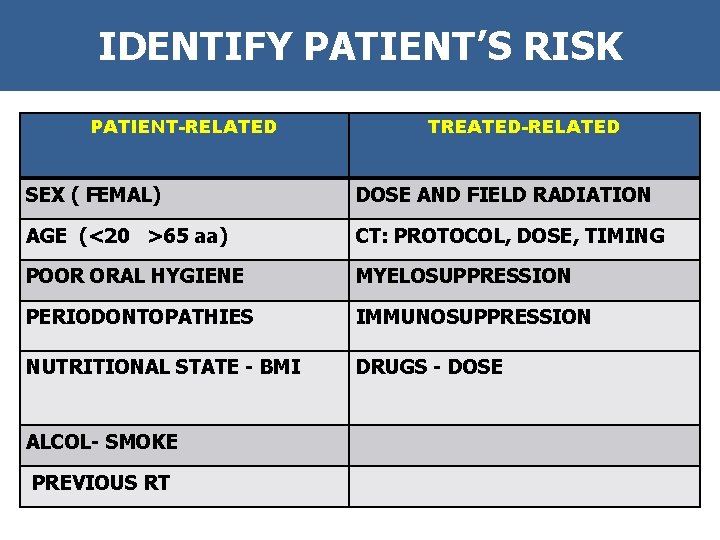
IDENTIFY PATIENT’S RISK PATIENT-RELATED TREATED-RELATED SEX ( FEMAL) DOSE AND FIELD RADIATION AGE (<20 >65 aa) CT: PROTOCOL, DOSE, TIMING POOR ORAL HYGIENE MYELOSUPPRESSION PERIODONTOPATHIES IMMUNOSUPPRESSION NUTRITIONAL STATE - BMI DRUGS - DOSE ALCOL- SMOKE PREVIOUS RT

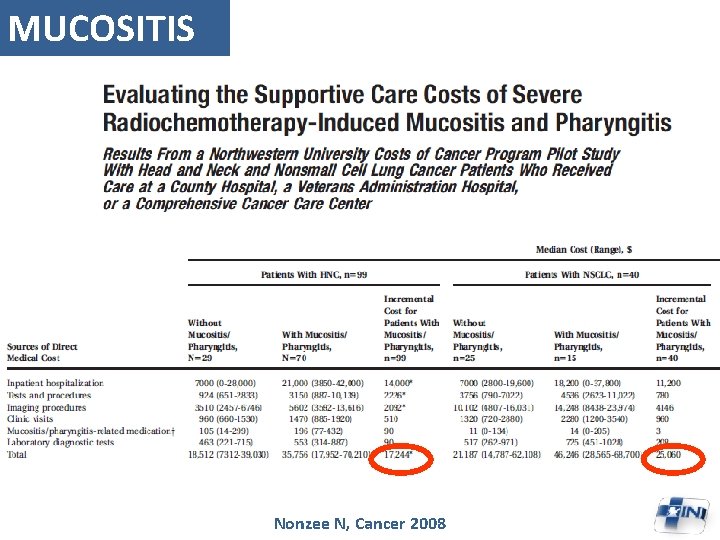
MUCOSITIS Nonzee N, Cancer 2008


MUCOSITIS Lalla et al. Cancer 2014


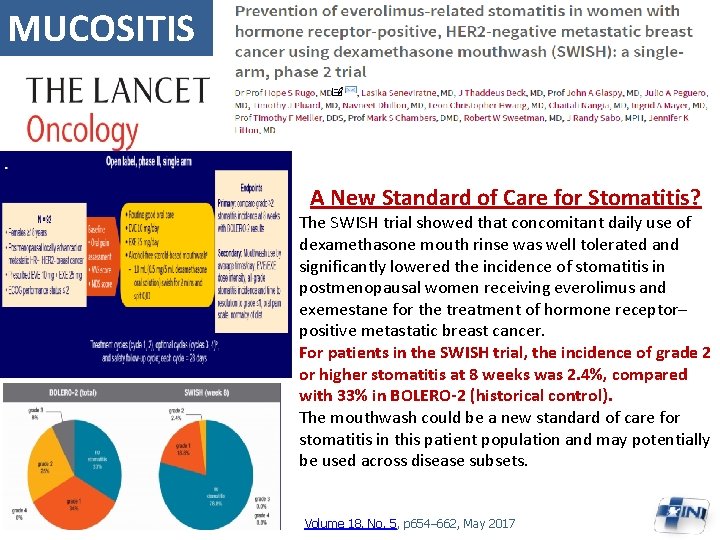
MUCOSITIS A New Standard of Care for Stomatitis? The SWISH trial showed that concomitant daily use of dexamethasone mouth rinse was well tolerated and significantly lowered the incidence of stomatitis in postmenopausal women receiving everolimus and exemestane for the treatment of hormone receptor– positive metastatic breast cancer. For patients in the SWISH trial, the incidence of grade 2 or higher stomatitis at 8 weeks was 2. 4%, compared with 33% in BOLERO-2 (historical control). The mouthwash could be a new standard of care for stomatitis in this patient population and may potentially be used across disease subsets. Volume 18, No. 5, p 654– 662, May 2017

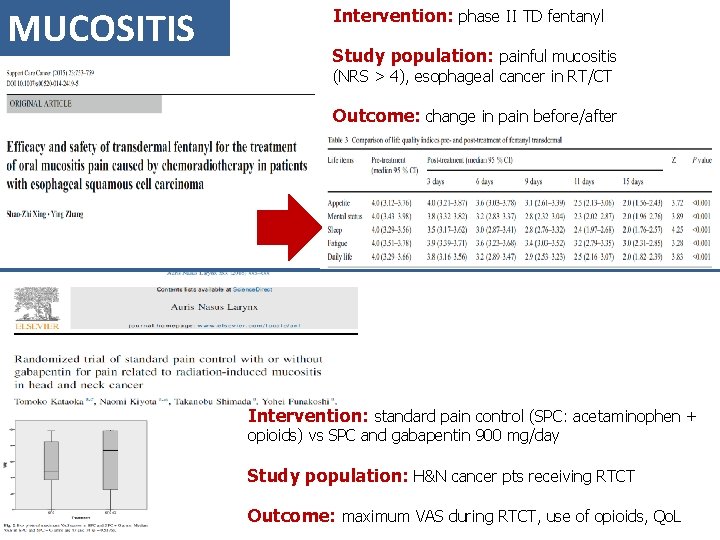
MUCOSITIS Intervention: phase II TD fentanyl Study population: painful mucositis (NRS > 4), esophageal cancer in RT/CT Outcome: change in pain before/after Intervention: standard pain control (SPC: acetaminophen + opioids) vs SPC and gabapentin 900 mg/day Study population: H&N cancer pts receiving RTCT Outcome: maximum VAS during RTCT, use of opioids, Qo. L

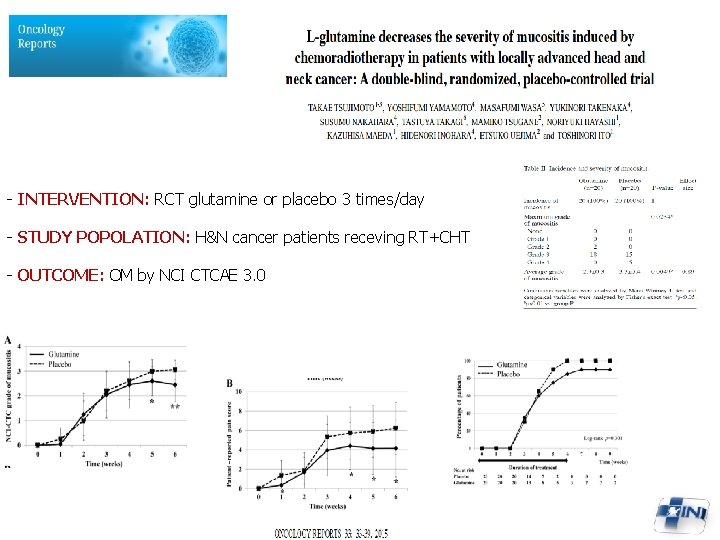
- INTERVENTION: RCT glutamine or placebo 3 times/day - STUDY POPOLATION: H&N cancer patients receving RT+CHT - OUTCOME: OM by NCI CTCAE 3. 0

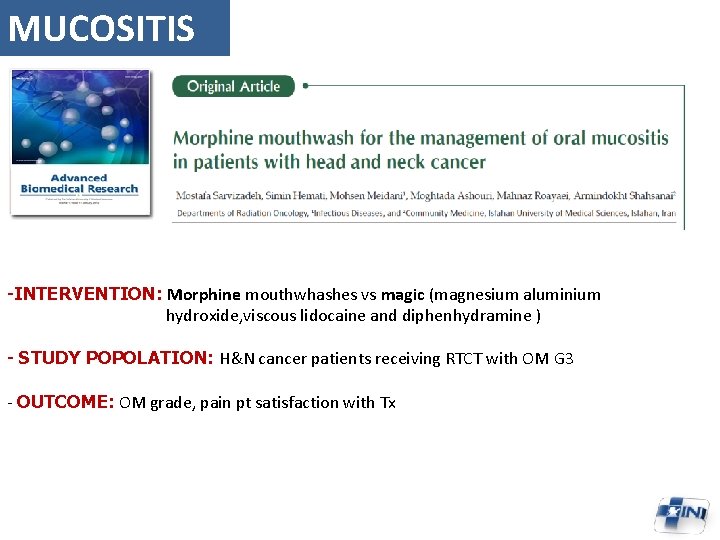
MUCOSITIS -INTERVENTION: Morphine mouthwhashes vs magic (magnesium aluminium hydroxide, viscous lidocaine and diphenhydramine ) - STUDY POPOLATION: H&N cancer patients receiving RTCT with OM G 3 - OUTCOME: OM grade, pain pt satisfaction with Tx

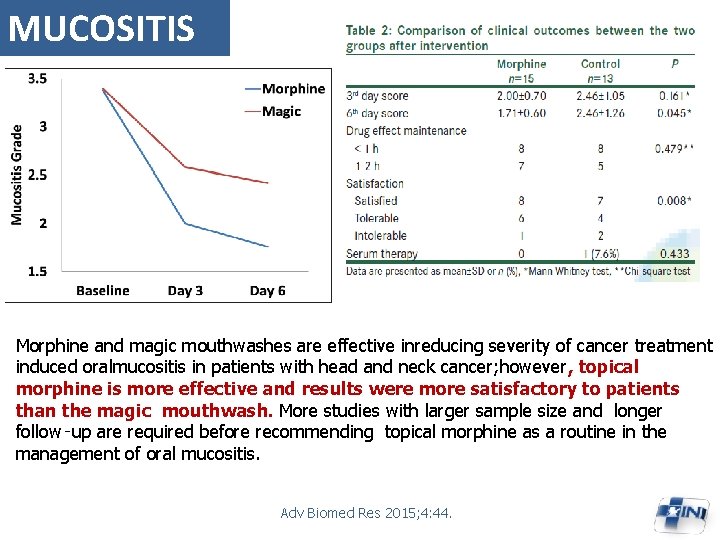
MUCOSITIS Morphine and magic mouthwashes are effective inreducing severity of cancer treatment induced oralmucositis in patients with head and neck cancer; however, topical morphine is more effective and results were more satisfactory to patients than the magic mouthwash. More studies with larger sample size and longer follow‑up are required before recommending topical morphine as a routine in the management of oral mucositis. Adv Biomed Res 2015; 4: 44.

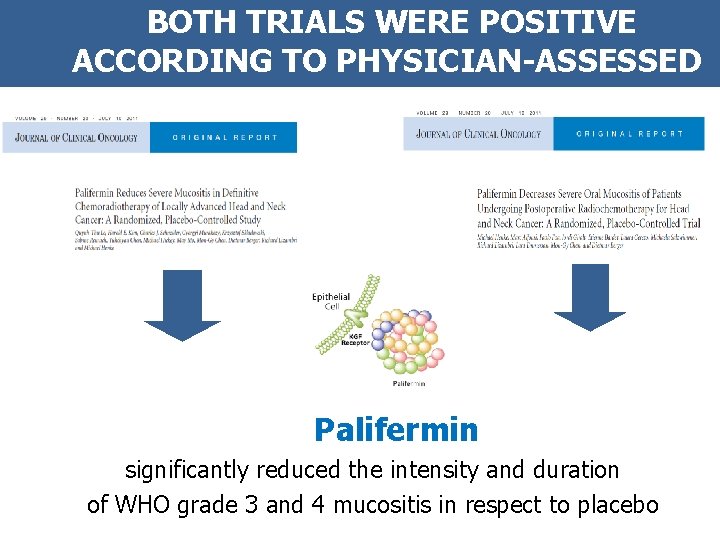
BOTH TRIALS WERE POSITIVE ACCORDING TO PHYSICIAN-ASSESSED MUCOSITIS Palifermin significantly reduced the intensity and duration of WHO grade 3 and 4 mucositis in respect to placebo

CONCLUSIONS q Multifactorial pathogenesis q Patient reported outcome measurable measure q Oral care is essential, before, during and after therapy q Beware of targeted therapies q Several individual-based interventions (surveys) q Impulse to clinical trials!

q MUCOSITIS q CINV q COSTIPATION q. IMMUNECHECK POINT INHIBITOR NAUSEA

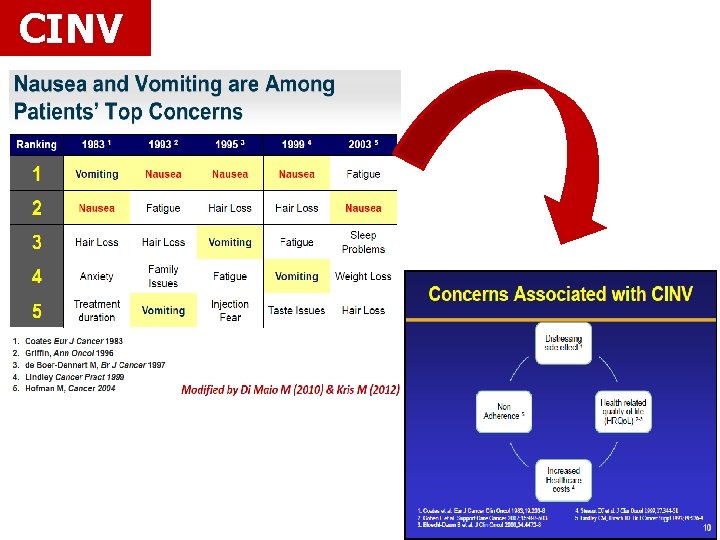
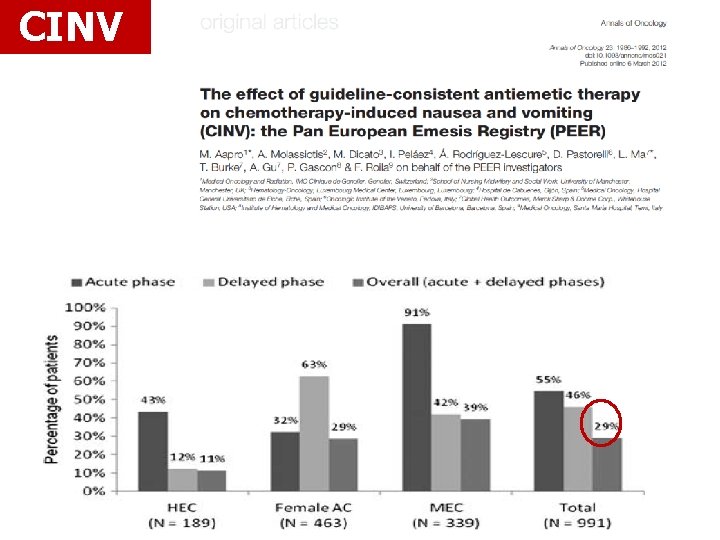
CINV

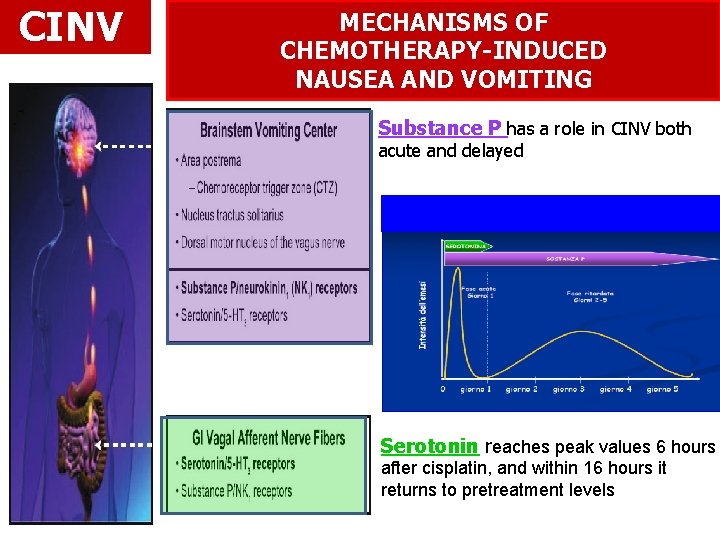
CINV MECHANISMS OF CHEMOTHERAPY-INDUCED NAUSEA AND VOMITING Substance P has a role in CINV both acute and delayed Serotonin reaches peak values 6 hours after cisplatin, and within 16 hours it returns to pretreatment levels

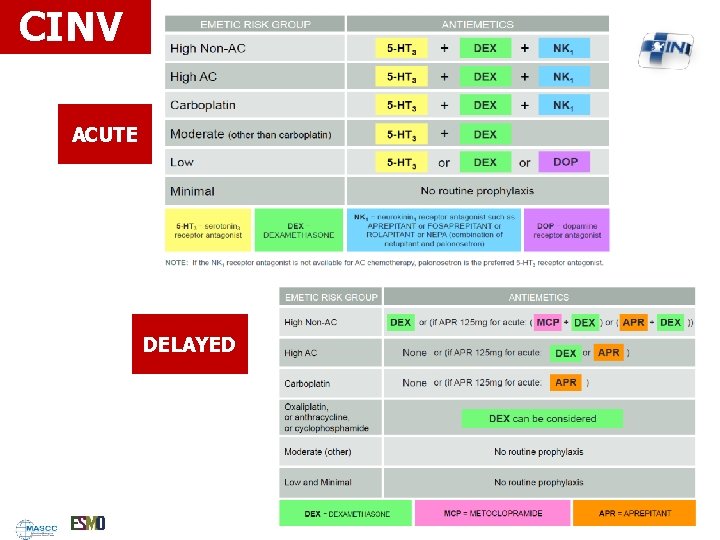
CINV ACUTE DELAYED

CINV

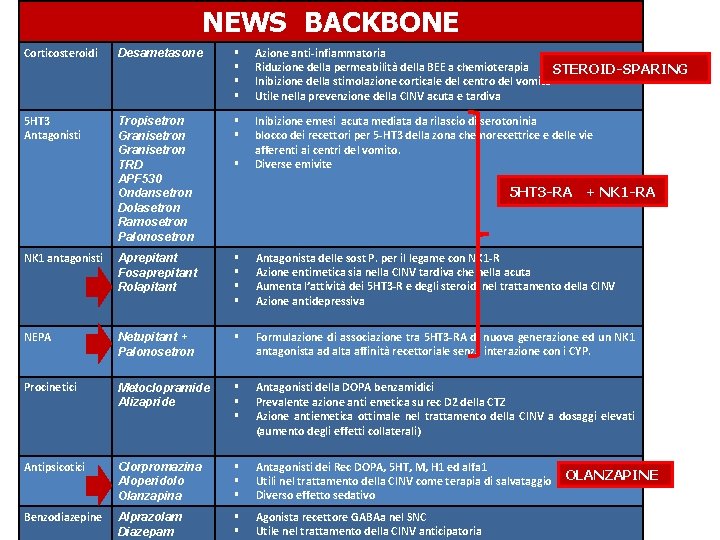
NEWS BACKBONE Corticosteroidi Desametasone § § Azione anti-infiammatoria Riduzione della permeabilità della BEE a chemioterapia STEROID-SPARING Inibizione della stimolazione corticale del centro del vomito Utile nella prevenzione della CINV acuta e tardiva 5 HT 3 Antagonisti Tropisetron Granisetron TRD APF 530 Ondansetron Dolasetron Ramosetron Palonosetron § § Inibizione emesi acuta mediata da rilascio di serotoninia blocco dei recettori per 5 -HT 3 della zona chemorecettrice e delle vie afferenti ai centri del vomito. Diverse emivite NK 1 antagonisti Aprepitant Fosaprepitant Rolapitant § § Antagonista delle sost P. per il legame con NK 1 -R Azione entimetica sia nella CINV tardiva che nella acuta Aumenta l’attività dei 5 HT 3 -R e degli steroidi nel trattamento della CINV Azione antidepressiva NEPA Netupitant + Palonosetron § Formulazione di associazione tra 5 HT 3 -RA di nuova generazione ed un NK 1 antagonista ad alta affinità recettoriale senza interazione con i CYP. Procinetici Metoclopramide Alizapride § § § Antagonisti della DOPA benzamidici Prevalente azione anti emetica su rec D 2 della CTZ Azione antiemetica ottimale nel trattamento della CINV a dosaggi elevati (aumento degli effetti collaterali) Antipsicotici Clorpromazina Aloperidolo Olanzapina § § § Antagonisti dei Rec DOPA, 5 HT, M, H 1 ed alfa 1 Utili nel trattamento della CINV come terapia di salvataggio Diverso effetto sedativo Benzodiazepine Alprazolam Diazepam § § Agonista recettore GABAa nel SNC Utile nel trattamento della CINV anticipatoria § 5 HT 3 -RA + NK 1 -RA OLANZAPINE

ARE THEY ALL THE SAME? 5 -HT 3 ANTAGONIST PALONOSETRON NK 1 -R ANTAGONSIT NEPA

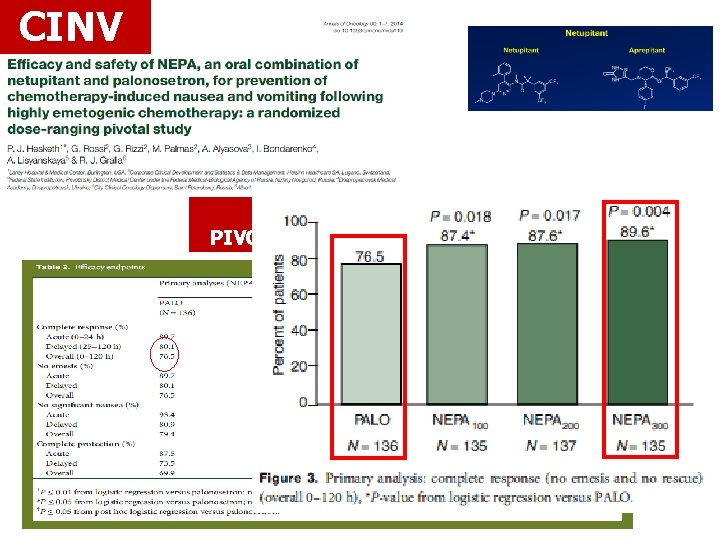
CINV AKYNZEO PIVOTAL STUDY HEC

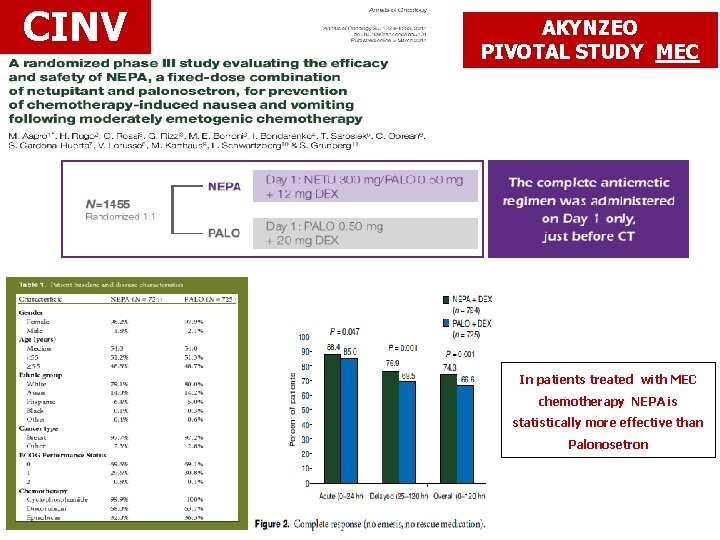
CINV AKYNZEO PIVOTAL STUDY MEC In patients treated with MEC chemotherapy NEPA is statistically more effective than Palonosetron

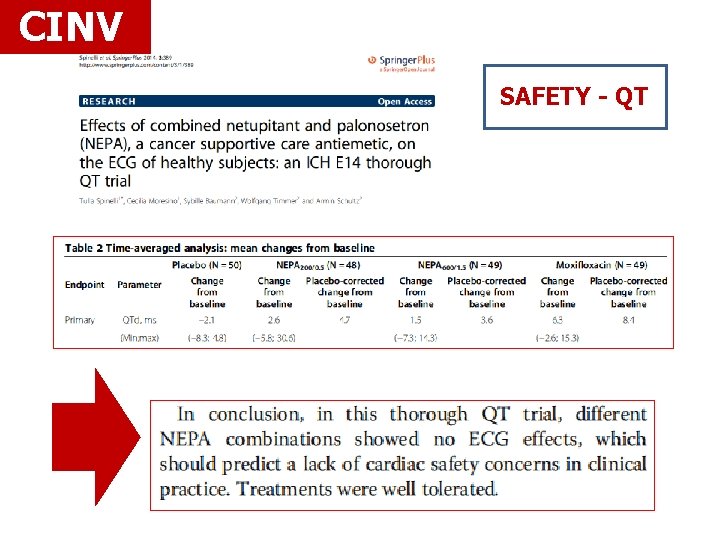
CINV SAFETY - QT

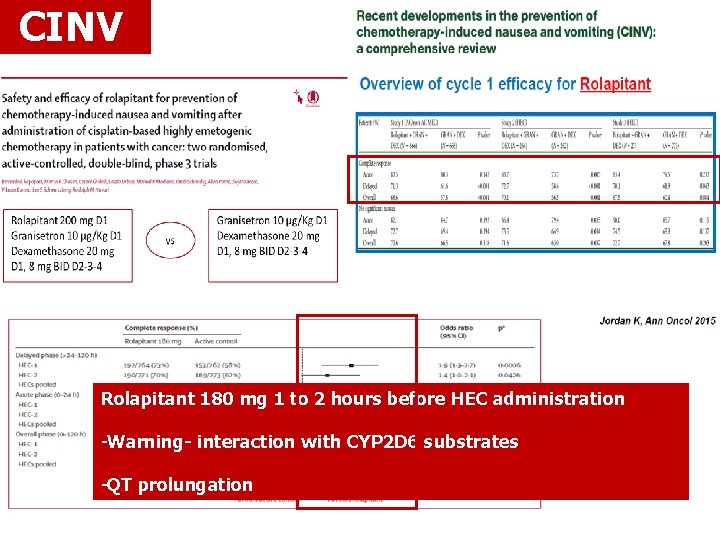
CINV Rolapitant 180 mg 1 to 2 hours before HEC administration -Warning- interaction with CYP 2 D 6 substrates -QT prolungation

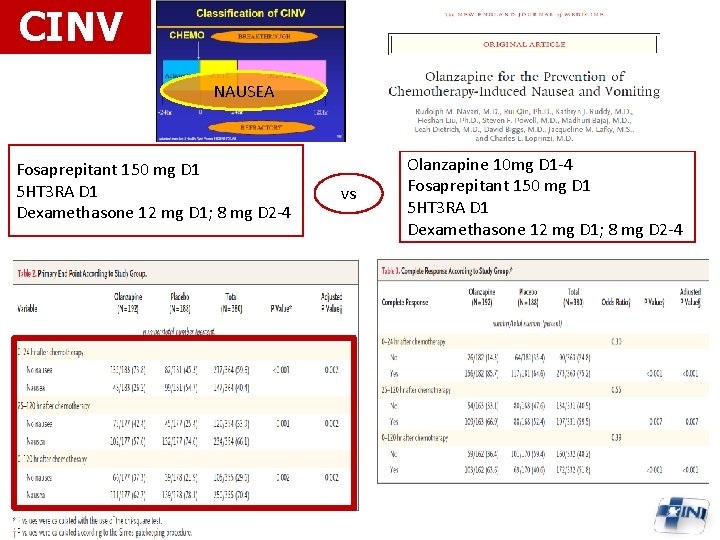
CINV NAUSEA Fosaprepitant 150 mg D 1 5 HT 3 RA D 1 Dexamethasone 12 mg D 1; 8 mg D 2 -4 vs Olanzapine 10 mg D 1 -4 Fosaprepitant 150 mg D 1 5 HT 3 RA D 1 Dexamethasone 12 mg D 1; 8 mg D 2 -4

CINV

CONCLUSIONS

q MUCOSITIS q CINV q OPIOD-INDUCED COSTIPATION q. IMMUNECHECK POINT INHIBITOR

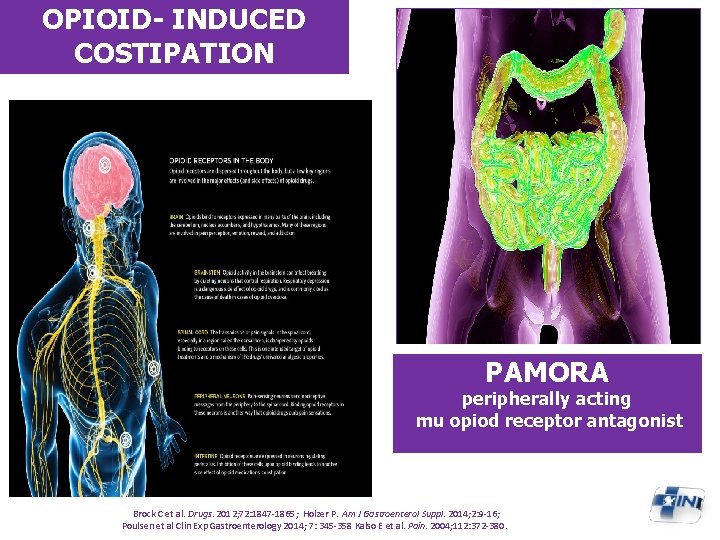
OPIOID- INDUCED COSTIPATION PAMORA peripherally acting mu opiod receptor antagonist Brock C et al. Drugs. 2012; 72: 1847 -1865; Holzer P. Am J Gastroenterol Suppl. 2014; 2: 9 -16; Poulsen et al Clin Exp Gastroenterology 2014; 7: 345 -358 Kalso E et al. Pain. 2004; 112: 372 -380.

OPIOID- INDUCED COSTIPATION

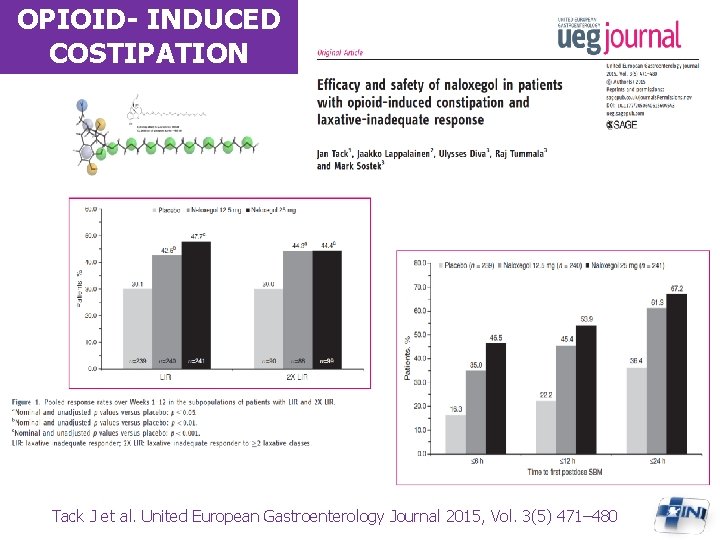
OPIOID- INDUCED COSTIPATION Tack J et al. United European Gastroenterology Journal 2015, Vol. 3(5) 471– 480

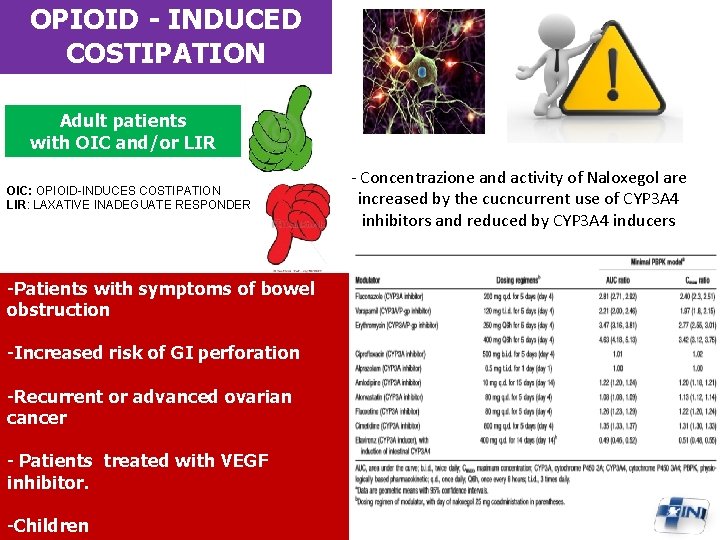
OPIOID - INDUCED COSTIPATION Adult patients with OIC and/or LIR OIC: OPIOID-INDUCES COSTIPATION LIR: LAXATIVE INADEGUATE RESPONDER -Patients with symptoms of bowel obstruction -Increased risk of GI perforation -Recurrent or advanced ovarian cancer - Patients treated with VEGF inhibitor. -Children - Concentrazione and activity of Naloxegol are increased by the cucncurrent use of CYP 3 A 4 inhibitors and reduced by CYP 3 A 4 inducers

Naloxegol 25 mg /day

q MUCOSITIS q CINV q COSTIPATION q. IMMUNE CHECKPOINT INHIBITORS

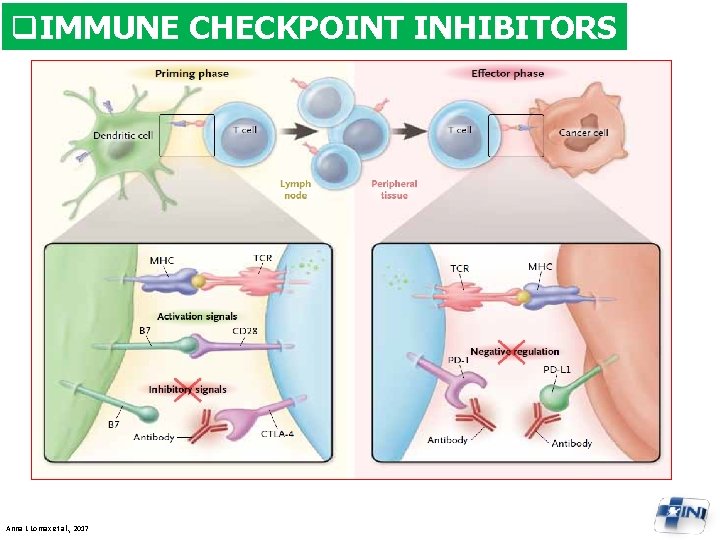
q. IMMUNE CHECKPOINT INHIBITORS Anna L Lomax et al. , 2017

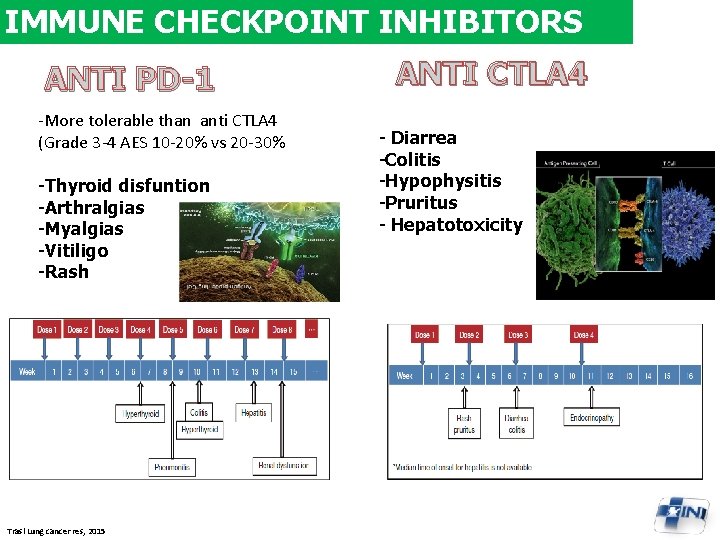
IMMUNE CHECKPOINT INHIBITORS ANTI PD-1 -More tolerable than anti CTLA 4 (Grade 3 -4 AES 10 -20% vs 20 -30% -Thyroid disfuntion -Arthralgias -Myalgias -Vitiligo -Rash Trasl Lung cancer res, 2015 ANTI CTLA 4 - Diarrea -Colitis -Hypophysitis -Pruritus - Hepatotoxicity

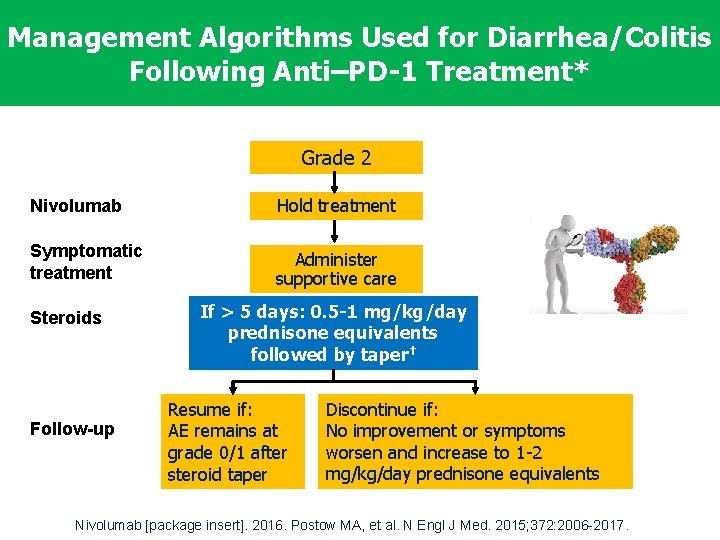
Management Algorithms Used for Diarrhea/Colitis Following Anti–PD-1 Treatment* Grade 2 Nivolumab Hold treatment Symptomatic treatment Administer supportive care Steroids Follow-up If > 5 days: 0. 5 -1 mg/kg/day prednisone equivalents followed by taper† Resume if: AE remains at grade 0/1 after steroid taper Discontinue if: No improvement or symptoms worsen and increase to 1 -2 mg/kg/day prednisone equivalents *Diarrhea and colitis to varying severity. Grades correspond to NCI CTCAE v 4. 0. †Consider prophylactic antibiotics. Nivolumab [package insert]. 2016. Postow MA, et al. N Engl J Med. 2015; 372: 2006 -2017.

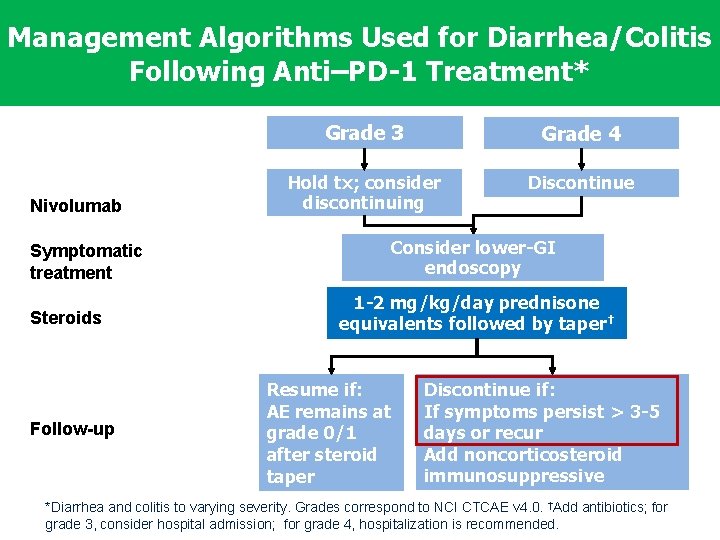
Management Algorithms Used for Diarrhea/Colitis Following Anti–PD-1 Treatment* Nivolumab Symptomatic treatment Steroids Follow-up Grade 3 Grade 4 Hold tx; consider discontinuing Discontinue Consider lower-GI endoscopy 1 -2 mg/kg/day prednisone equivalents followed by taper† Resume if: AE remains at grade 0/1 after steroid taper Discontinue if: If symptoms persist > 3 -5 days or recur Add noncorticosteroid immunosuppressive *Diarrhea and colitis to varying severity. Grades correspond to NCI CTCAE v 4. 0. †Add antibiotics; for grade 3, consider hospital admission; for grade 4, hospitalization is recommended.

NEW NCCN/ASCO GUIDELINES ON MANAGING IMMUNE-RELATED AES

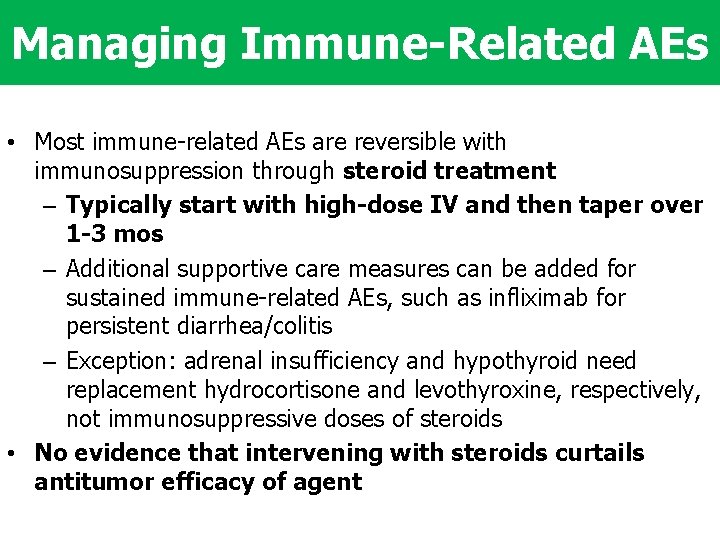
Managing Immune-Related AEs • Most immune-related AEs are reversible with immunosuppression through steroid treatment – Typically start with high-dose IV and then taper over 1 -3 mos – Additional supportive care measures can be added for sustained immune-related AEs, such as infliximab for persistent diarrhea/colitis – Exception: adrenal insufficiency and hypothyroid need replacement hydrocortisone and levothyroxine, respectively, not immunosuppressive doses of steroids • No evidence that intervening with steroids curtails antitumor efficacy of agent

THANKS FOR YOUR ATTENTION vvvvv
 Gaetano lanzetta
Gaetano lanzetta Kenneth lanzetta
Kenneth lanzetta Hypertension
Hypertension Non pharmacological management of hypertension
Non pharmacological management of hypertension Non pharmacological treatment for copd
Non pharmacological treatment for copd Paul ehrlich
Paul ehrlich Serotaxonomy classification of crude drugs
Serotaxonomy classification of crude drugs Lipid classification
Lipid classification Endocrine axis
Endocrine axis The pharmacological basis of therapeutics
The pharmacological basis of therapeutics Pharmacological and parenteral therapies
Pharmacological and parenteral therapies Levels of nursing care primary secondary tertiary
Levels of nursing care primary secondary tertiary Gaetano antonio lanza curriculum
Gaetano antonio lanza curriculum The joker gaetano bellei
The joker gaetano bellei Gaetano raiola
Gaetano raiola Gaetano marrone
Gaetano marrone Gaetano domenici
Gaetano domenici Andrew gaetano
Andrew gaetano Itet salvemini molfetta
Itet salvemini molfetta Inserra gaetano gastroenterologo catania
Inserra gaetano gastroenterologo catania Salvemini sorrento
Salvemini sorrento Liceo gaetano de sanctis
Liceo gaetano de sanctis Resna opera italijansko
Resna opera italijansko Gaetano di stefano
Gaetano di stefano New approaches to organizing hr
New approaches to organizing hr New approaches to organizing hr
New approaches to organizing hr English
English Develop new approaches to public governance and engagement
Develop new approaches to public governance and engagement New approaches to sme financing
New approaches to sme financing Principle of supportive relationship
Principle of supportive relationship Supportive approach examples
Supportive approach examples Aggression escalation continuum stages
Aggression escalation continuum stages What is supportive communication
What is supportive communication Functions of indirect retainer
Functions of indirect retainer Supportive communication style
Supportive communication style 4 social styles
4 social styles Examples of corrective discipline in the classroom
Examples of corrective discipline in the classroom Creating a strategy supportive culture
Creating a strategy supportive culture Dominance continuum
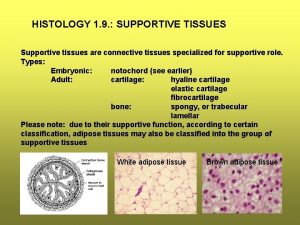
Dominance continuum Type of tissue
Type of tissue Reluctant contributor
Reluctant contributor Creating supportive environments smoking
Creating supportive environments smoking Models of casework practice
Models of casework practice Mapa holds
Mapa holds High quality supportive environments
High quality supportive environments Supportive behavior
Supportive behavior Office of supportive housing santa clara county
Office of supportive housing santa clara county Auxiliary team member duties cpi
Auxiliary team member duties cpi Assignment of supportive model
Assignment of supportive model Supportive tissue
Supportive tissue Cpi coping model control phase
Cpi coping model control phase The supportive techniques are
The supportive techniques are Supportive
Supportive