Metallic Bonds and Metallic Properties The valence electrons
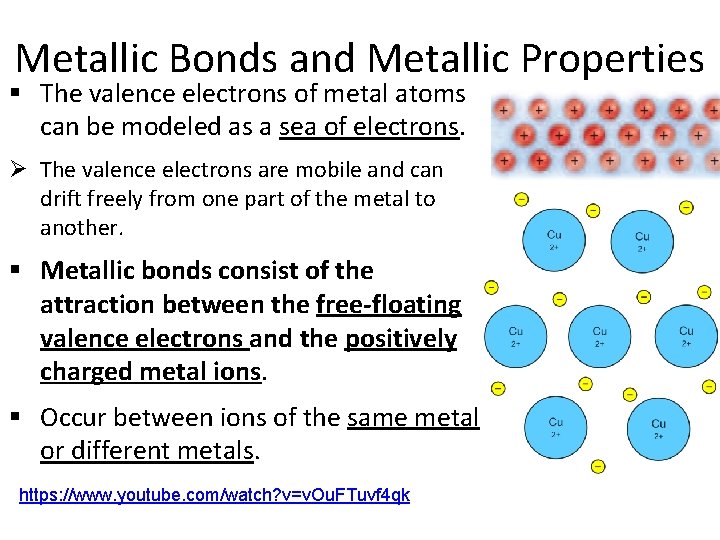
Metallic Bonds and Metallic Properties § The valence electrons of metal atoms can be modeled as a sea of electrons. Ø The valence electrons are mobile and can drift freely from one part of the metal to another. § Metallic bonds consist of the attraction between the free-floating valence electrons and the positively charged metal ions. § Occur between ions of the same metal or different metals. https: //www. youtube. com/watch? v=v. Ou. FTuvf 4 qk
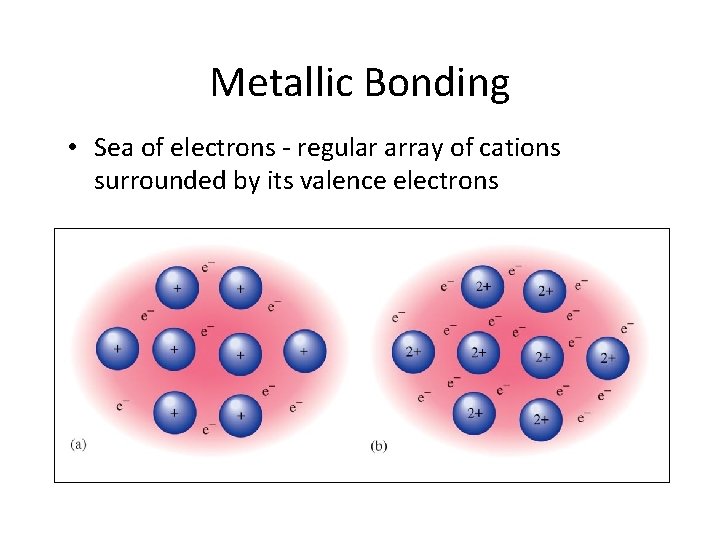
Metallic Bonding • Sea of electrons - regular array of cations surrounded by its valence electrons
Metal Structure Model: Atoms form a non-rigid “crystal” structure to best use available space.
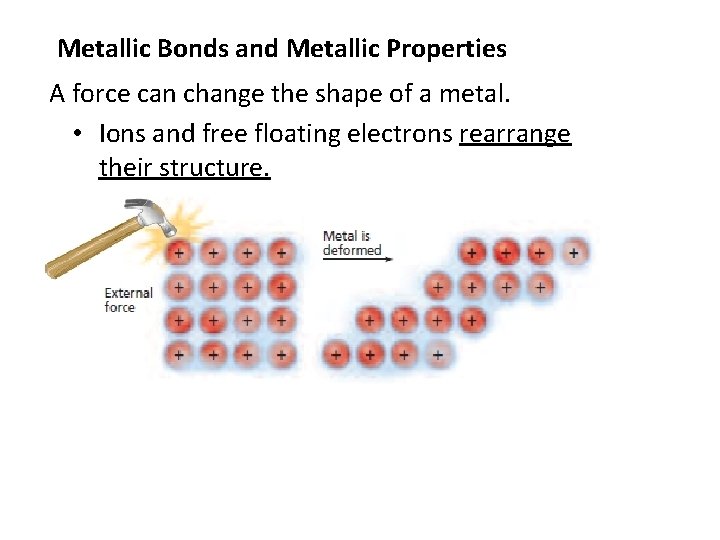
Metallic Bonds and Metallic Properties A force can change the shape of a metal. • Ions and free floating electrons rearrange their structure.
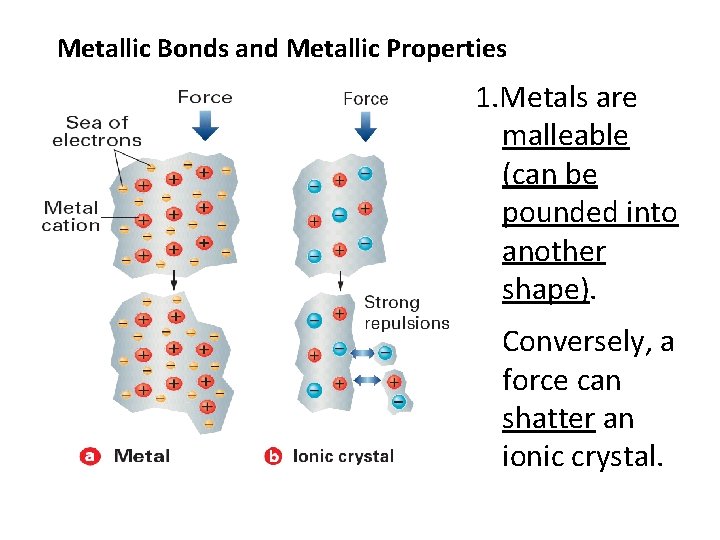
Metallic Bonds and Metallic Properties 1. Metals are malleable (can be pounded into another shape). Conversely, a force can shatter an ionic crystal.
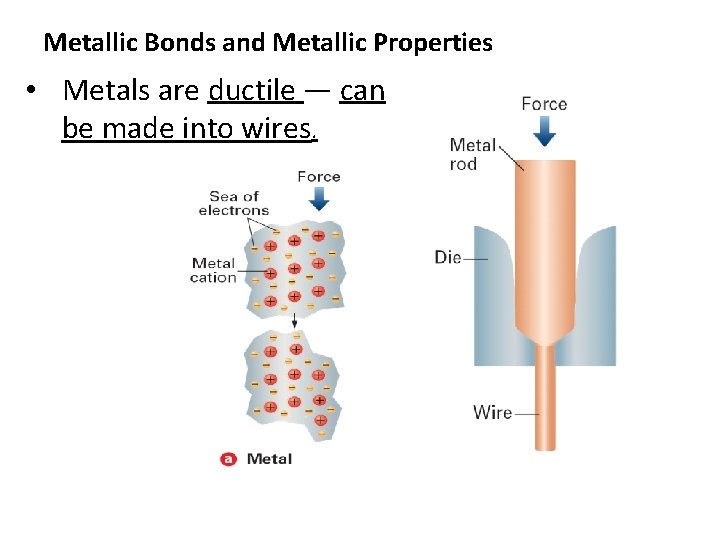
Metallic Bonds and Metallic Properties • Metals are ductile — can be made into wires.
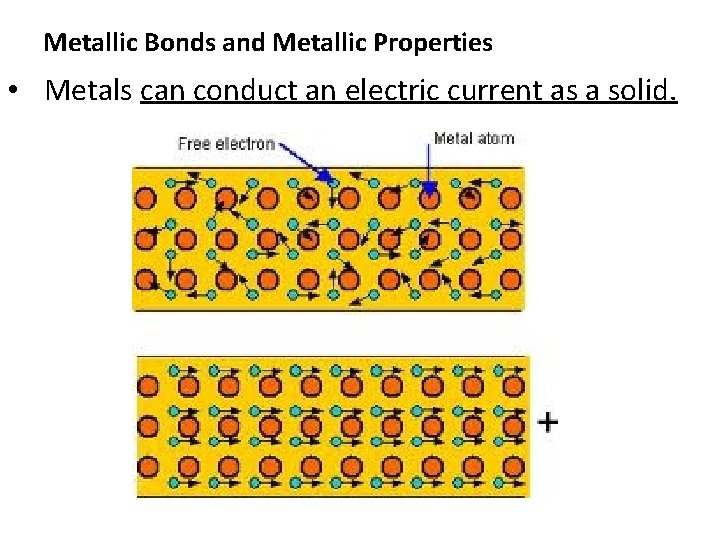
Metallic Bonds and Metallic Properties • Metals can conduct an electric current as a solid.
Metal Alloys • Alloys are mixtures of two or more elements, at least one of which is a metal. – Alloys are important because their properties are often superior to those of the individual elements.

Metal alloys • Metals melted together to make a solution (homogeneous solid!!) - 2 types – Substitutional Alloy – some of the host metal atoms are replaced by other metal atoms of similar size. – Interstitial Alloy – some of the holes in the closest packed metal structure are occupied by small atoms. – https: //www. youtube. com/watch? v=9 LH DSB 1 n 11 k
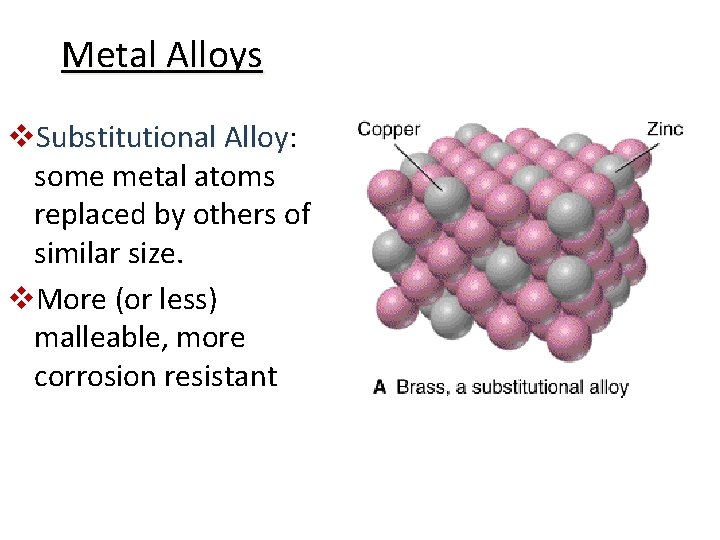
Metal Alloys v. Substitutional Alloy: Alloy some metal atoms replaced by others of similar size. v. More (or less) malleable, more corrosion resistant
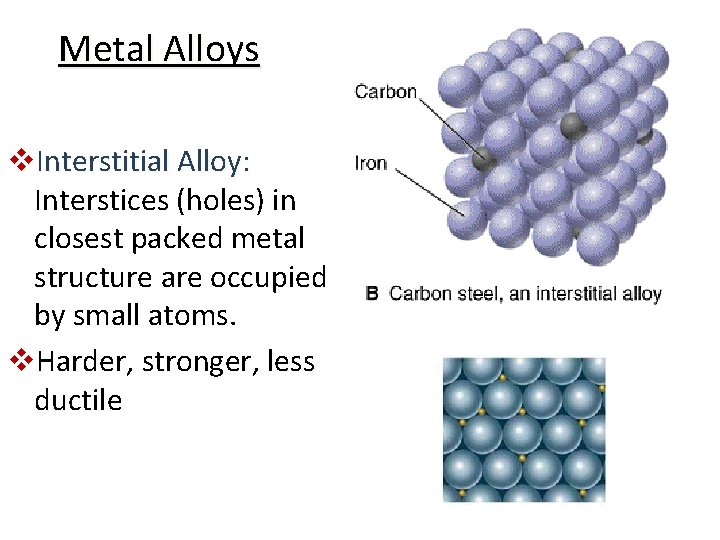
Metal Alloys v. Interstitial Alloy: Interstices (holes) in closest packed metal structure are occupied by small atoms. v. Harder, stronger, less ductile
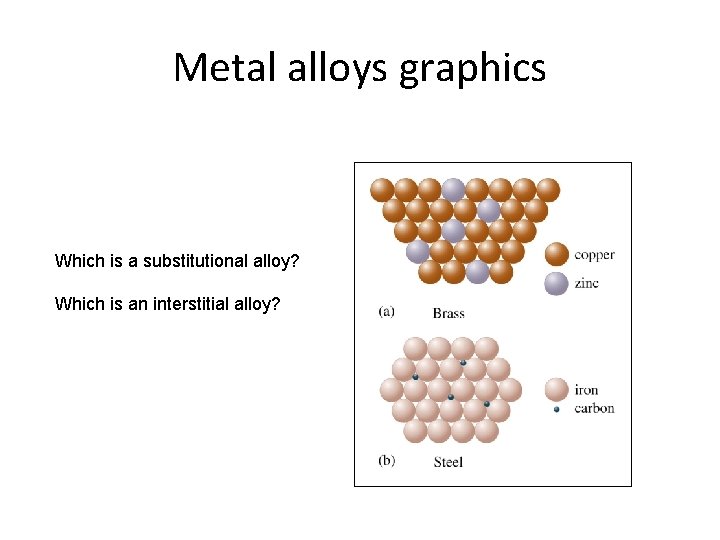
Metal alloys graphics Which is a substitutional alloy? Which is an interstitial alloy?
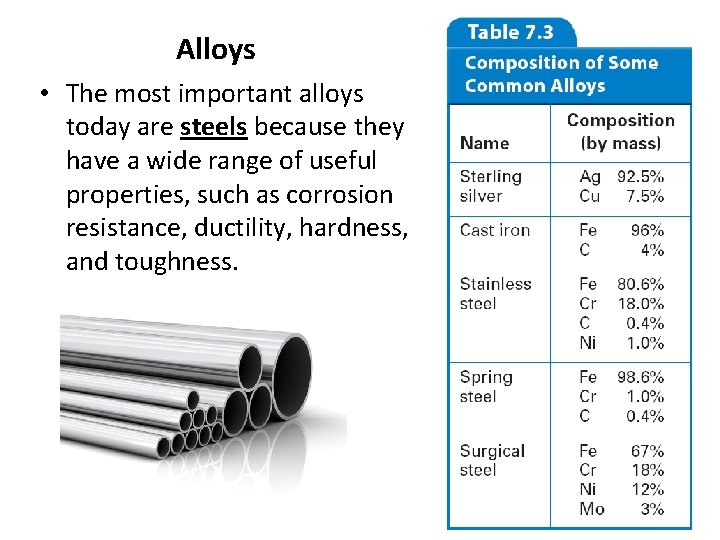
Alloys • The most important alloys today are steels because they have a wide range of useful properties, such as corrosion resistance, ductility, hardness, and toughness.

Other Important Alloys • Pewter (tin, silver, antimony, bismuth) • Bronze (copper and tin) (Allowed ancient people in the Bronze Age to build stronger, more durable tools) • Sterling silver (silver, copper)

Alloys • In 1812, Napoleon tried to invade Russia. • All soldiers had shiny tin buttons. • In the Siberian cold, the tin disintegrated in to a fine powder. Uniforms and boots would not stay closed.
- Slides: 15