New Magnesium Alloy Programs Ron Waksman MD FACC







![Unity BDS Crimping and Crossing Profile Characteristics Crossing Profile of the crimped stent [mm] Unity BDS Crimping and Crossing Profile Characteristics Crossing Profile of the crimped stent [mm]](https://slidetodoc.com/presentation_image_h/94cb1f28fa551c47a86f845f05fefc72/image-24.jpg)



- Slides: 28

New Magnesium Alloy Programs Ron Waksman, MD, FACC, FSCAI Professor of Medicine, (Cardiology) Georgetown University Director, Cardiovascular Research Advanced Education Med. Star Heart &Vascular Institute, Washington DC

Ron Waksman, MD

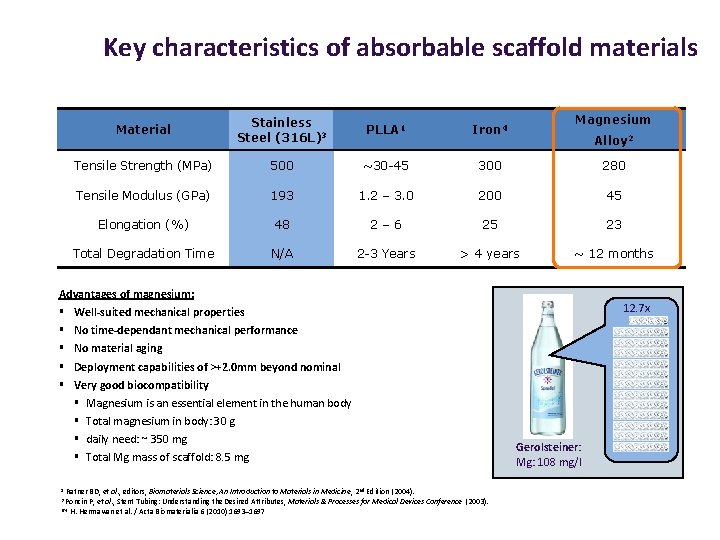
Key characteristics of absorbable scaffold materials Magnesium Material Stainless Steel (316 L)3 PLLA 1 Iron 4 Tensile Strength (MPa) 500 ~30 -45 300 280 Tensile Modulus (GPa) 193 1. 2 – 3. 0 200 45 Elongation (%) 48 2– 6 25 23 Total Degradation Time N/A 2 -3 Years > 4 years ~ 12 months Alloy 2 Advantages of magnesium: § § § No time-dependant mechanical performance No material aging Deployment capabilities of >+2. 0 mm beyond nominal Very good biocompatibility § § Magnesium is an essential element in the human body Total magnesium in body: 30 g daily need: ~ 350 mg Total Mg mass of scaffold: 8. 5 mg Ratner BD, et al. , editors, Biomaterials Science, An Introduction to Materials in Medicine , 2 nd Edition (2004). Poncin P, et al. , Stent Tubing: Understanding the Desired Attributes, Materials & Processes for Medical Devices Conference (2003). 2/4 H. Hermawan et al. / Acta Biomaterialia 6 (2010) 1693– 1697 1 3 12. 7 x Well-suited mechanical properties Gerolsteiner: Mg: 108 mg/l

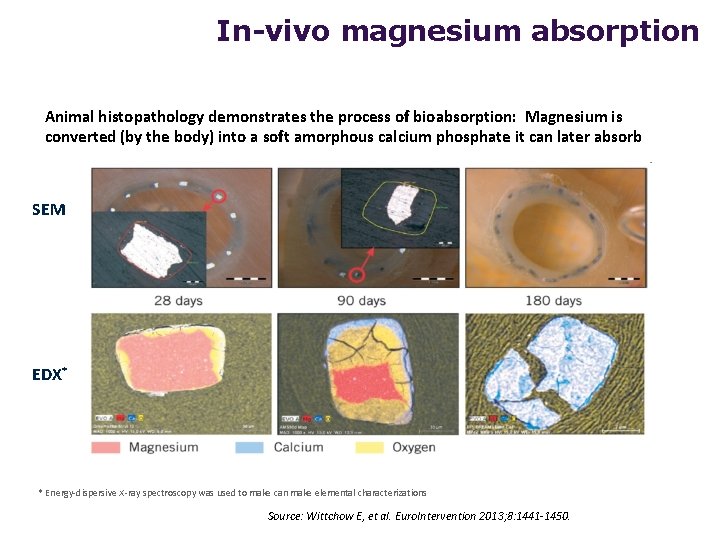
In-vivo magnesium absorption Animal histopathology demonstrates the process of bioabsorption: Magnesium is converted (by the body) into a soft amorphous calcium phosphate it can later absorb SEM EDX* * Energy-dispersive X-ray spectroscopy was used to make can make elemental characterizations Source: Wittchow E, et al. Euro. Intervention 2013; 8: 1441 -1450.

Absorbable Mg Scaffold Programs Scaffold Biotronik AMS Biotronik DREAMS II Mg-Alloy + Paclitaxel Mg-Alloy + Sirolimus Medtronic Mg-Alloy + Sirolimus Mg-Alloy Quali. Med UNITY Mg-Alloy + Polymer (Hybrid) Mg Alloy + Coating Resoloy Meko

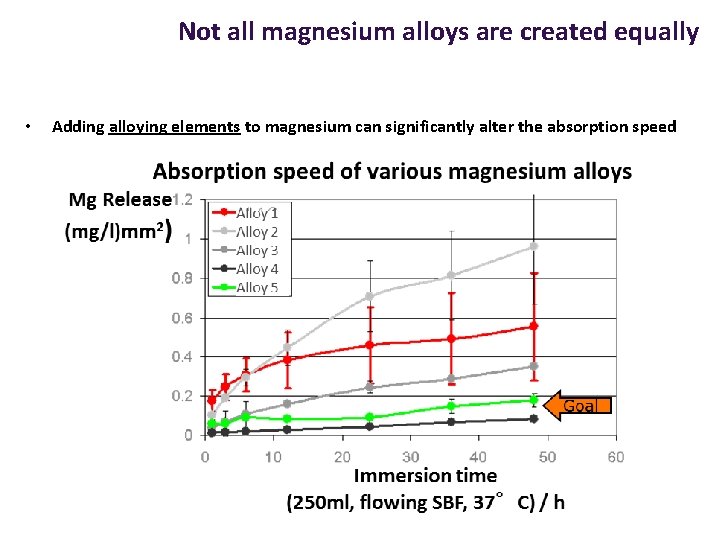
Not all magnesium alloys are created equally • Adding alloying elements to magnesium can significantly alter the absorption speed

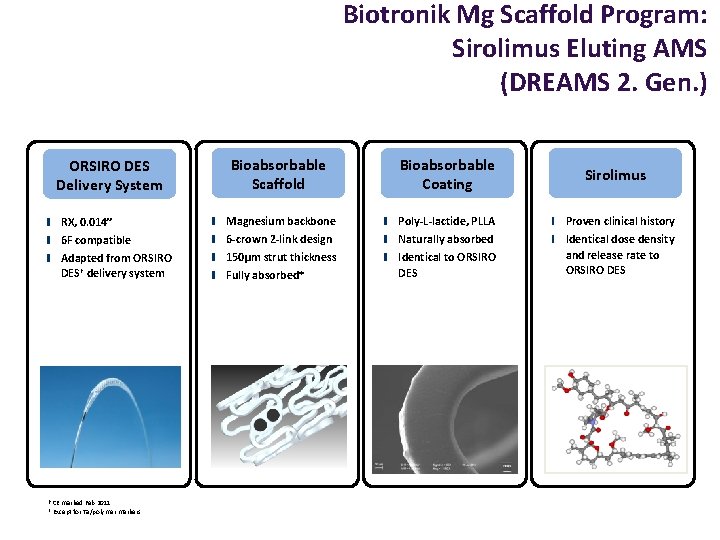
Biotronik Mg Scaffold Program: Sirolimus Eluting AMS (DREAMS 2. Gen. ) Bioabsorbable Scaffold Bioabsorbable Coating RX, 0. 014” Magnesium backbone Poly-L-lactide, PLLA Proven clinical history 6 F compatible 6 -crown 2 -link design Naturally absorbed Adapted from ORSIRO DES† delivery system 150µm strut thickness Fully absorbed* Identical to ORSIRO DES Identical dose density and release rate to ORSIRO DES Delivery System † CE marked Feb 2011 * Except for Ta/polymer markers Sirolimus

Biotronik Mg Scaffold Program: Sirolimus Eluting AMS (DREAMS 2. Gen. ) DREAMS 1 st generation BIOSOLVE-I DREAMS 2 nd generation BIOSOLVE-II • 120 -150µm strut thickness Magnesium • Addition of radiopaque Magnesium markers at both ends PLGA Polymer +Paclitaxel • Increased post-dilatation capabilities PLLA Polymer +Sirolimus • PLLA polymer carrier • Sirolimus drug elution 90 day faxitron

Biotronik Mg Scaffold Program: Sirolimus Eluting AMS (DREAMS 2. Gen. ) Prolonged scaffolding Faxitron imaging 90 days DREAMS 2 nd Generation DREAMS 1 st Generation 90 d Accel. LAB preclinical studies

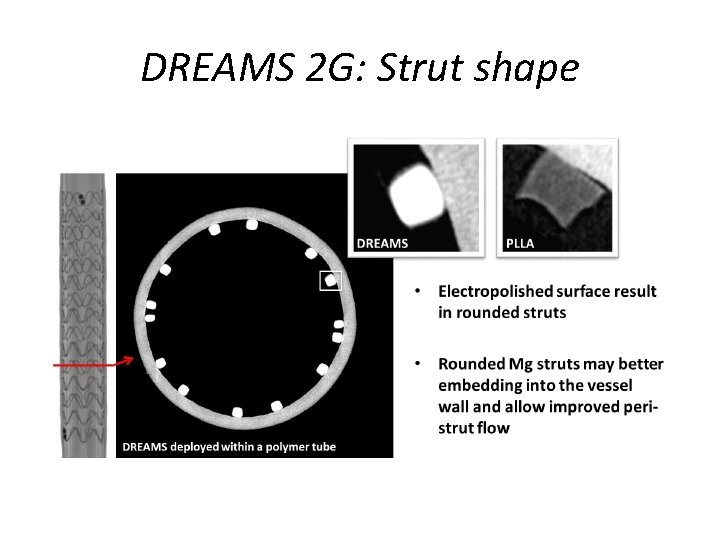
DREAMS 2 G: Strut shape

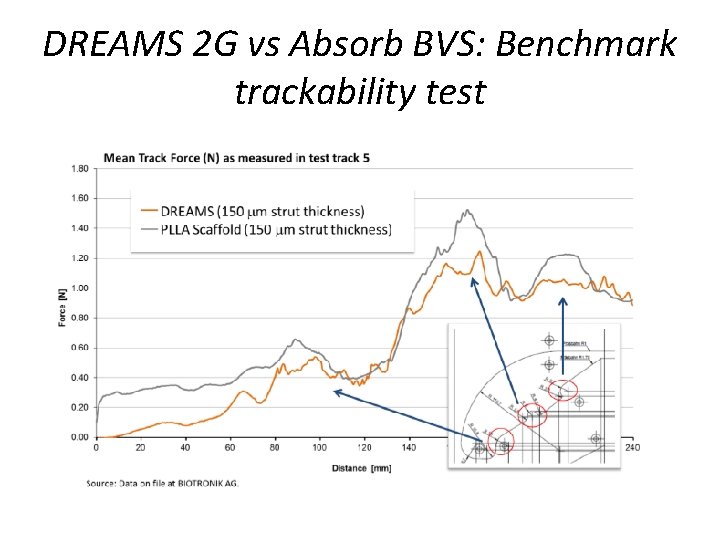
DREAMS 2 G vs Absorb BVS: Benchmark trackability test

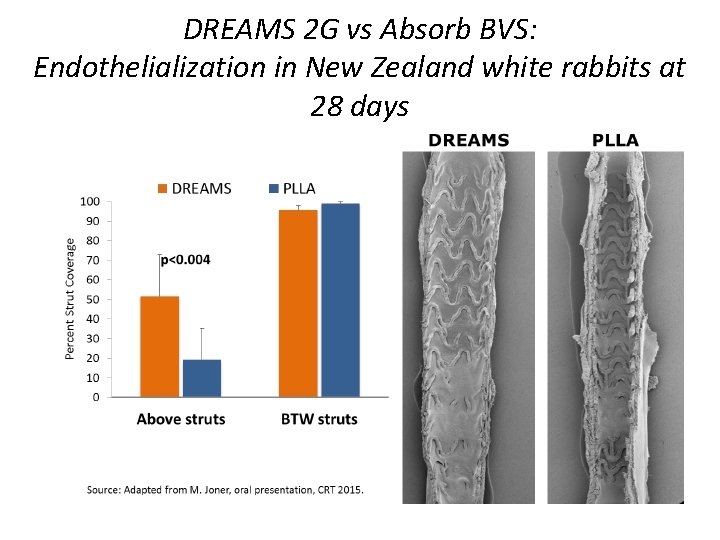
DREAMS 2 G vs Absorb BVS: Endothelialization in New Zealand white rabbits at 28 days

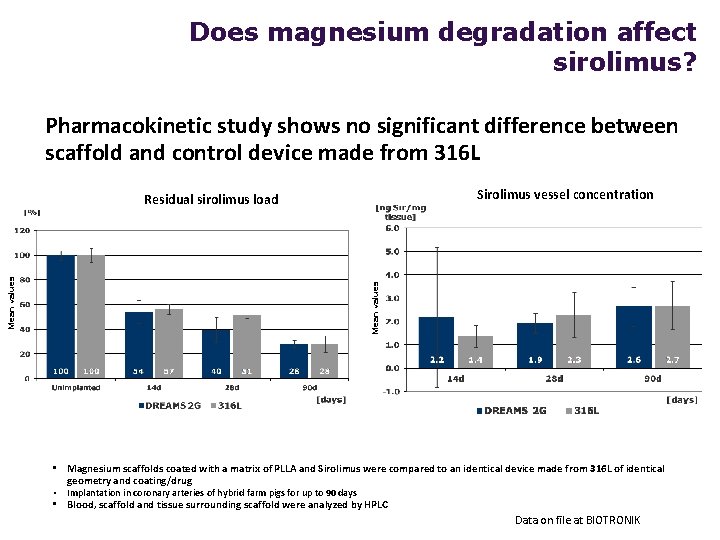
Does magnesium degradation affect sirolimus? Pharmacokinetic study shows no significant difference between scaffold and control device made from 316 L Sirolimus vessel concentration Mean values Residual sirolimus load • Magnesium scaffolds coated with a matrix of PLLA and Sirolimus were compared to an identical device made from 316 L of identical geometry and coating/drug • Implantation in coronary arteries of hybrid farm pigs for up to 90 days • Blood, scaffold and tissue surrounding scaffold were analyzed by HPLC Data on file at BIOTRONIK

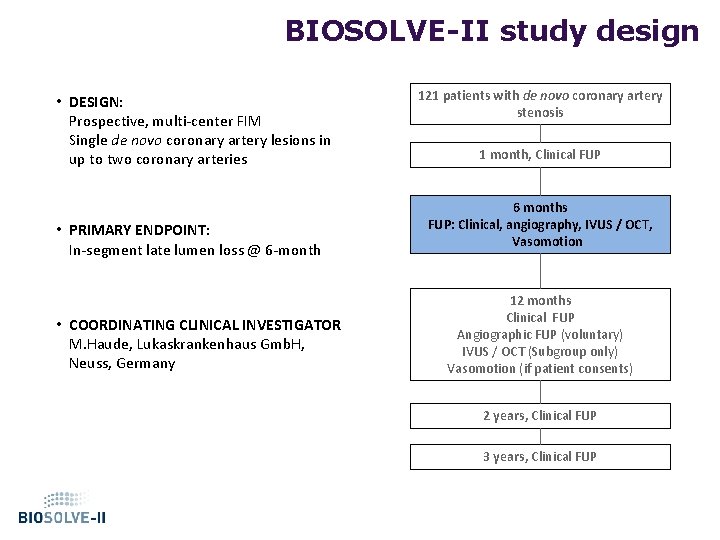
BIOSOLVE-II study design • DESIGN: Prospective, multi-center FIM Single de novo coronary artery lesions in up to two coronary arteries • PRIMARY ENDPOINT: In-segment late lumen loss @ 6 -month • COORDINATING CLINICAL INVESTIGATOR M. Haude, Lukaskrankenhaus Gmb. H, Neuss, Germany 121 patients with de novo coronary artery stenosis 1 month, Clinical FUP 6 months FUP: Clinical, angiography, IVUS / OCT, Vasomotion 12 months Clinical FUP Angiographic FUP (voluntary) IVUS / OCT (Subgroup only) Vasomotion (if patient consents) 2 years, Clinical FUP 3 years, Clinical FUP

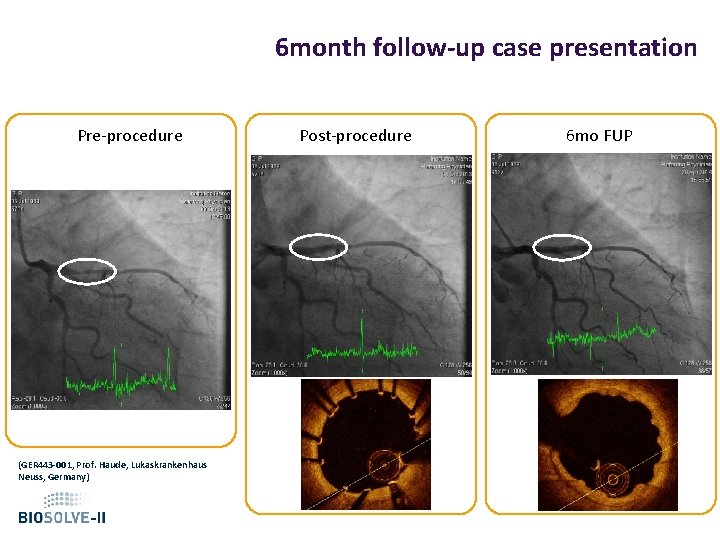
6 month follow-up case presentation Pre-procedure (GER 443 -001, Prof. Haude, Lukaskrankenhaus Neuss, Germany) Post-procedure 6 mo FUP

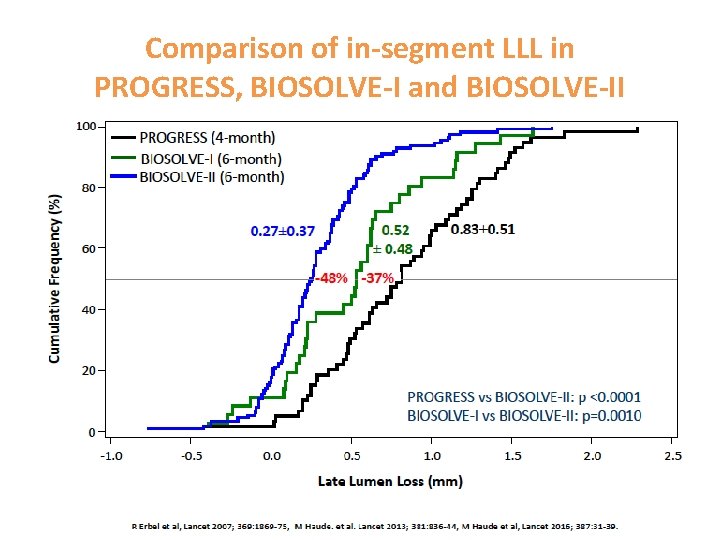
Comparison of in-segment LLL in PROGRESS, BIOSOLVE-I and BIOSOLVE-II

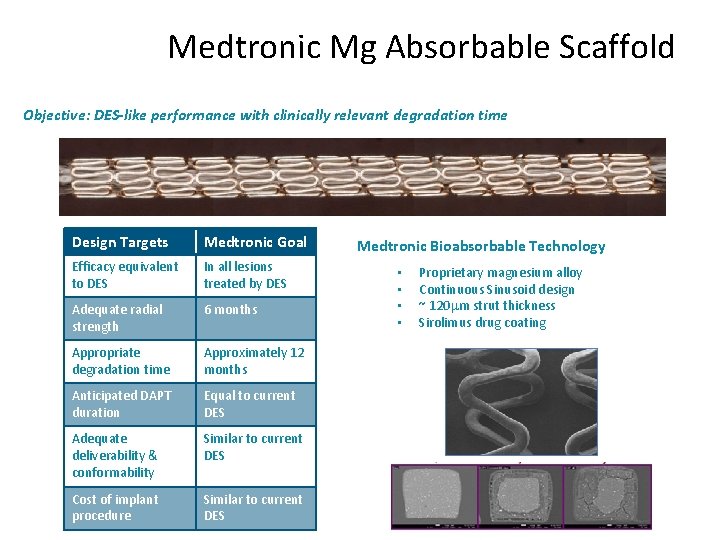
Medtronic Mg Absorbable Scaffold Objective: DES-like performance with clinically relevant degradation time Design Targets Medtronic Goal Efficacy equivalent to DES In all lesions treated by DES Adequate radial strength 6 months Appropriate degradation time Approximately 12 months Anticipated DAPT duration Equal to current DES Adequate deliverability & conformability Similar to current DES Cost of implant procedure Similar to current DES Medtronic Bioabsorbable Technology • • Proprietary magnesium alloy Continuous Sinusoid design ~ 120 m strut thickness Sirolimus drug coating

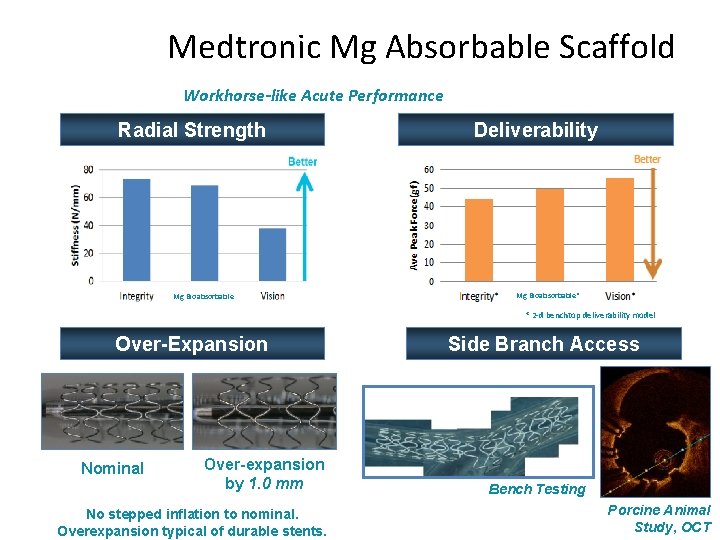
Medtronic Mg Absorbable Scaffold Workhorse-like Acute Performance Radial Strength Mg Bioabsorbable Deliverability Mg Bioabsorbable* * 2 -d benchtop deliverability model Over-Expansion Nominal Over-expansion by 1. 0 mm No stepped inflation to nominal. Overexpansion typical of durable stents. Side Branch Access Bench Testing Porcine Animal Study, OCT

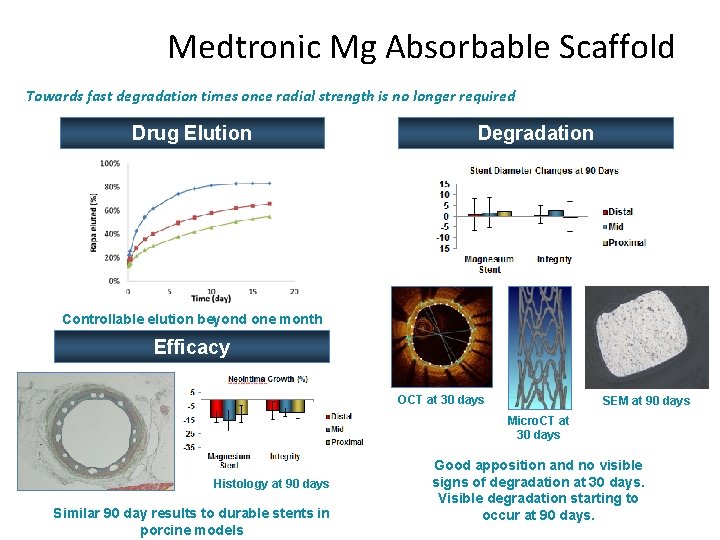
Medtronic Mg Absorbable Scaffold Towards fast degradation times once radial strength is no longer required Drug Elution Degradation Controllable elution beyond one month Efficacy OCT at 30 days SEM at 90 days Micro. CT at 30 days Histology at 90 days Similar 90 day results to durable stents in porcine models Good apposition and no visible signs of degradation at 30 days. Visible degradation starting to occur at 90 days.

Resoloy a new Magnesium Scaffold

RESOLOY® A new bioresorbable Mg-alloy

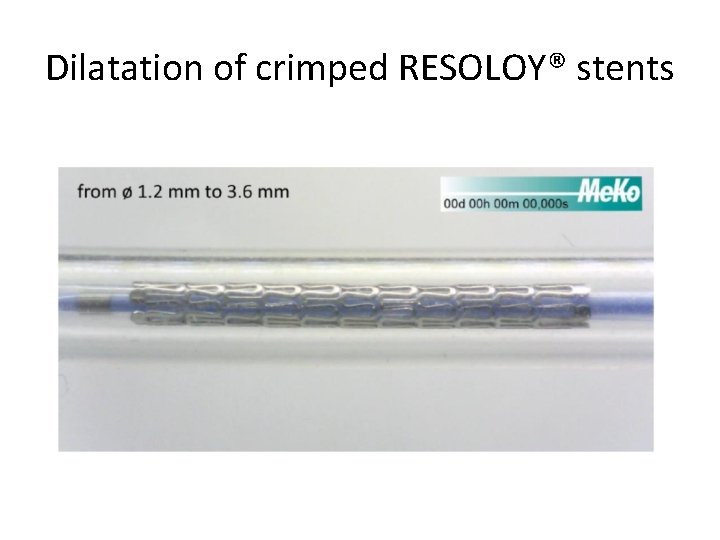
Dilatation of crimped RESOLOY® stents

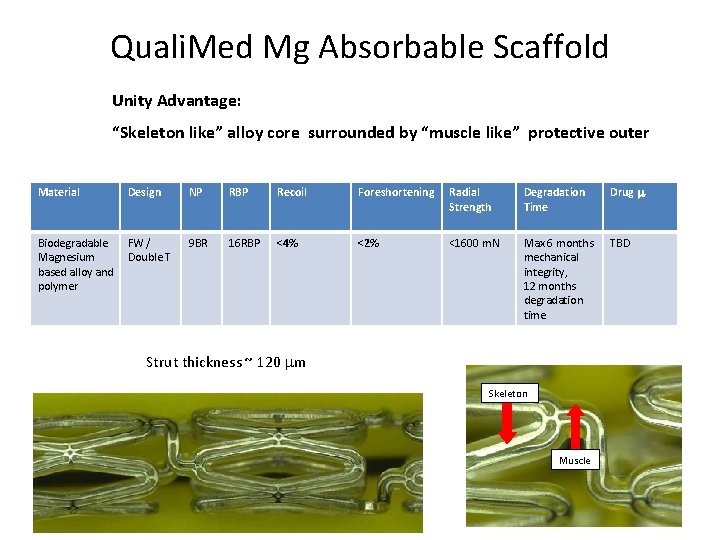
Quali. Med Mg Absorbable Scaffold Unity Advantage: “Skeleton like” alloy core surrounded by “muscle like” protective outer Material Design NP RBP Recoil Foreshortening Radial Strength Degradation Time Drug Biodegradable Magnesium based alloy and polymer FW / Double T 9 BR 16 RBP <4% <2% <1600 m. N Max 6 months mechanical integrity, 12 months degradation time TBD Strut thickness ~ 120 m Skeleton Muscle
![Unity BDS Crimping and Crossing Profile Characteristics Crossing Profile of the crimped stent mm Unity BDS Crimping and Crossing Profile Characteristics Crossing Profile of the crimped stent [mm]](https://slidetodoc.com/presentation_image_h/94cb1f28fa551c47a86f845f05fefc72/image-24.jpg)
Unity BDS Crimping and Crossing Profile Characteristics Crossing Profile of the crimped stent [mm] Quali. Med “Unity BDS” Abbott Absorb BVS 1. 13 – 1. 18 mm 1. 34 – 1. 37 mm 1. 6 1. 4 B 1. 2 E 1 T 0. 8 T 0. 6 E 0. 4 R 0. 2 0 Unity ABSORB

Unity BRS K 2 Animal Study – OCT Investigations Controls ABSORB + BMS Planned for 54 pigs with two control groups: Absorb + BMS Follow-up at 7 days, 1 month, 3 months, 6 months, 12 months and 18 months 25 25

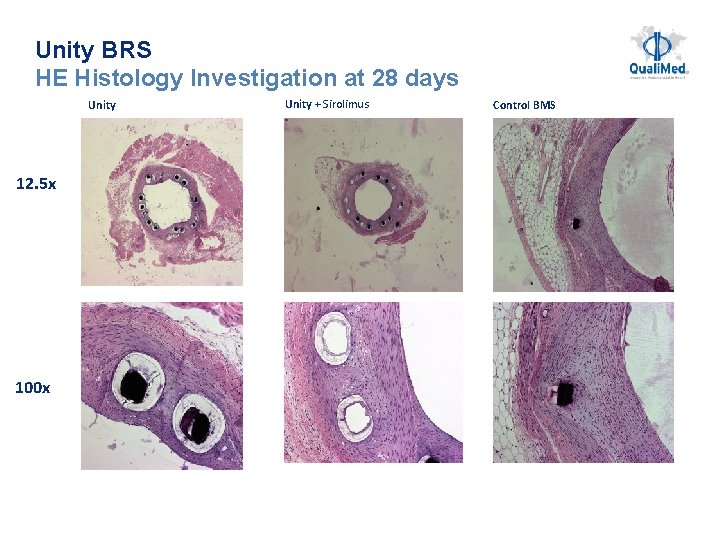
Unity BRS HE Histology Investigation at 28 days Unity + Sirolimus Control BMS 12. 5 x 100 x 26 26

Summary • Magnesium offers an ideal balance between biocompatibility, mechanical performances and absorption. • Magnesium has better deliverability and apposition when compared to PLLA • Not all Magnesium alloys are the same • The DREAMS program of Biotronik showed safety with no scaffold thrombosis and acceptable efficacy • DREAMS second generation with the PLLA and Sirolimus coating, is the most advanced Mg program and anticipated to have CE mark in 2016. • Other companies are also working on absorbable Mg scaffold programs, but are currently in the preclinical, and bench evaluation.

Thank you for your attention
 Adam waksman
Adam waksman The metalco company desires to blend a new alloy
The metalco company desires to blend a new alloy Cpmcd full form
Cpmcd full form Delayed expansion of amalgam
Delayed expansion of amalgam Steel alloy
Steel alloy Wikimedia
Wikimedia Fast fill electrode
Fast fill electrode Examples of alloy metals
Examples of alloy metals Steel interstitial alloy
Steel interstitial alloy Memory shape
Memory shape Lathe cut alloy
Lathe cut alloy Substitutional alloy
Substitutional alloy 1200 alloy
1200 alloy Low alloy steel
Low alloy steel Silver alloy in dentistry
Silver alloy in dentistry Lathe cut alloy
Lathe cut alloy Lathe cut alloy
Lathe cut alloy Chrome cobalt alloy in dentistry
Chrome cobalt alloy in dentistry Interionic distance formula
Interionic distance formula Fick's second law
Fick's second law Single phase alloy
Single phase alloy Steel interstitial alloy
Steel interstitial alloy Alloy nomenclature
Alloy nomenclature Alloy 718 api
Alloy 718 api Bronze is an alloy of
Bronze is an alloy of Alloy steel producers association of india
Alloy steel producers association of india Linen or cloth tape
Linen or cloth tape Bronze vs brass
Bronze vs brass Bronze is an alloy of
Bronze is an alloy of