Oxidation Magnesium oxygen Magnesium oxide Magnesium is OXIDISED


















- Slides: 72


Oxidation Magnesium + oxygen Magnesium oxide Magnesium is OXIDISED, because it has gained oxygen


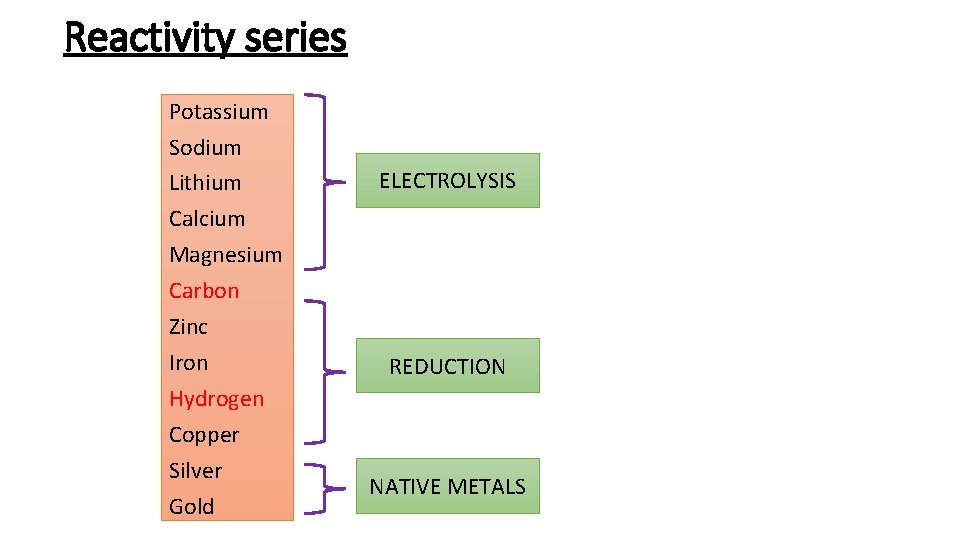
Reactivity series Potassium Sodium Lithium Calcium Magnesium Carbon Zinc Iron Hydrogen Copper Silver Gold ELECTROLYSIS REDUCTION NATIVE METALS


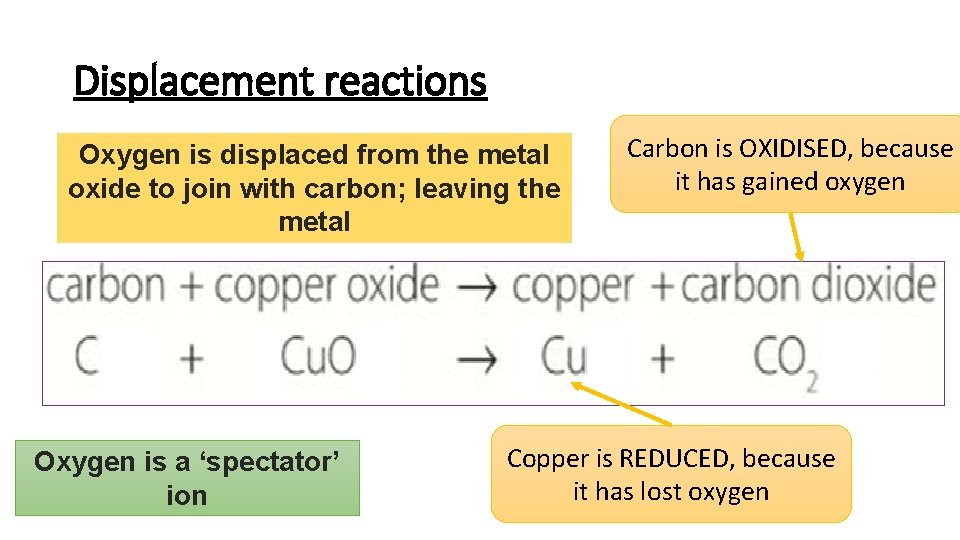
Displacement reactions Oxygen is displaced from the metal oxide to join with carbon; leaving the metal Oxygen is a ‘spectator’ ion Carbon is OXIDISED, because it has gained oxygen Copper is REDUCED, because it has lost oxygen


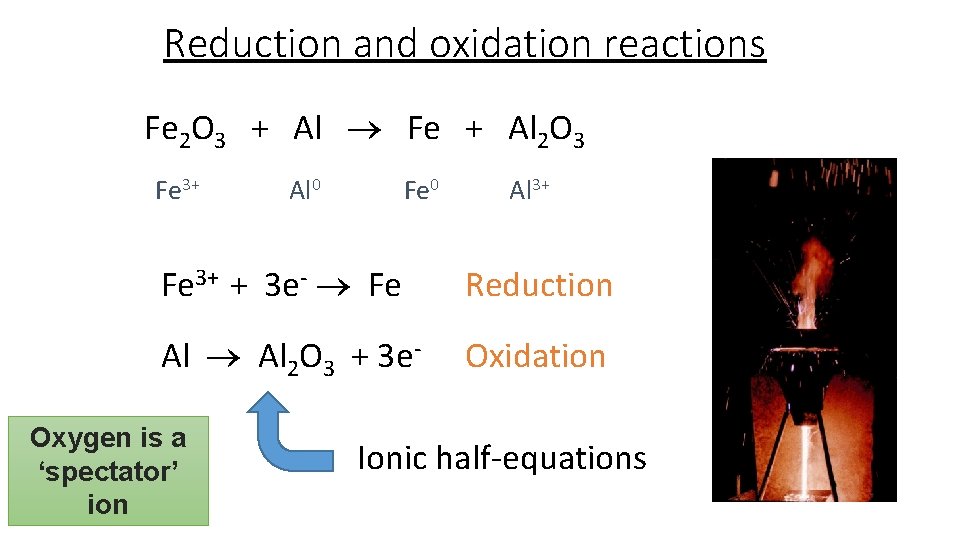
Reduction and oxidation reactions Fe 2 O 3 + Al Fe + Al 2 O 3 Fe 3+ Al 0 Fe 0 Al 3+ Fe 3+ + 3 e- Fe Reduction Al 2 O 3 + 3 e- Oxidation Oxygen is a ‘spectator’ ion Ionic half-equations

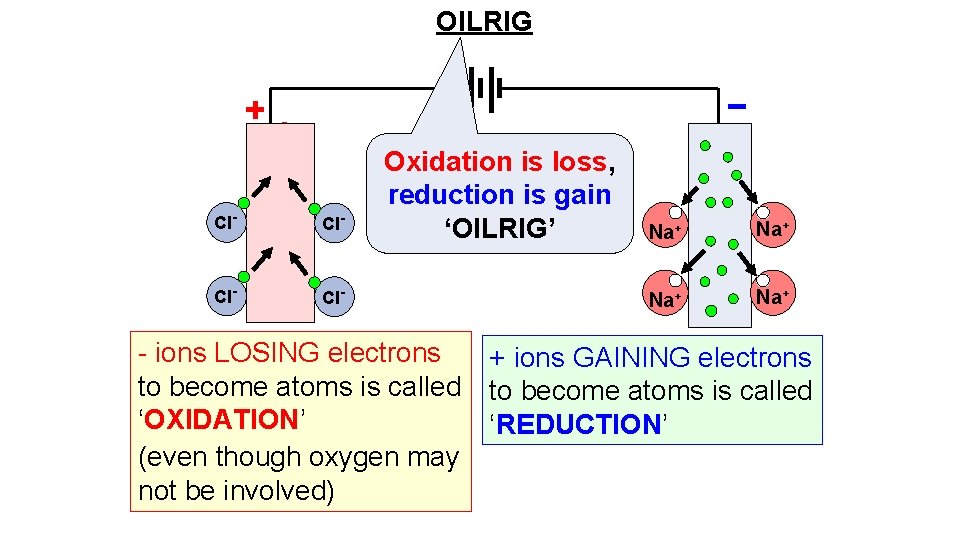
OIL RIG OXIDATION is LOSS (of electrons) REDUCTION is GAIN (of electrons)

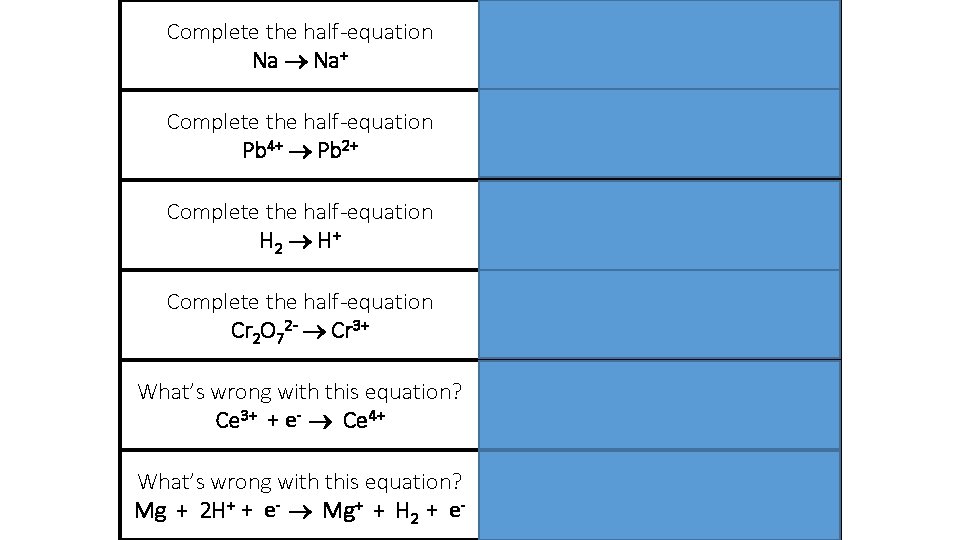
Complete the half-equation Na Na++ e- Complete the half-equation Pb 4+ Pb 2+ Pb 4+ + 2 e- Pb 2+ Complete the half-equation H 2 H + H 2 2 H+ + 2 e- Complete the half-equation Cr 2 O 72 - Cr 3+ Cr 2 O 72 - + 6 e- 2 Cr 3+ What’s wrong with this equation? Ce 3+ + e- Ce 4+ Electron is on the wrong side! What’s wrong with this equation? Mg + 2 H+ + e- Mg+ + H 2 + e- Should be Mg 2+ Electrons should be cancelled out


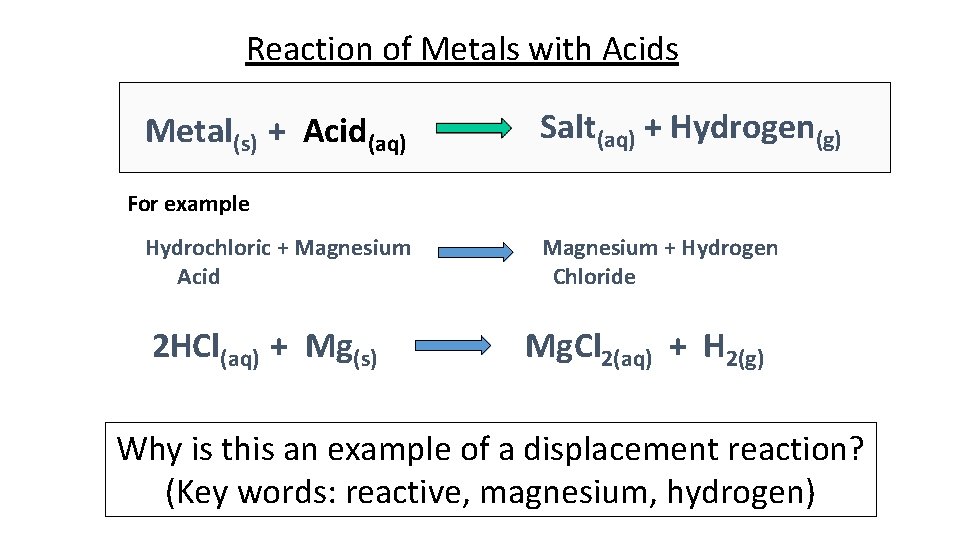
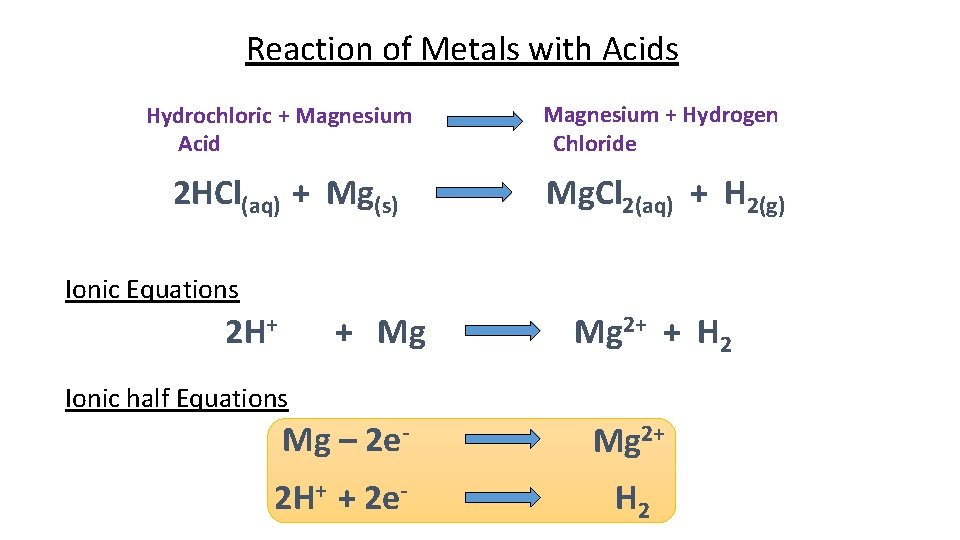
Reaction of Metals with Acids Metal(s) + Acid(aq) Salt(aq) + Hydrogen(g) For example Hydrochloric + Magnesium Acid 2 HCl(aq) + Mg(s) Magnesium + Hydrogen Chloride Mg. Cl 2(aq) + H 2(g) Why is this an example of a displacement reaction? (Key words: reactive, magnesium, hydrogen)

Reaction of Metals with Acids Hydrochloric + Magnesium Acid Magnesium + Hydrogen Chloride 2 HCl(aq) + Mg(s) Mg. Cl 2(aq) + H 2(g) Ionic Equations 2 H+ + Mg Mg 2+ + H 2 Ionic half Equations Mg – 2 e- Mg 2+ 2 H+ + 2 e- H 2


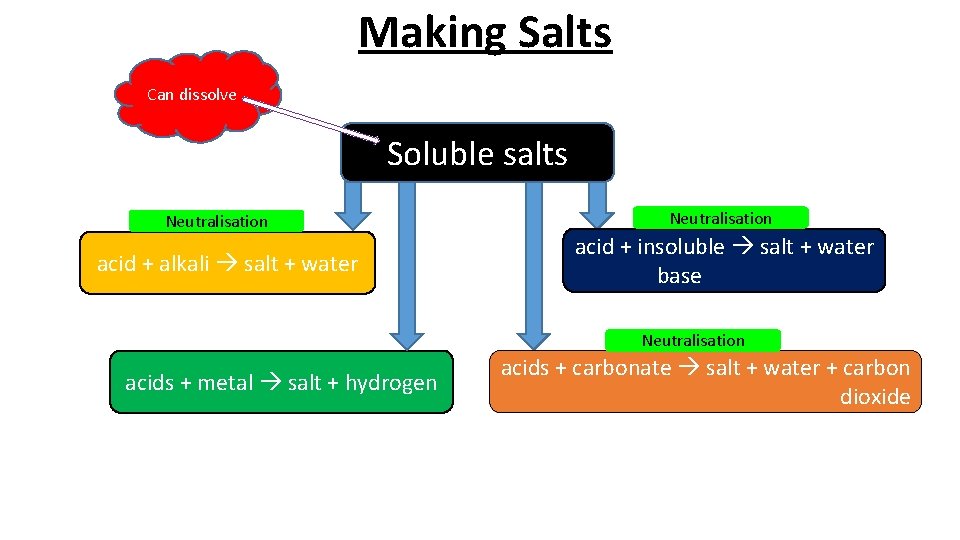
Making Salts Can dissolve Soluble salts Neutralisation acid + alkali salt + water Neutralisation acid + insoluble salt + water base Neutralisation acids + metal salt + hydrogen acids + carbonate salt + water + carbon dioxide

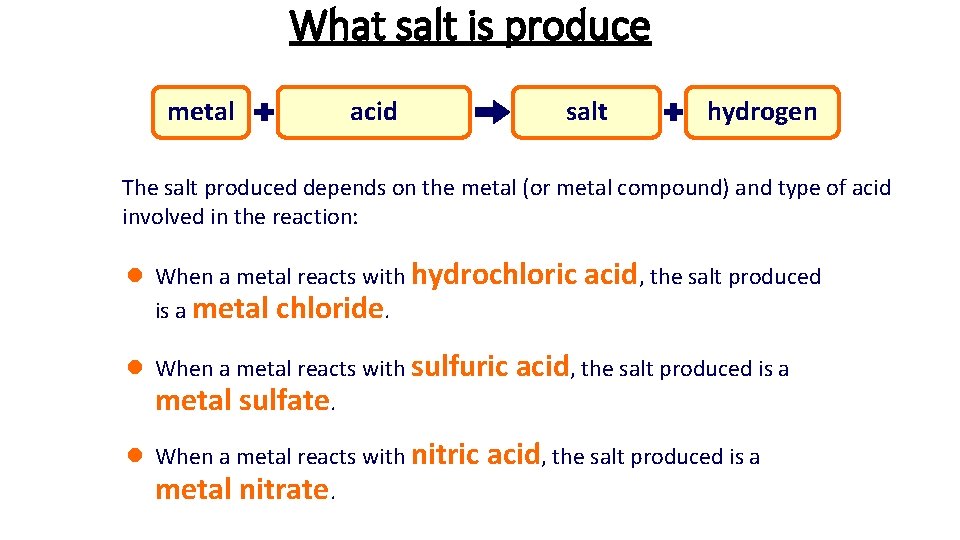
What salt is produce metal hydrochloric sulfuric nitric acid metal salt chloride sulfate nitrate hydrogen The salt produced depends on the metal (or metal compound) and type of acid involved in the reaction: l When a metal reacts with hydrochloric acid, the salt produced is a metal chloride. l When a metal reacts with sulfuric acid, the salt produced is a metal sulfate. l When a metal reacts with nitric acid, the salt produced is a metal nitrate.

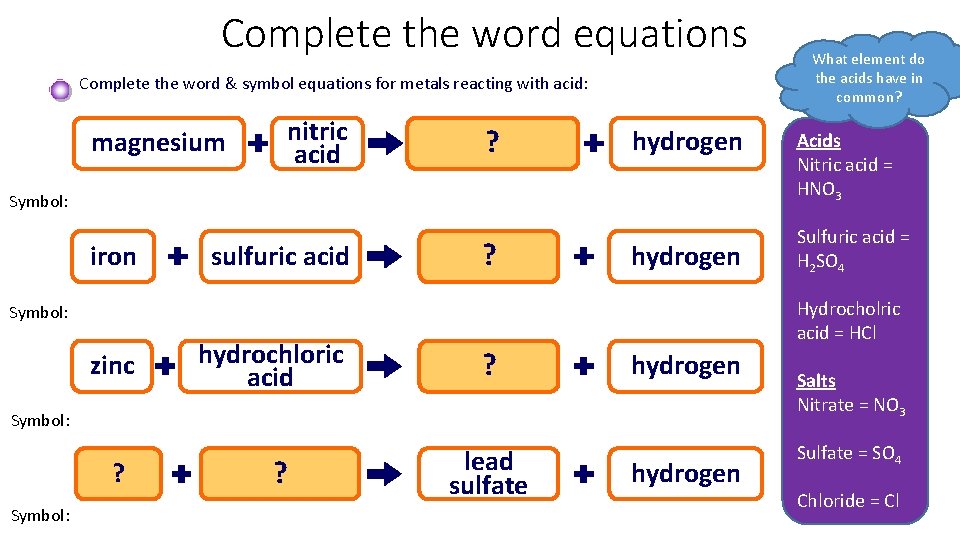
Complete the word equations Complete the word & symbol equations for metals reacting with acid: magnesium nitric acid magnesium ? nitrate hydrogen Symbol: iron sulfuric acid iron ? sulfate hydrogen hydrochloric acid zinc ? chloride sulfuric ? acid lead sulfate hydrogen Symbol: lead ? Symbol: Acids Nitric acid = HNO 3 Sulfuric acid = H 2 SO 4 Hydrocholric acid = HCl Symbol: zinc What element do the acids have in common? hydrogen Salts Nitrate = NO 3 Sulfate = SO 4 Chloride = Cl


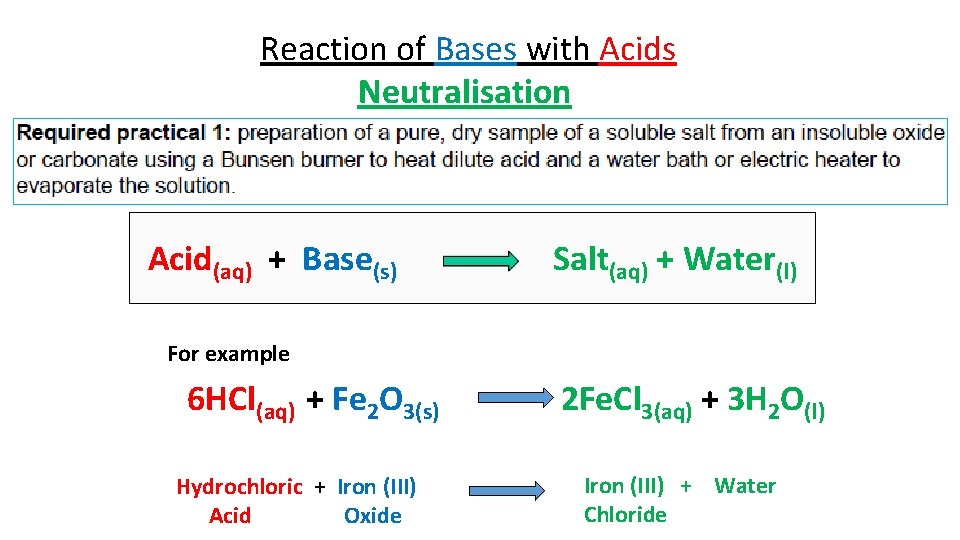
Reaction of Bases with Acids Neutralisation Acid(aq) + Base(s) Salt(aq) + Water(l) For example 6 HCl(aq) + Fe 2 O 3(s) Hydrochloric + Iron (III) Acid Oxide 2 Fe. Cl 3(aq) + 3 H 2 O(l) Iron (III) + Water Chloride





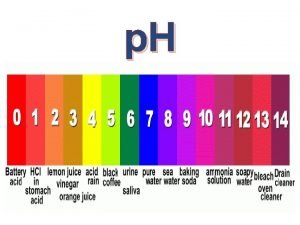
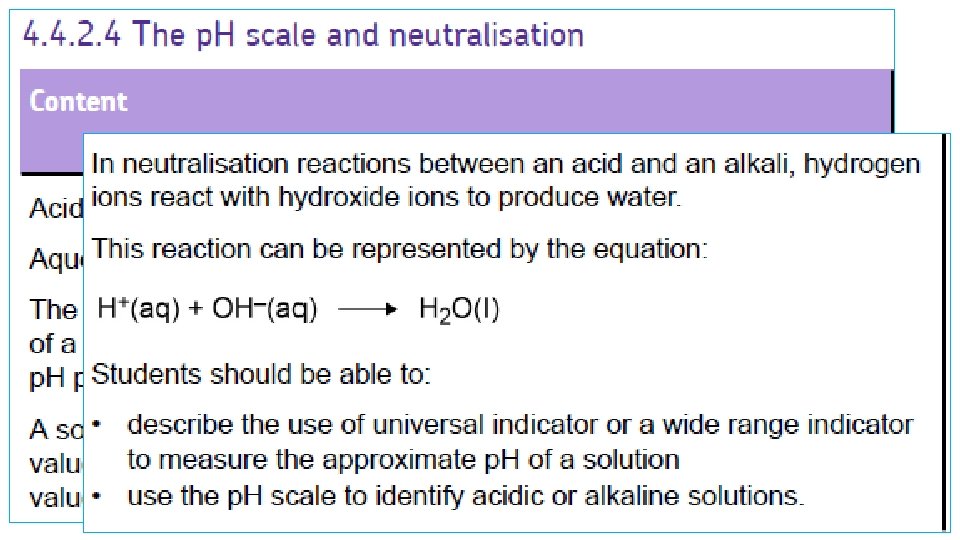
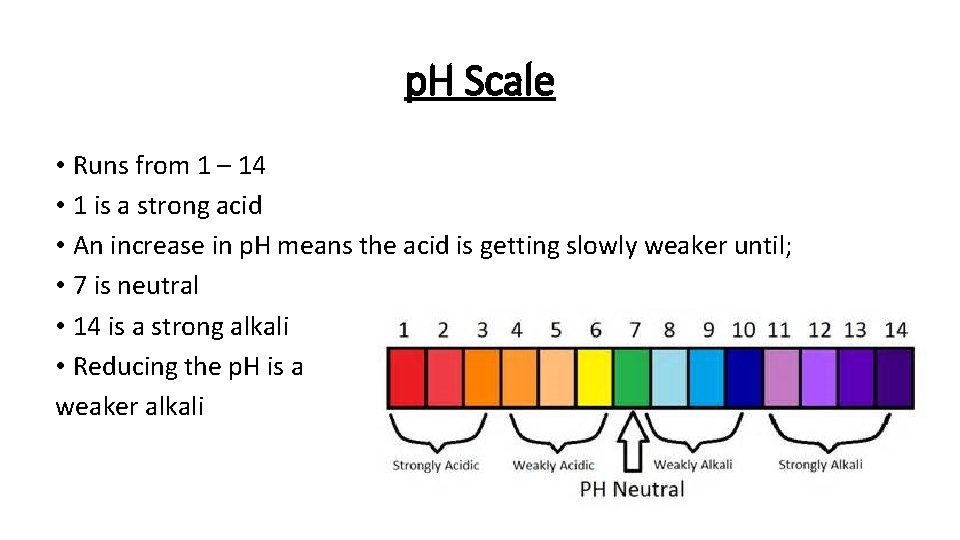
p. H Scale • Runs from 1 – 14 • 1 is a strong acid • An increase in p. H means the acid is getting slowly weaker until; • 7 is neutral • 14 is a strong alkali • Reducing the p. H is a weaker alkali

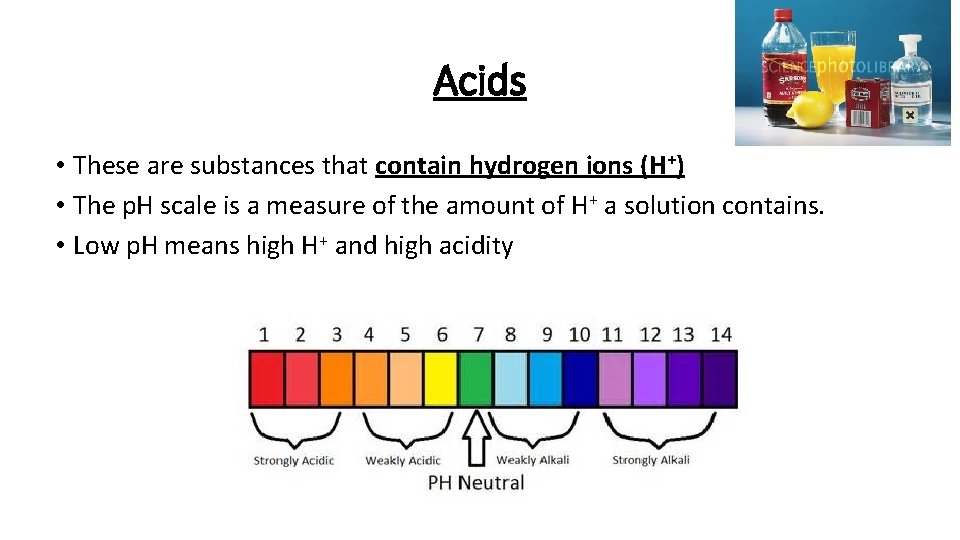
Acids • These are substances that contain hydrogen ions (H+) • The p. H scale is a measure of the amount of H+ a solution contains. • Low p. H means high H+ and high acidity

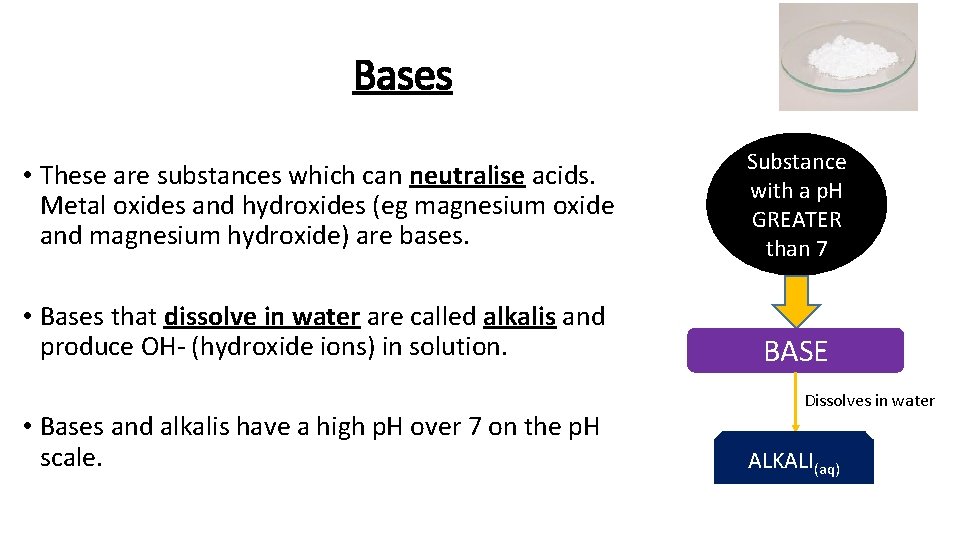
Bases • These are substances which can neutralise acids. Metal oxides and hydroxides (eg magnesium oxide and magnesium hydroxide) are bases. Substance with a p. H GREATER than 7 • Bases that dissolve in water are called alkalis and produce OH- (hydroxide ions) in solution. BASE • Bases and alkalis have a high p. H over 7 on the p. H scale. Dissolves in water ALKALI(aq)

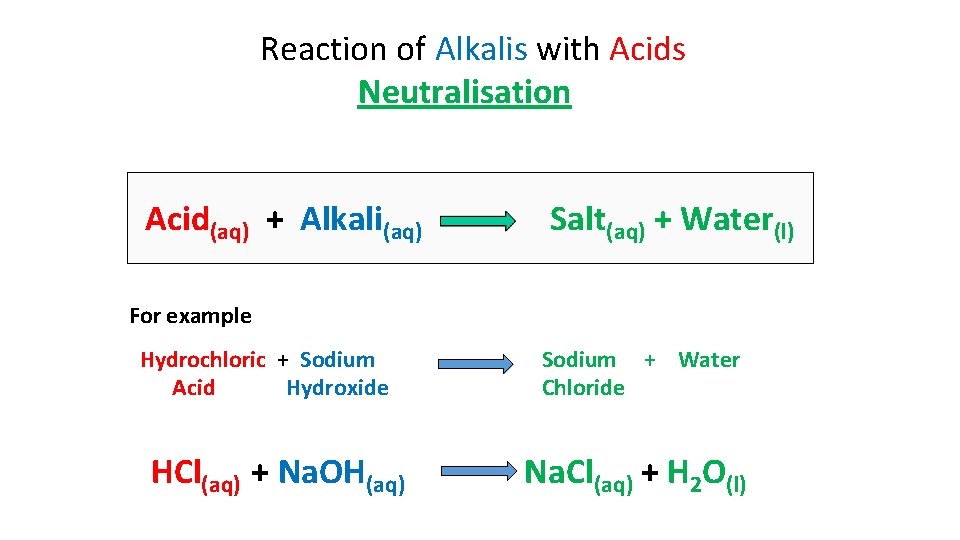
Reaction of Alkalis with Acids Neutralisation Acid(aq) + Alkali(aq) Salt(aq) + Water(l) For example Hydrochloric + Sodium Acid Hydroxide HCl(aq) + Na. OH(aq) Sodium + Water Chloride Na. Cl(aq) + H 2 O(l)

Creating a salt from an acid and alkali

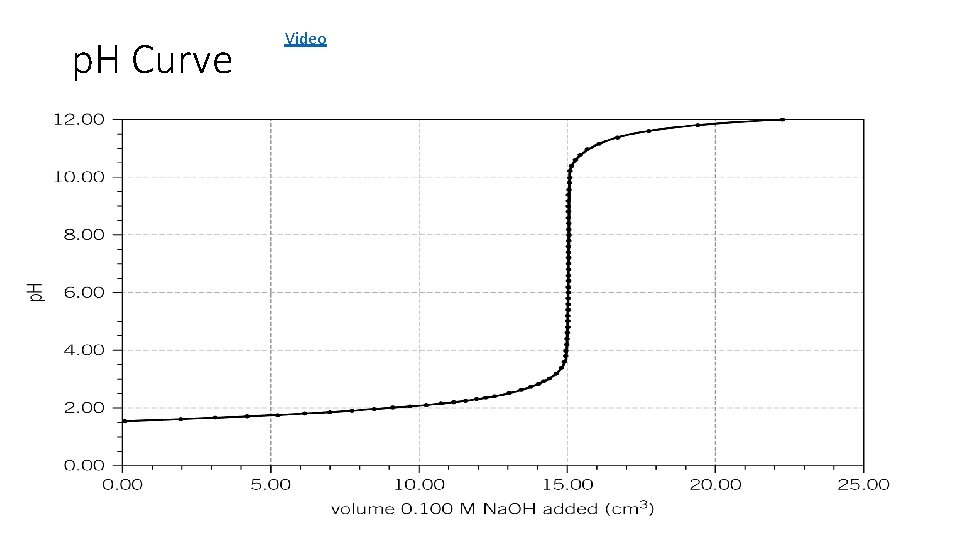
p. H Curve Video



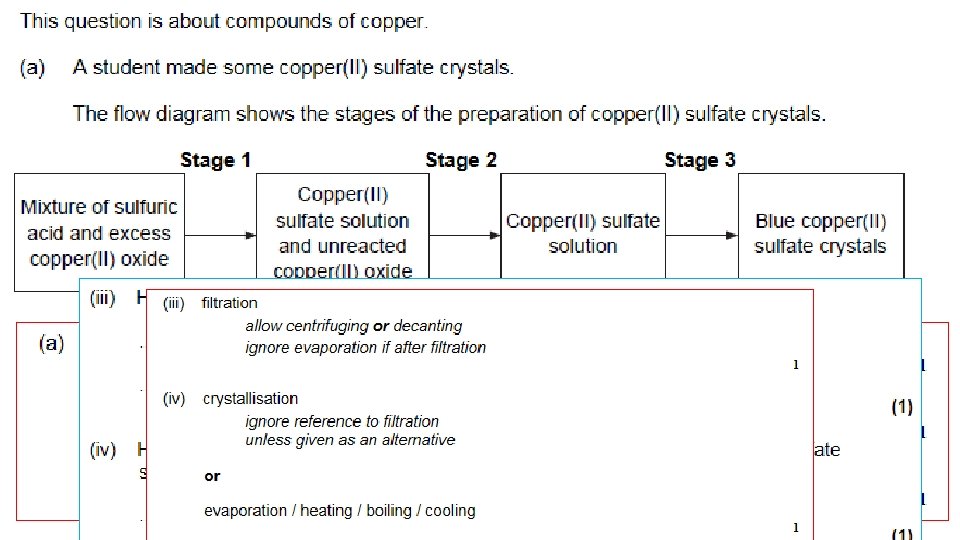
1. Use the pipette and pipette filler to put exactly 25 cm 3 sodium hydroxide solution into the conical flask. 2. Put the flask on a white tile. 3. Use the small funnel to carefully fill the burette with dilute sulfuric acid. Before it completely fills put a small beaker underneath the tap, gently open it to allow acid to fill the tap, before closing again and filling the burette to the 0. 00 cm 3 line. Remove the funnel. 4. Put 5– 10 drops of phenolphthalein indicator into the conical flask. Swirl the flask to mix and put under the burette on top of the tile. The contents of the flask will go pink. 5. Carefully open the burette tap so that 10 cm 3 sulfuric acid slowly flows into the flask. Constantly swirl the flask when adding the acid. Then add the acid drop by drop until you see a permanent colour change from pink to colourless in the flask. 6. You need to be able to shut the tap immediately after a single drop of acid causes the colour to become permanently colourless.

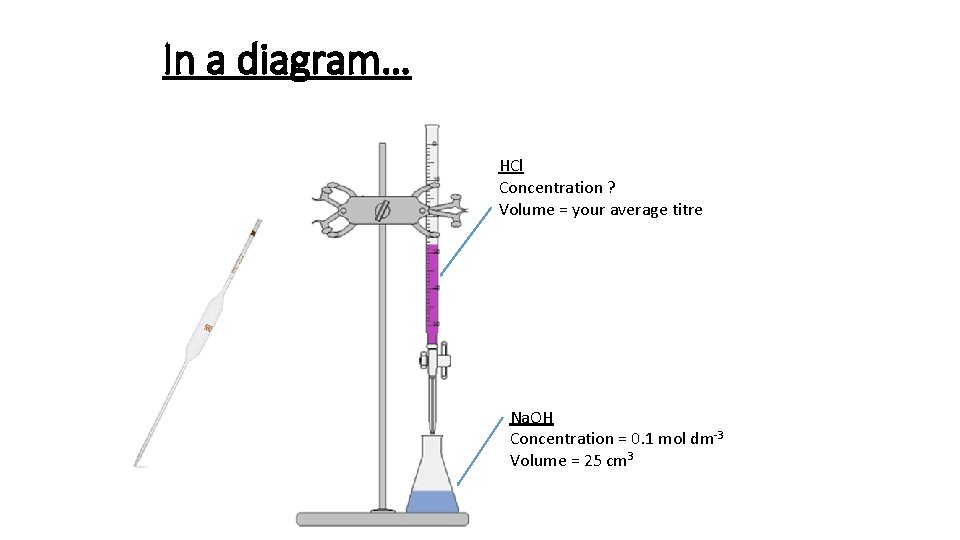
In a diagram… HCl Concentration ? Volume = your average titre Na. OH Concentration = 0. 1 mol dm-3 Volume = 25 cm 3

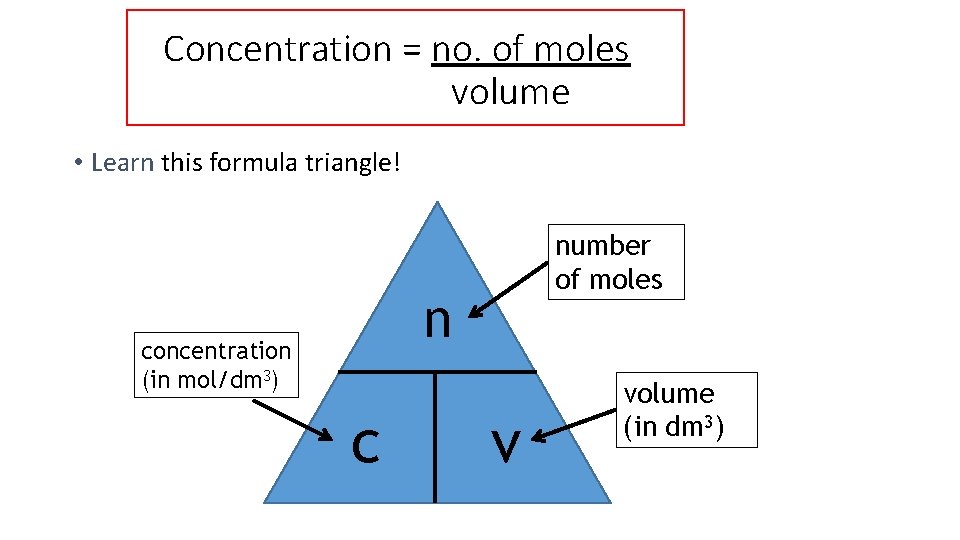
Concentration = no. of moles volume • Learn this formula triangle! number of moles n concentration (in mol/dm 3) c v volume (in dm 3)

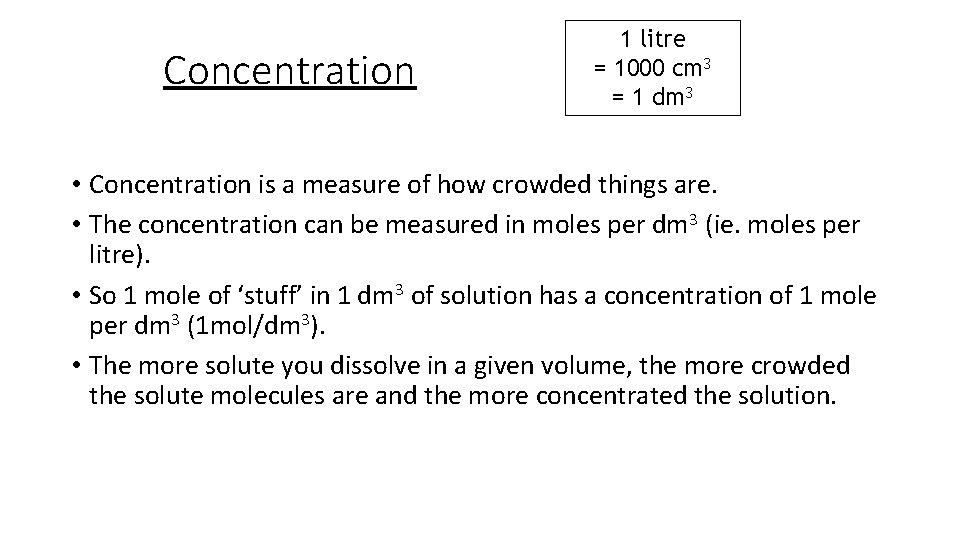
Concentration 1 litre = 1000 cm 3 = 1 dm 3 • Concentration is a measure of how crowded things are. • The concentration can be measured in moles per dm 3 (ie. moles per litre). • So 1 mole of ‘stuff’ in 1 dm 3 of solution has a concentration of 1 mole per dm 3 (1 mol/dm 3). • The more solute you dissolve in a given volume, the more crowded the solute molecules are and the more concentrated the solution.

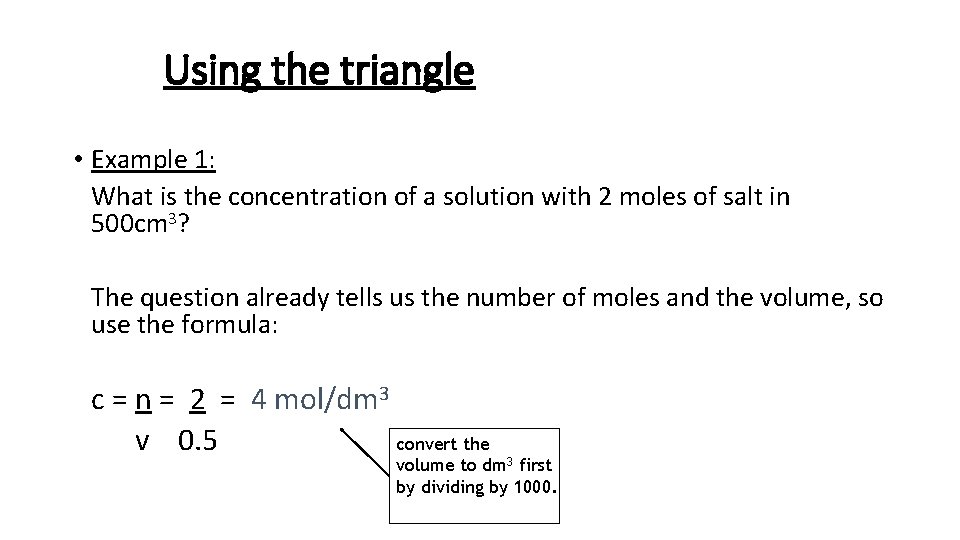
Using the triangle • Example 1: What is the concentration of a solution with 2 moles of salt in 500 cm 3? The question already tells us the number of moles and the volume, so use the formula: c = n = 2 = 4 mol/dm 3 convert the v 0. 5 volume to dm 3 first by dividing by 1000.

Using the triangle • Example 2: How many moles of sodium chloride are in 250 cm 3 of a 3 mol/dm 3 solution of sodium chloride? The question tells us the volume and concentration, so use the formula: n = c x v = 3 x 0. 25 = 0. 75 moles convert the volume to dm 3 first by dividing by 1000.


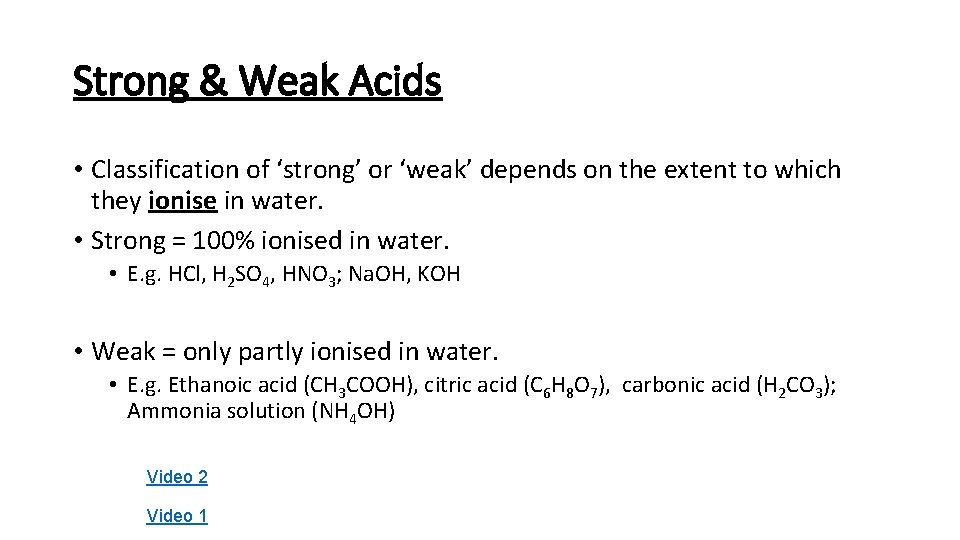
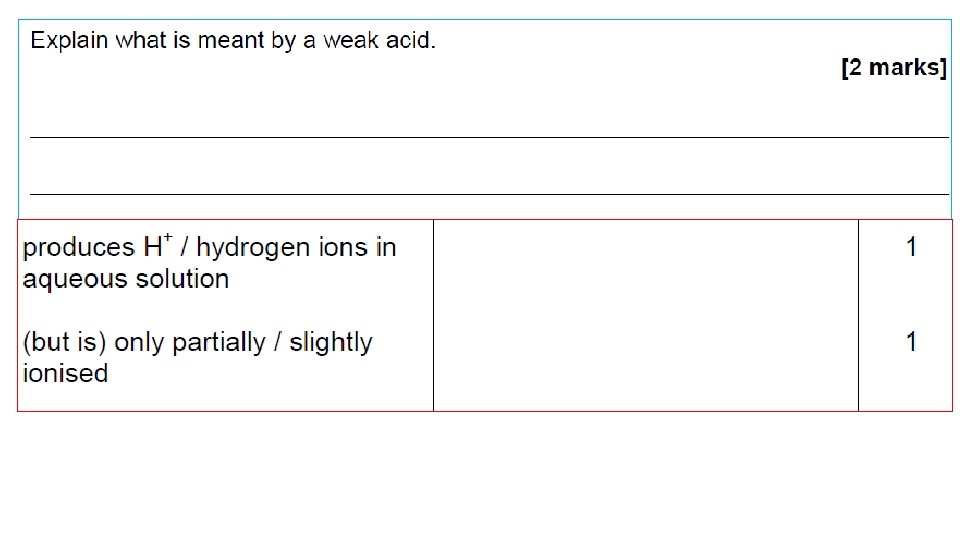
Strong & Weak Acids • Classification of ‘strong’ or ‘weak’ depends on the extent to which they ionise in water. • Strong = 100% ionised in water. • E. g. HCl, H 2 SO 4, HNO 3; Na. OH, KOH • Weak = only partly ionised in water. • E. g. Ethanoic acid (CH 3 COOH), citric acid (C 6 H 8 O 7), carbonic acid (H 2 CO 3); Ammonia solution (NH 4 OH) Video 2 Video 1

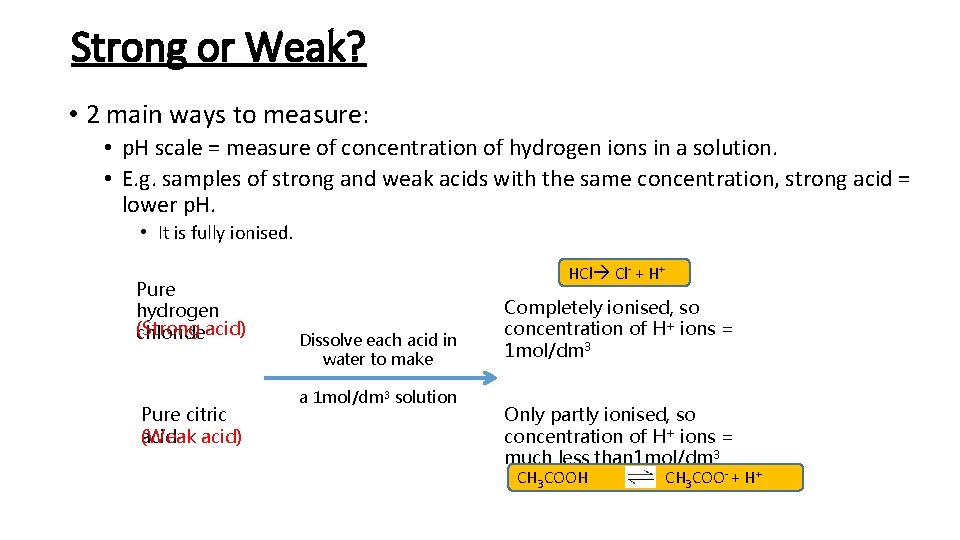
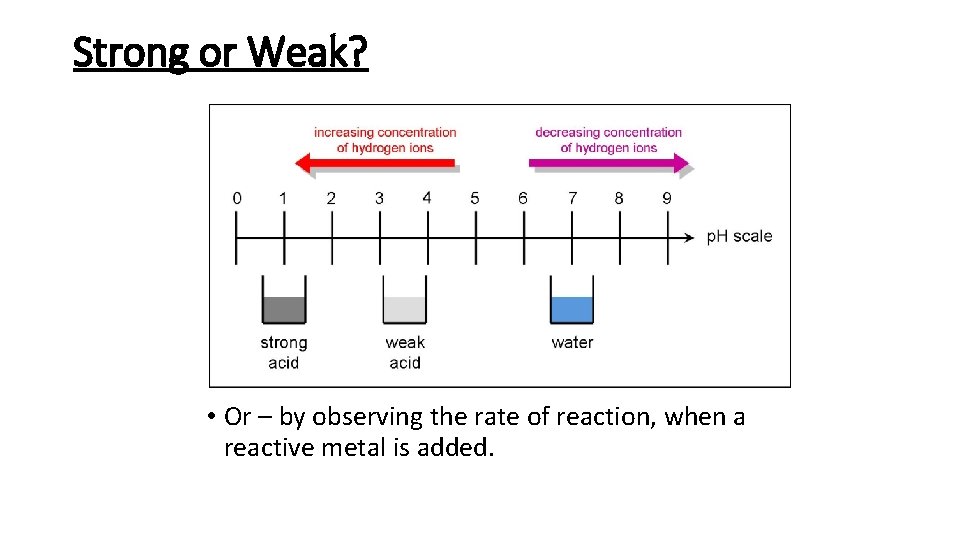
Strong or Weak? • 2 main ways to measure: • p. H scale = measure of concentration of hydrogen ions in a solution. • E. g. samples of strong and weak acids with the same concentration, strong acid = lower p. H. • It is fully ionised. Pure hydrogen (Strong chlorideacid) Pure citric acid) (Weak HCl Cl- + H+ Dissolve each acid in water to make a 1 mol/dm 3 solution Completely ionised, so concentration of H+ ions = 1 mol/dm 3 Only partly ionised, so concentration of H+ ions = much less than 1 mol/dm 3 CH 3 COOH CH 3 COO- + H+

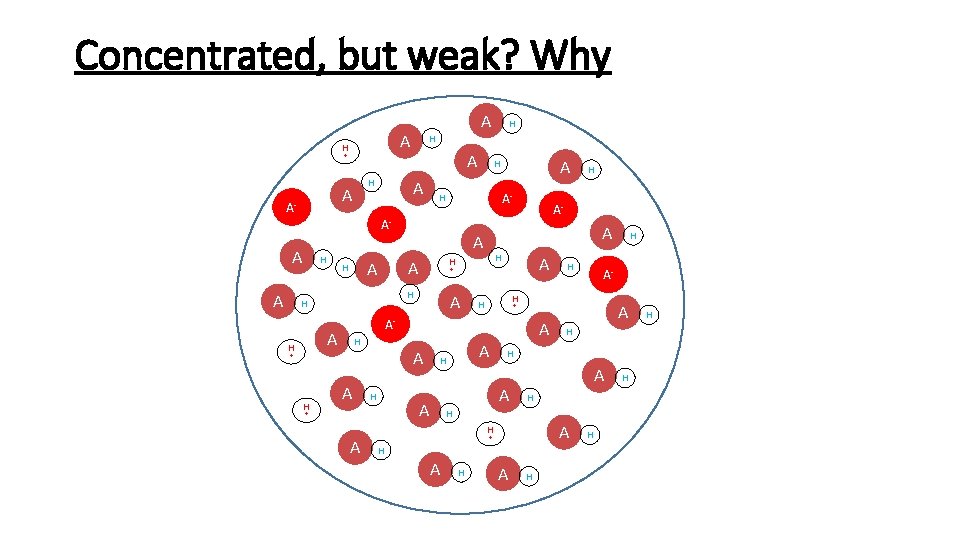
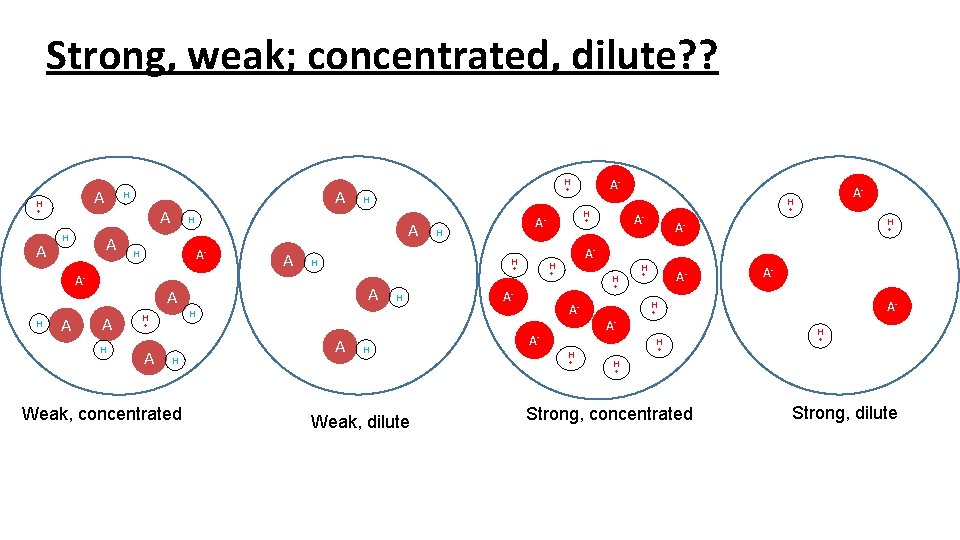
Concentrated, but weak? Why A H + H A A- A H A A A H H A- H H H A + A + H A A H H A H A + H A H H A- + A A + H H H A- A- A H A H H H H

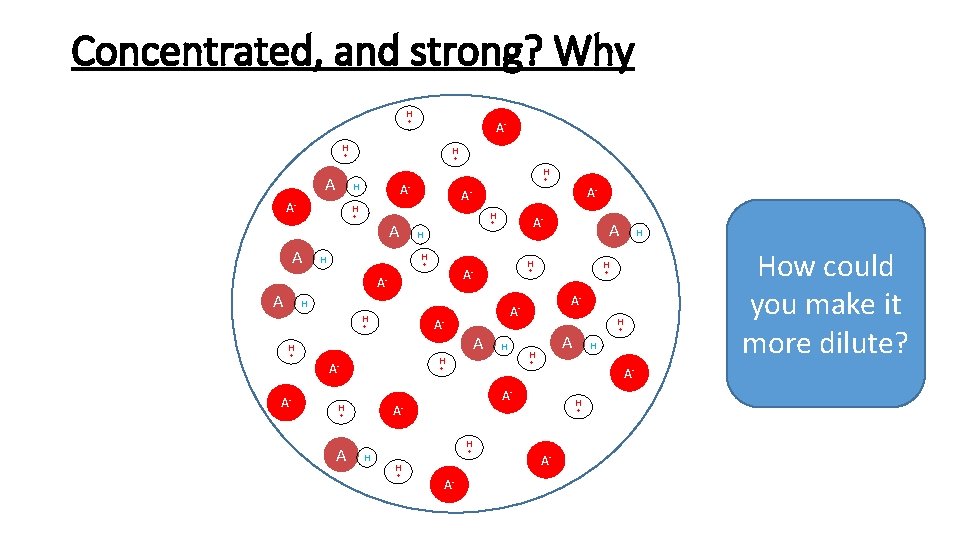
Concentrated, and strong? Why H + A- H H + + H A- + A- H H + A A A- + H H H + AA- H H + + H A- H H + A H A- A- + + H A H H A- How could you make it more dilute? + A- A- + H H A H A- A- A-

Strong or Weak? • Or – by observing the rate of reaction, when a reactive metal is added.

Strong, weak; concentrated, dilute? ? A H + A H A A A H H A H A- + A H Weak, concentrated A- H A- + + + AA- H + AH H H A- + A- H H + A- + H + A- H H A A- + A A- A A H H H AH A H H + + H + Weak, dilute Strong, concentrated Strong, dilute



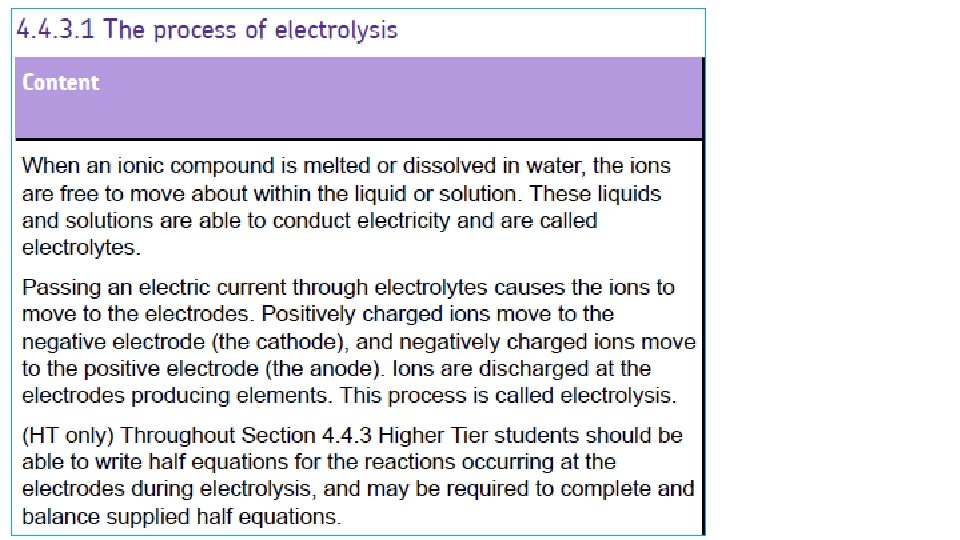
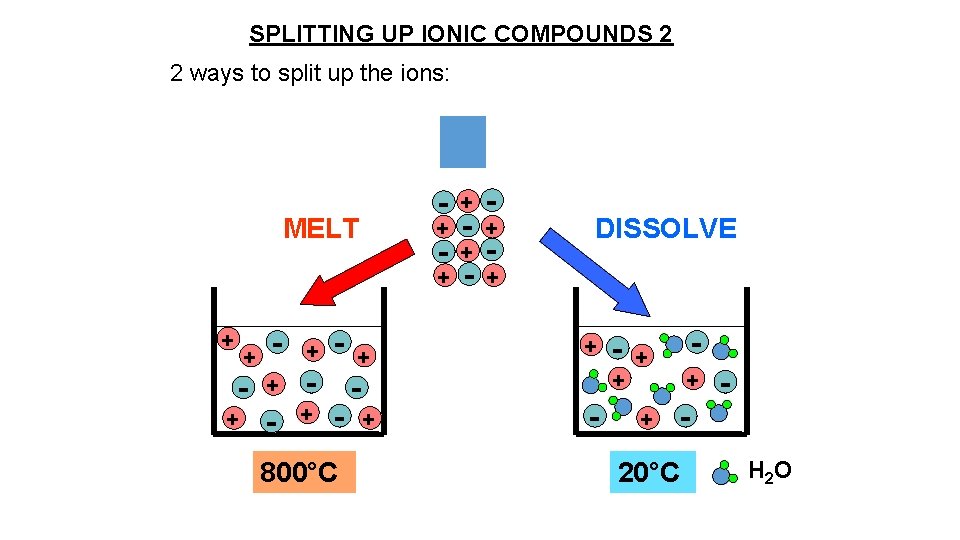
SPLITTING UP IONIC COMPOUNDS 2 2 ways to split up the ions: MELT + + - + - + 800°C - + + - - + - DISSOLVE + - + + + - - - + 20°C H 2 O

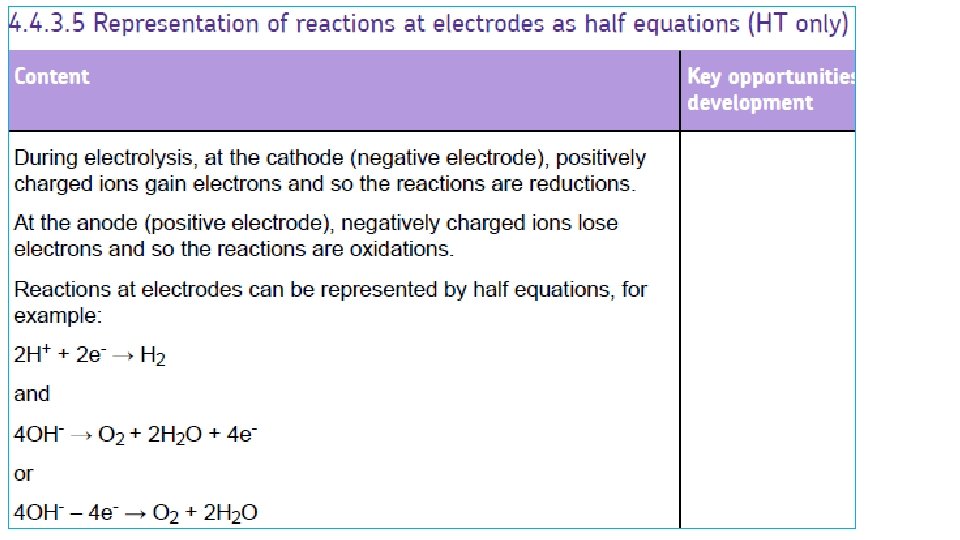
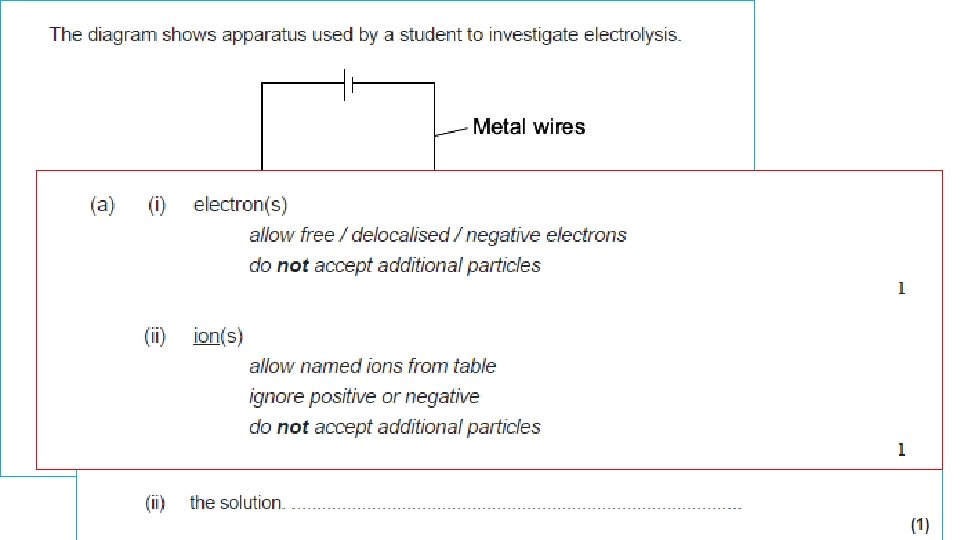
SEPARATING THE IONS 2 + MOLTEN IONIC COMPOUND - + ANODE + + - + + + - CATHODE When the battery is switched on, the + IONS move to the – CATHODE the – IONS move to the + ANODE This gives a way to SPLIT UP IONIC COMPOUNDS: “ELECTROLYSIS”


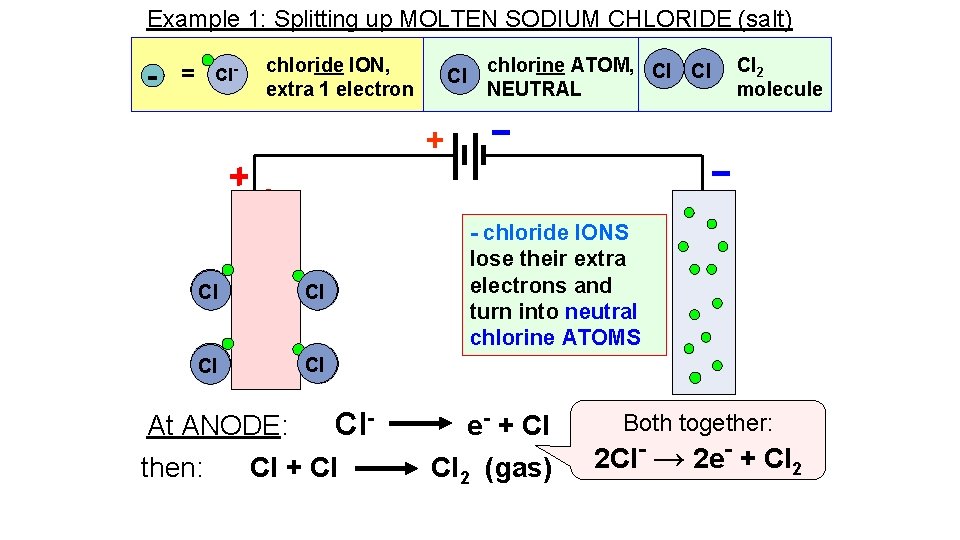
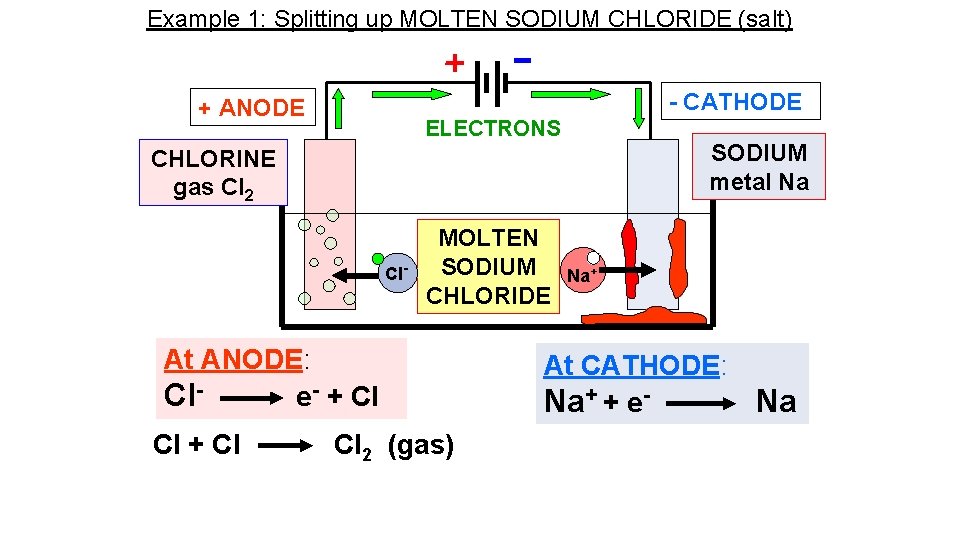
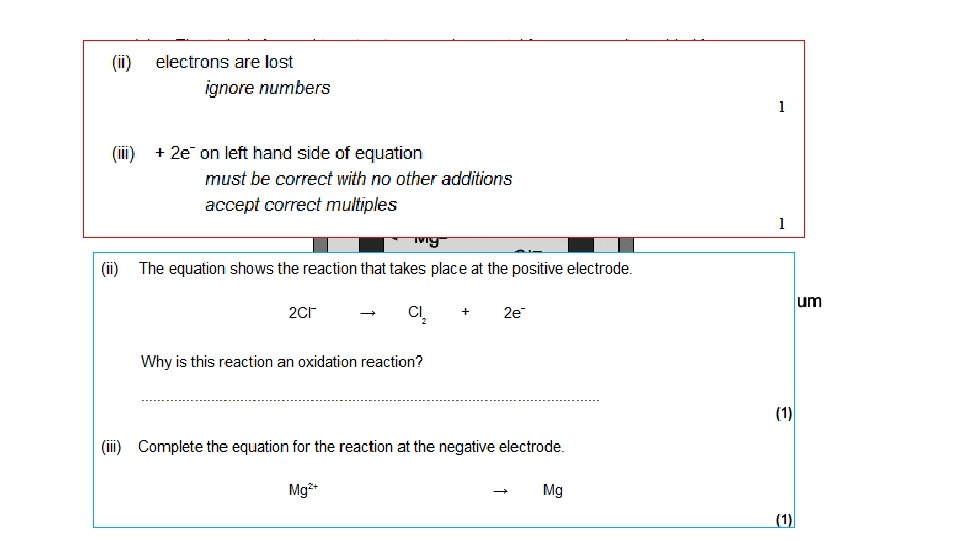
Example 1: Splitting up MOLTEN SODIUM CHLORIDE (salt) - = Cl- chloride ION, extra 1 electron Cl chlorine ATOM, Cl Cl NEUTRAL Cl 2 molecule + Cl Cl Cl- - Cl Cl At ANODE: Clthen: Cl + Cl - chloride IONS lose their extra electrons and turn into neutral chlorine ATOMS e- + Cl Cl 2 (gas) Both together: 2 Cl- → 2 e- + Cl 2

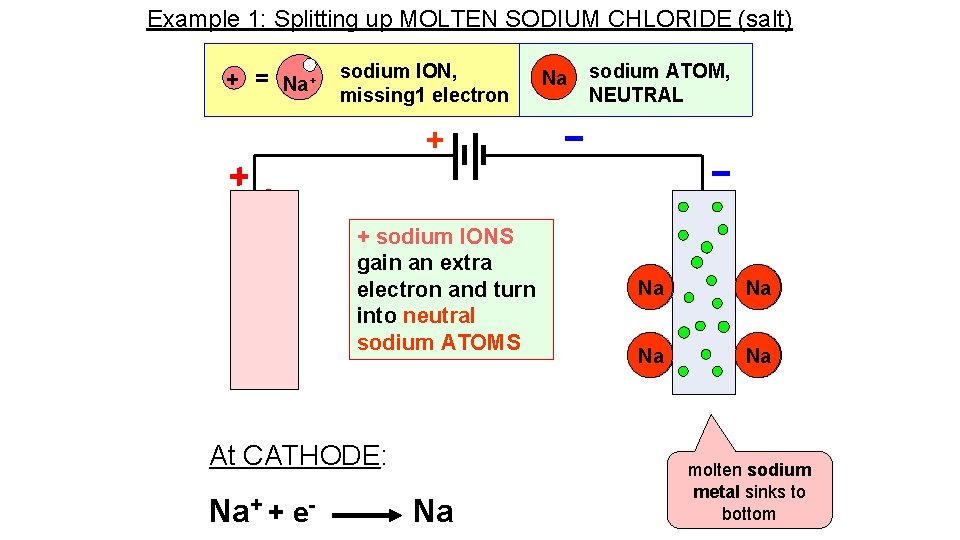
Example 1: Splitting up MOLTEN SODIUM CHLORIDE (salt) + = Na+ sodium ION, missing 1 electron Na sodium ATOM, NEUTRAL + + sodium IONS gain an extra electron and turn into neutral sodium ATOMS At CATHODE: Na+ + e- Na Na+ Na molten sodium metal sinks to bottom

Example 1: Splitting up MOLTEN SODIUM CHLORIDE (salt) - CATHODE + ANODE ELECTRONS SODIUM metal Na CHLORINE gas Cl 2 Cl- MOLTEN SODIUM CHLORIDE At ANODE: Cle- + Cl Cl 2 (gas) Na+ At CATHODE: Na+ + e- Na

OILRIG Cl- Cl- Oxidation is loss, reduction is gain ‘OILRIG’ Na+ Na+ - ions LOSING electrons + ions GAINING electrons to become atoms is called ‘OXIDATION’ ‘REDUCTION’ (even though oxygen may not be involved)

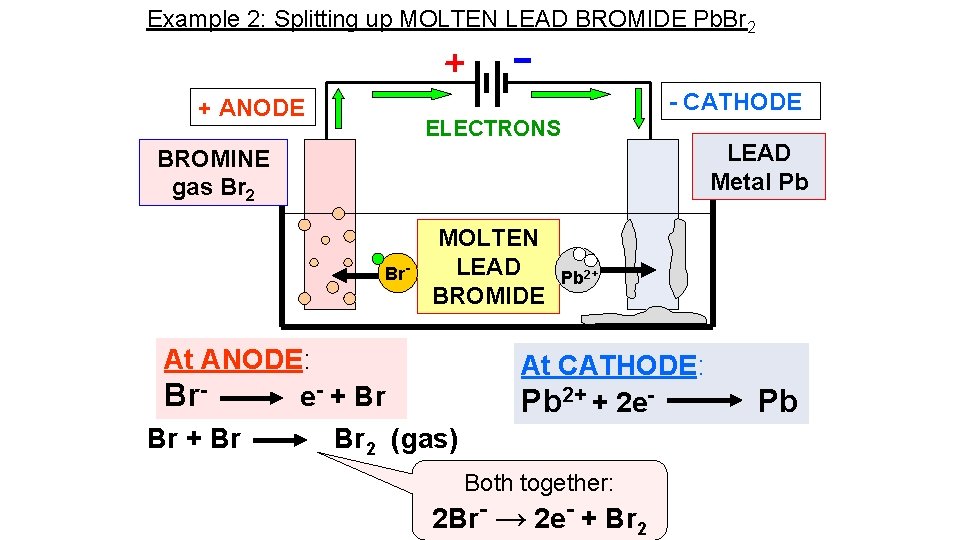
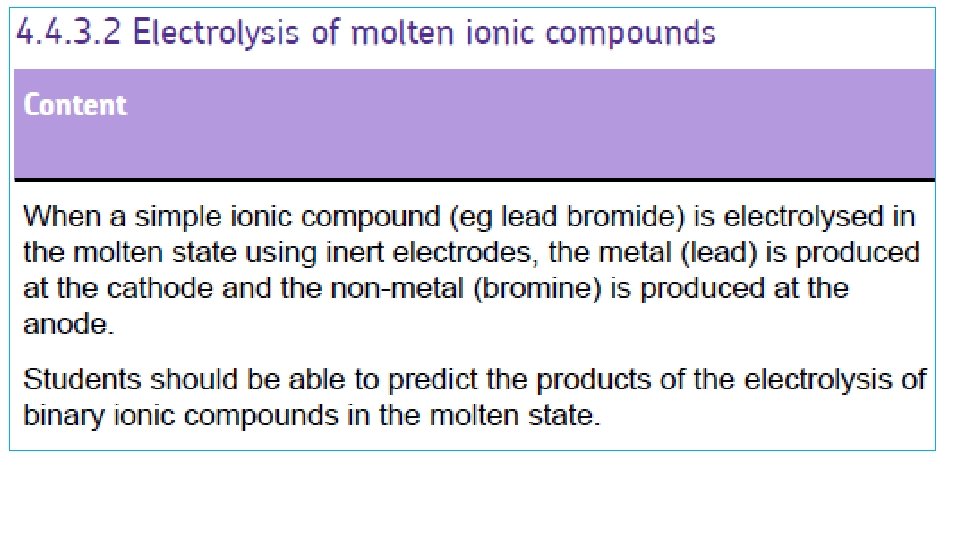
Example 2: Splitting up MOLTEN LEAD BROMIDE Pb. Br 2 - CATHODE + ANODE ELECTRONS LEAD Metal Pb BROMINE gas Br 2 Br- MOLTEN LEAD BROMIDE At ANODE: Bre- + Br Br 2 (gas) Pb 2+ At CATHODE: Pb 2+ + 2 e- Both together: 2 Br- → 2 e- + Br 2 Pb




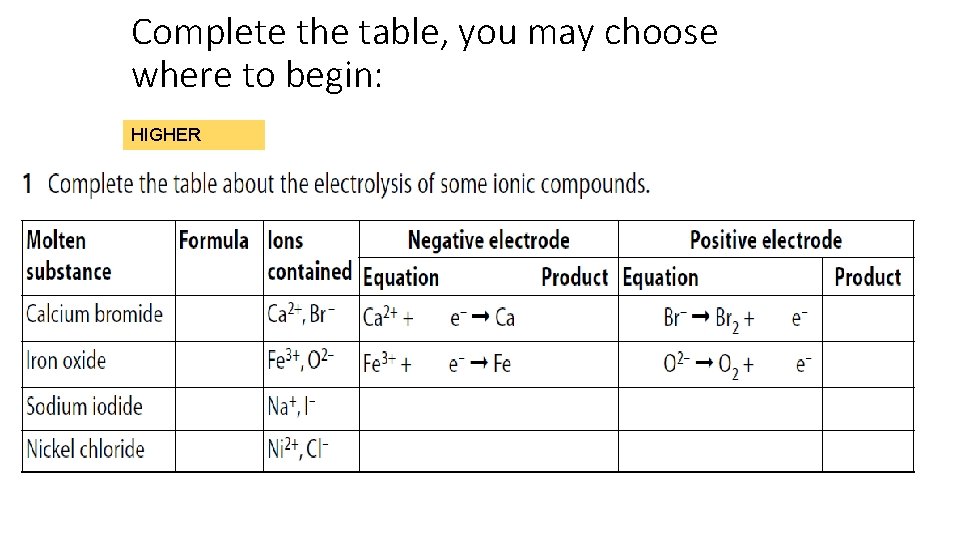
Complete the table, you may choose where to begin: HIGHER


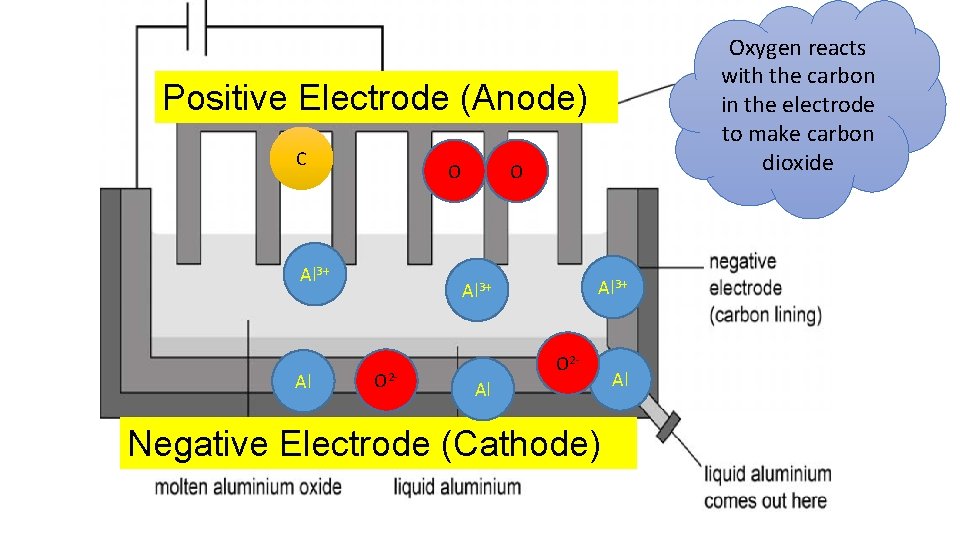
To reduce melting point

Oxygen reacts with the carbon in the electrode to make carbon dioxide Positive Electrode (Anode) C O Al 3+ O 2 - O 2 Al Negative Electrode (Cathode) Al


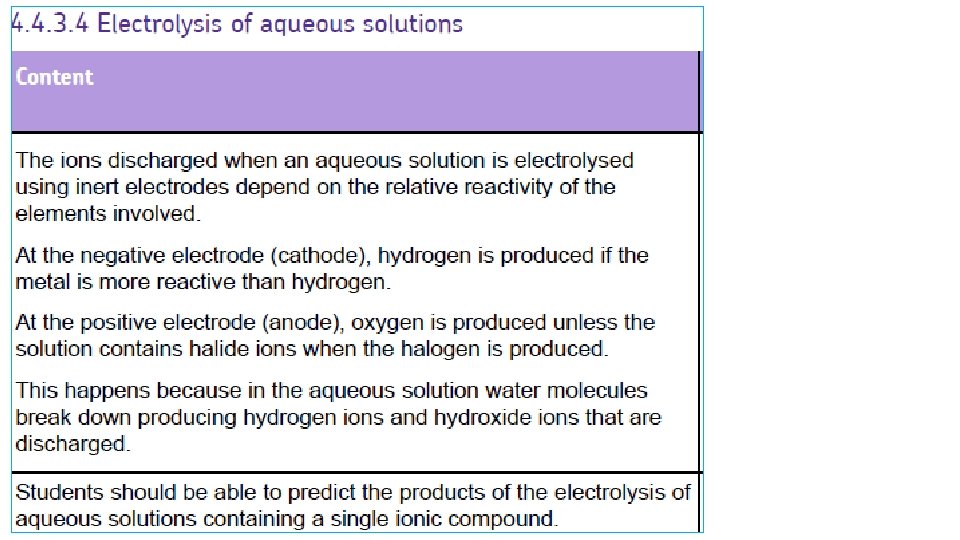
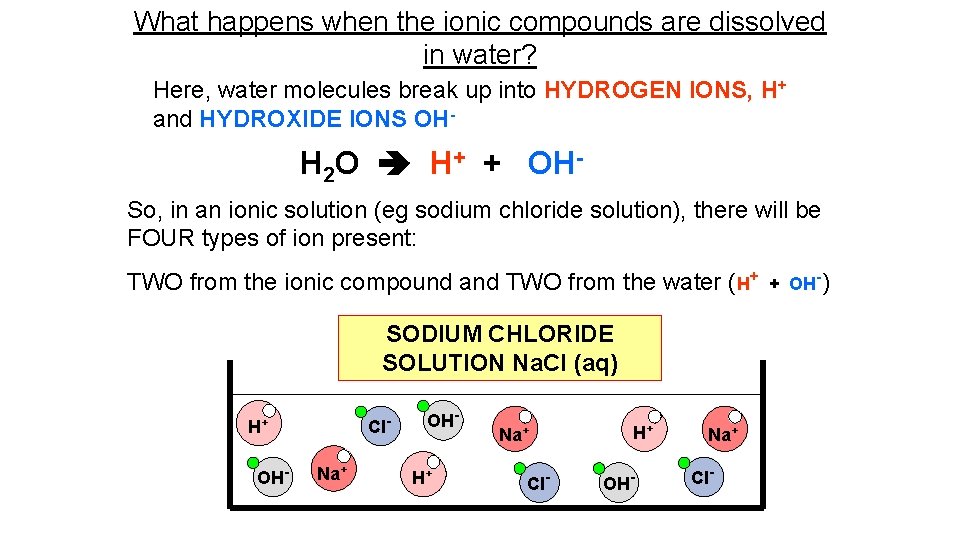
What happens when the ionic compounds are dissolved in water? Here, water molecules break up into HYDROGEN IONS, H+ and HYDROXIDE IONS OH- H 2 O H+ + OHSo, in an ionic solution (eg sodium chloride solution), there will be FOUR types of ion present: TWO from the ionic compound and TWO from the water (H+ SODIUM CHLORIDE SOLUTION Na. Cl (aq) Cl- H+ OH- Na+ OHH+ Na+ Cl- H+ OH- Na+ Cl- + OH-)

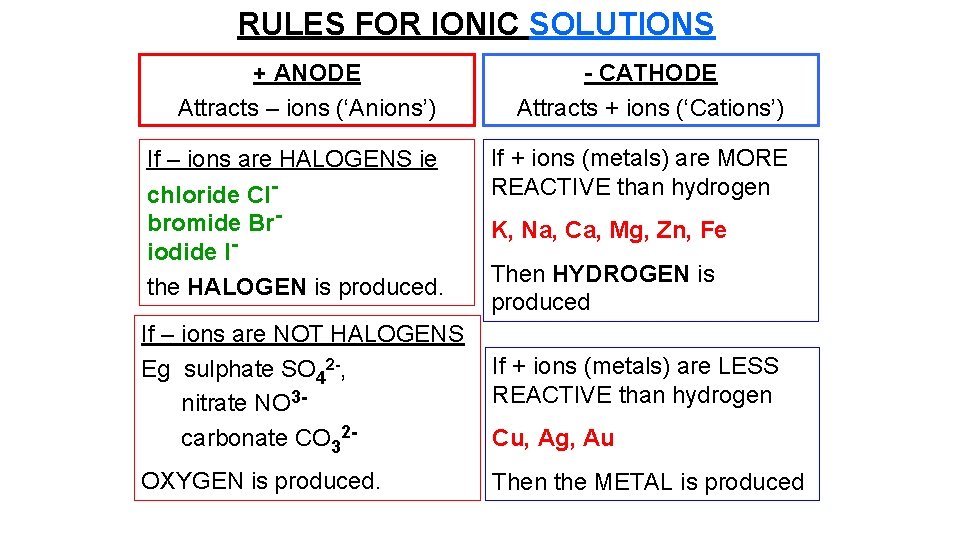
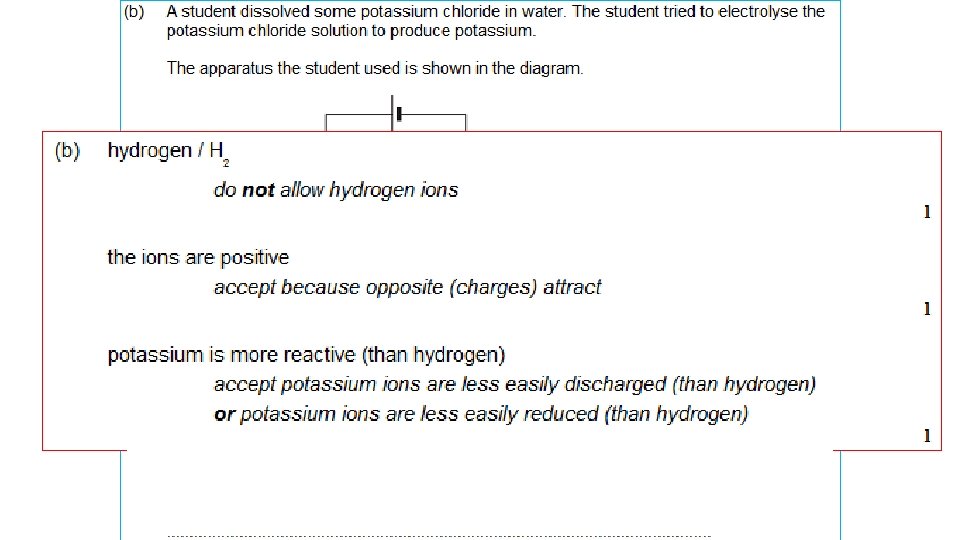
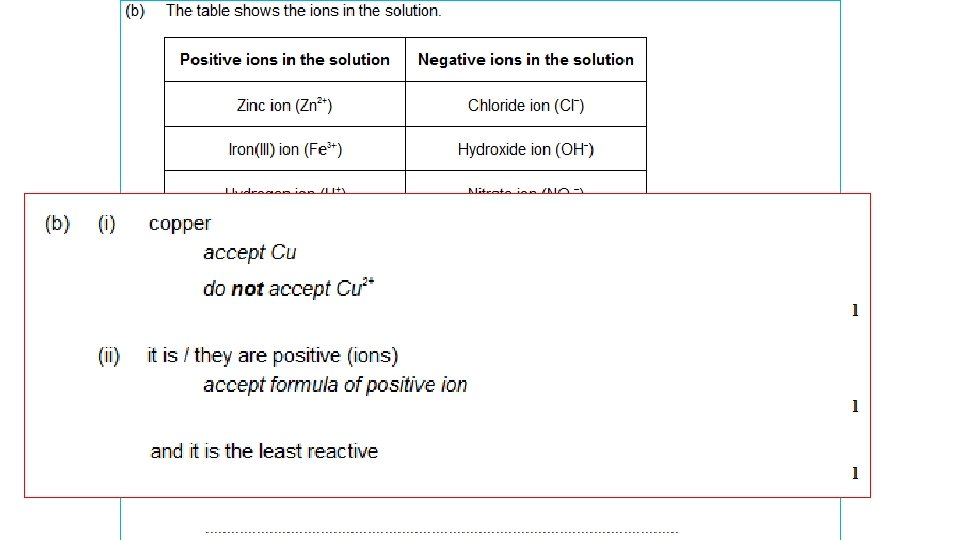
RULES FOR IONIC SOLUTIONS + ANODE Attracts – ions (‘Anions’) If – ions are HALOGENS ie chloride Clbromide Briodide Ithe HALOGEN is produced. - CATHODE Attracts + ions (‘Cations’) If + ions (metals) are MORE REACTIVE than hydrogen K, Na, Ca, Mg, Zn, Fe Then HYDROGEN is produced If – ions are NOT HALOGENS Eg sulphate SO 42 -, nitrate NO 3 carbonate CO 32 - If + ions (metals) are LESS REACTIVE than hydrogen OXYGEN is produced. Then the METAL is produced Cu, Ag, Au

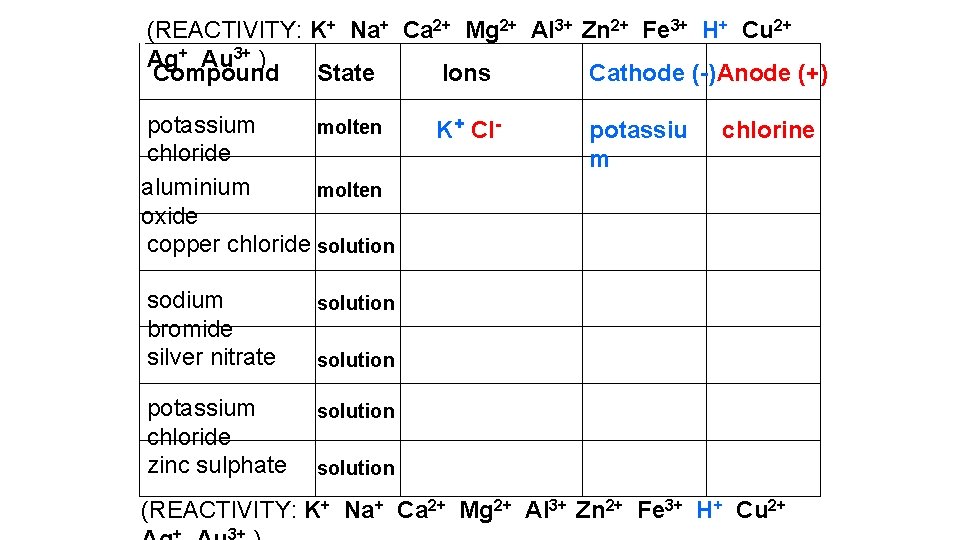
(REACTIVITY: K+ Na+ Ca 2+ Mg 2+ Al 3+ Zn 2+ Fe 3+ H+ Cu 2+ Ag+ Au 3+ ) Compound State Ions Cathode (-) Anode (+) potassium molten chloride aluminium molten oxide copper chloride solution sodium bromide silver nitrate solution potassium chloride zinc sulphate solution K+ Cl- potassiu m chlorine solution (REACTIVITY: K+ Na+ Ca 2+ Mg 2+ Al 3+ Zn 2+ Fe 3+ H+ Cu 2+ + 3+







 Basic oxides
Basic oxides Zinc and copper reaction
Zinc and copper reaction Empirical formula mgo
Empirical formula mgo Word equation of magnesium oxide
Word equation of magnesium oxide Dry oxidation vs wet oxidation
Dry oxidation vs wet oxidation Magnesium reacting with oxygen
Magnesium reacting with oxygen Nitrous oxide cylinder uses
Nitrous oxide cylinder uses Foncl chemistry
Foncl chemistry Metal oxide semiconductor field effect transistor
Metal oxide semiconductor field effect transistor Solid oxide fuel cell
Solid oxide fuel cell Mixed metal oxide anode
Mixed metal oxide anode Zinc oxide plaster
Zinc oxide plaster Denora smog
Denora smog Thin gate oxide
Thin gate oxide Word equation examples
Word equation examples Anisotropic vs isotropic etching
Anisotropic vs isotropic etching Ethylene oxide pcr tests
Ethylene oxide pcr tests Metal oxide semiconductor field effect transistor
Metal oxide semiconductor field effect transistor Nitrous oxide
Nitrous oxide Moscap
Moscap Hydrochloric acid and sodium hydroxide
Hydrochloric acid and sodium hydroxide Blanket field oxide
Blanket field oxide Oxide, peroxide superoxide formula
Oxide, peroxide superoxide formula Description
Description Oxide thickness color chart
Oxide thickness color chart Be2o compound name
Be2o compound name Salt + hydrogen gas
Salt + hydrogen gas Nitric oxide properties
Nitric oxide properties Blanket field oxide
Blanket field oxide Bradykinin nitric oxide
Bradykinin nitric oxide Double replacement example
Double replacement example Ecology unit review
Ecology unit review Ppo
Ppo Most inhalants are actually intended to be _______________
Most inhalants are actually intended to be _______________ The process described above led to
The process described above led to Lithium oxide ionic bonding diagram
Lithium oxide ionic bonding diagram Nitrous oxide
Nitrous oxide Pacific bio labs
Pacific bio labs Depressan
Depressan The boron family
The boron family Potassium oxide
Potassium oxide Zinc oxide eugenol bite registration
Zinc oxide eugenol bite registration Sio2 thickness color chart
Sio2 thickness color chart Chlorine shell configuration
Chlorine shell configuration Binary acid examples
Binary acid examples Native oxide
Native oxide Sodium carbonate reacts with hydrochloric acid
Sodium carbonate reacts with hydrochloric acid Ethylene oxide
Ethylene oxide Laboratory preparation of oxygen
Laboratory preparation of oxygen Palladium (iv) oxide formula
Palladium (iv) oxide formula Ionic compounds
Ionic compounds Aluminum and iron iii oxide balanced equation
Aluminum and iron iii oxide balanced equation Apex metal oxide coating
Apex metal oxide coating 29 cfr 1910
29 cfr 1910 Giant molecular structure vs simple molecular structure
Giant molecular structure vs simple molecular structure Ozone composition
Ozone composition Prime nitric oxide activator
Prime nitric oxide activator Beryllium oxide reaction with water
Beryllium oxide reaction with water Oxidation of alcohol
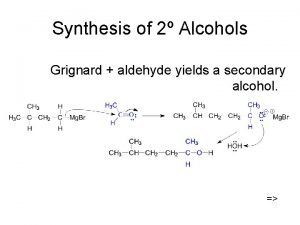
Oxidation of alcohol Oxide valency
Oxide valency Epoprotenol
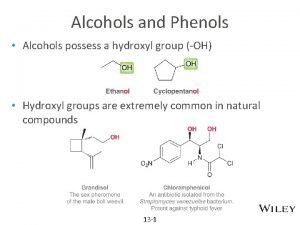
Epoprotenol Hydroxyl group
Hydroxyl group Elements with multiple oxidation states
Elements with multiple oxidation states How to combine two equations
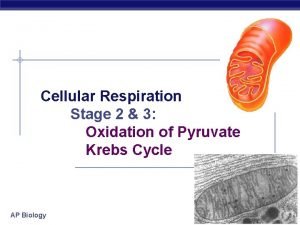
How to combine two equations Oxidation of pyruvate to acetyl coa
Oxidation of pyruvate to acetyl coa Leo the lion says ger
Leo the lion says ger Oxidation reduction
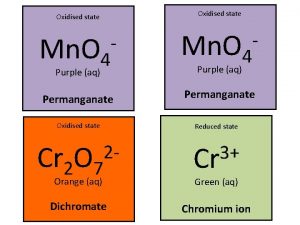
Oxidation reduction Reducing agent vs oxidizing agent
Reducing agent vs oxidizing agent Ipo4 oxidation number
Ipo4 oxidation number Kno3 salt bridge
Kno3 salt bridge Oxidation of food
Oxidation of food Reduction of alcohol to alkane
Reduction of alcohol to alkane Redox leo ger
Redox leo ger