Solids Image Wikimedia Commons User Alchemistryhp Types of

- Slides: 21

Solids Image: Wikimedia Commons User Alchemistry-hp

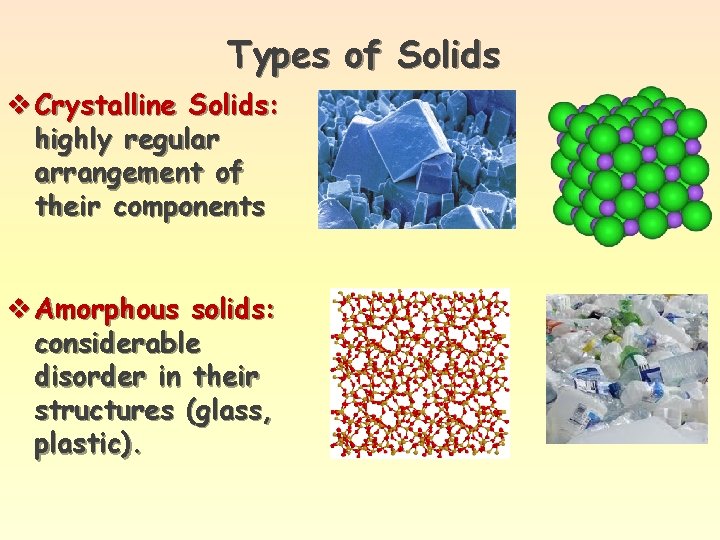
Types of Solids v Crystalline Solids: highly regular arrangement of their components v Amorphous solids: considerable disorder in their structures (glass, plastic).

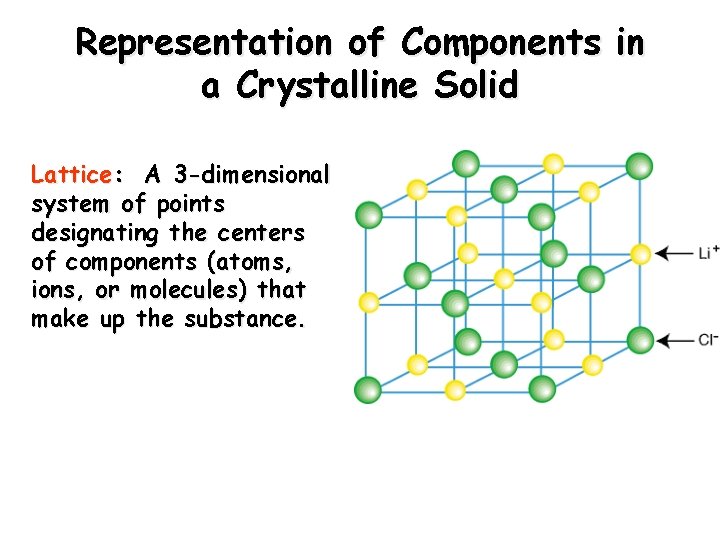
Representation of Components in a Crystalline Solid Lattice: A 3 -dimensional system of points designating the centers of components (atoms, ions, or molecules) that make up the substance.

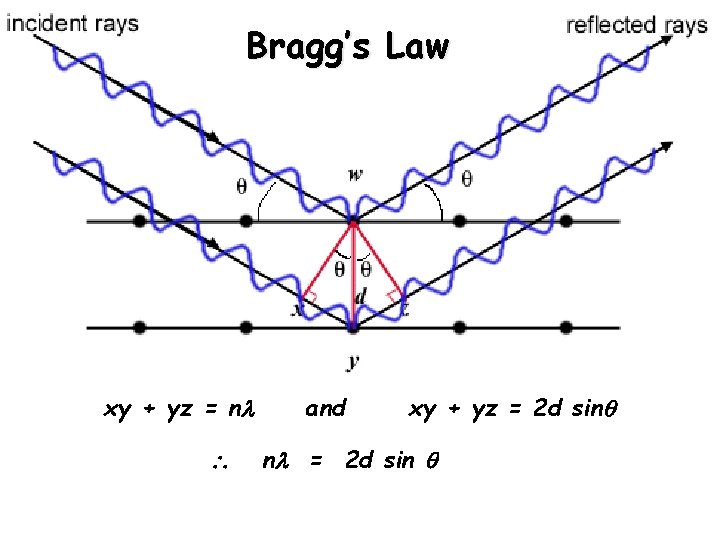
Bragg’s Law xy + yz = n and xy + yz = 2 d sin n = 2 d sin

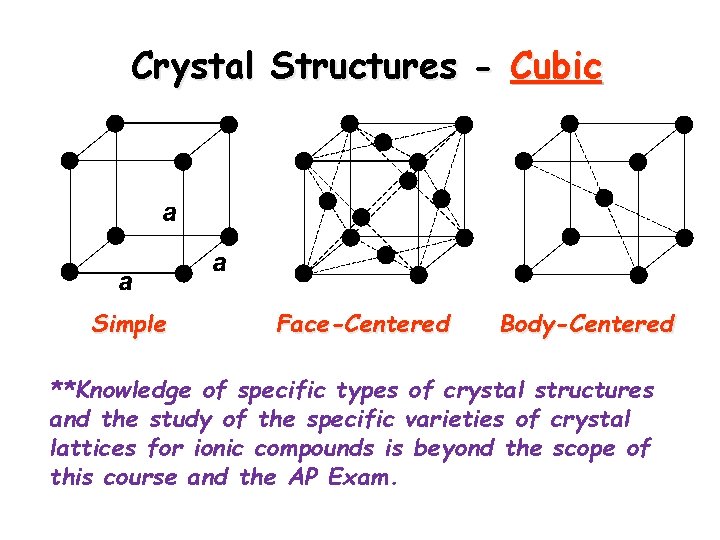
Crystal Structures - Cubic Simple Face-Centered Body-Centered **Knowledge of specific types of crystal structures and the study of the specific varieties of crystal lattices for ionic compounds is beyond the scope of this course and the AP Exam.

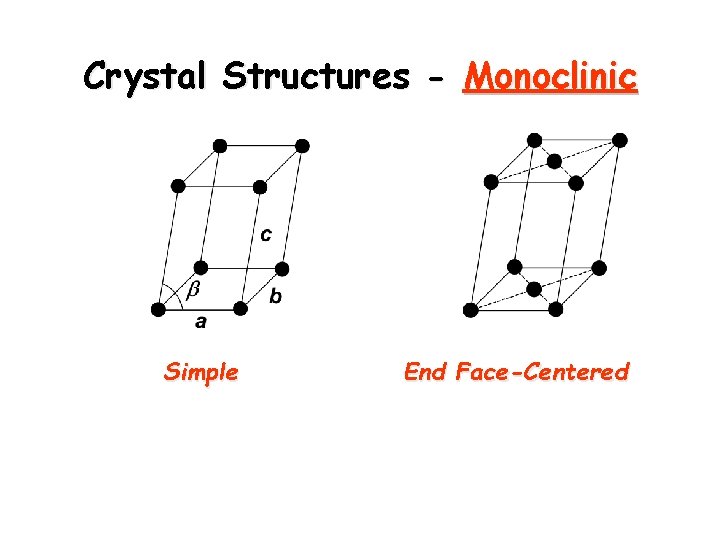
Crystal Structures - Monoclinic Simple End Face-Centered

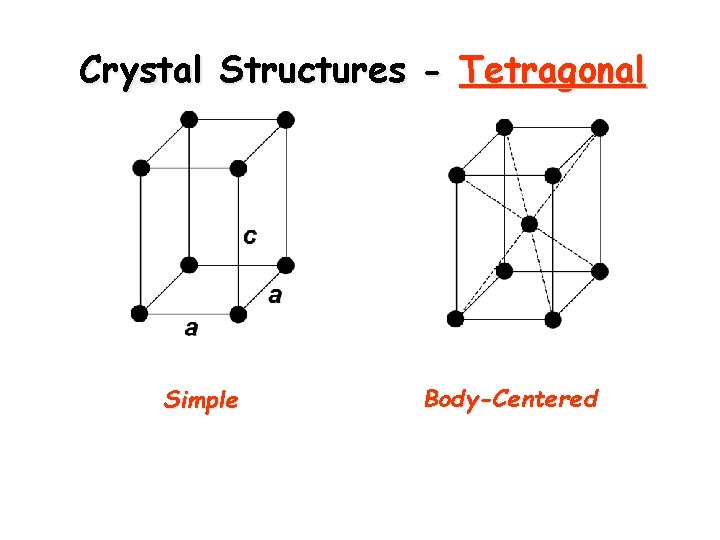
Crystal Structures - Tetragonal Simple Body-Centered

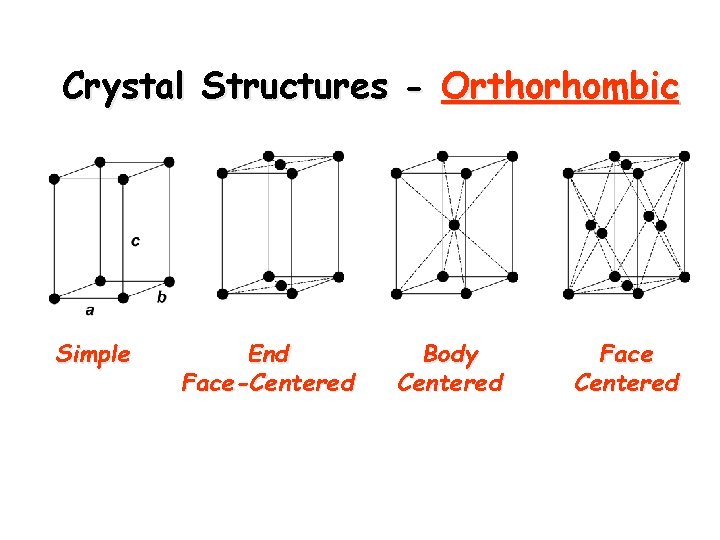
Crystal Structures - Orthorhombic Simple End Face-Centered Body Centered Face Centered

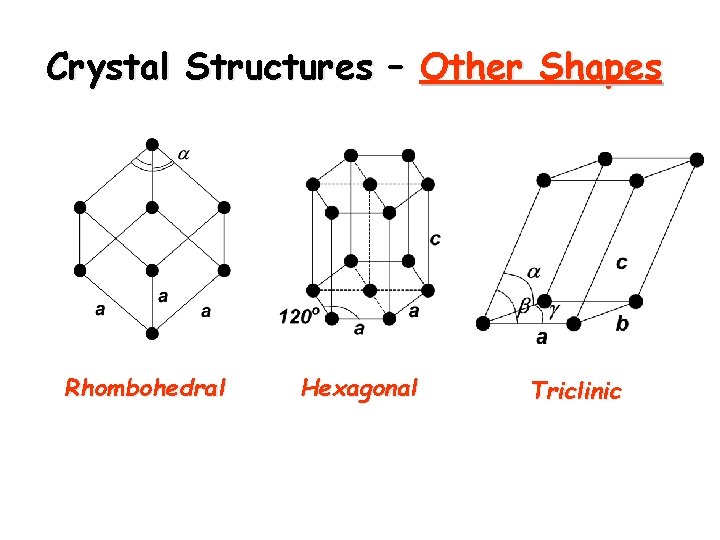
Crystal Structures – Other Shapes Rhombohedral Hexagonal Triclinic

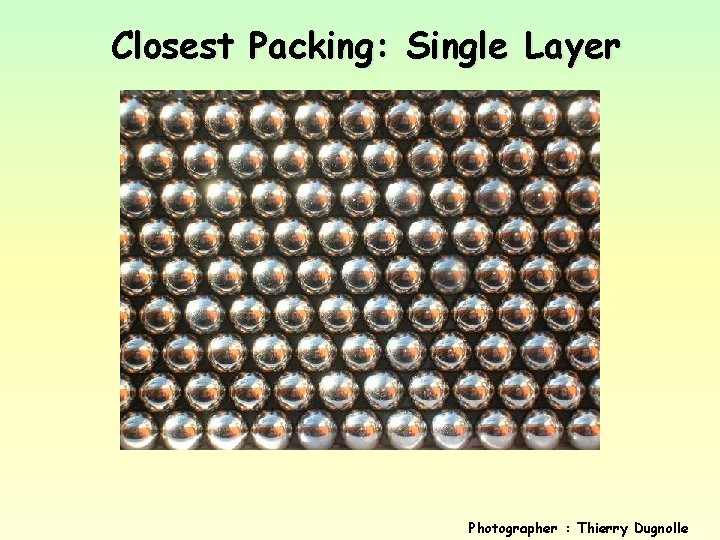
Closest Packing: Single Layer Photographer : Thierry Dugnolle

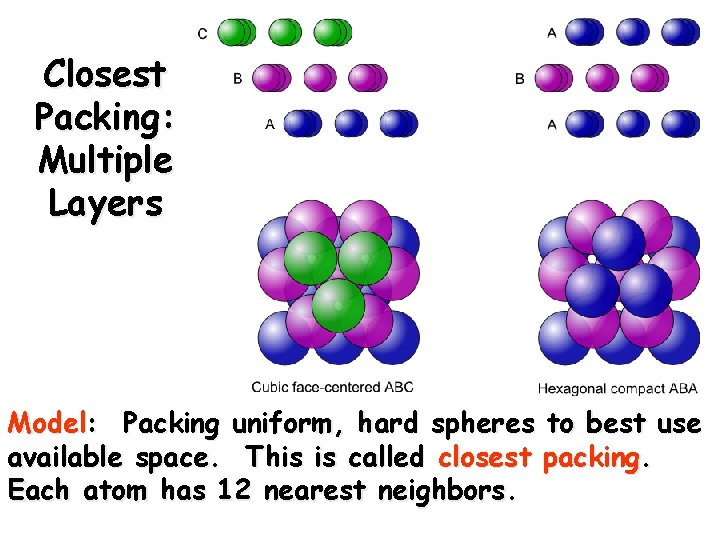
Closest Packing: Multiple Layers Model: Packing uniform, hard spheres to best use available space. This is called closest packing. Each atom has 12 nearest neighbors.

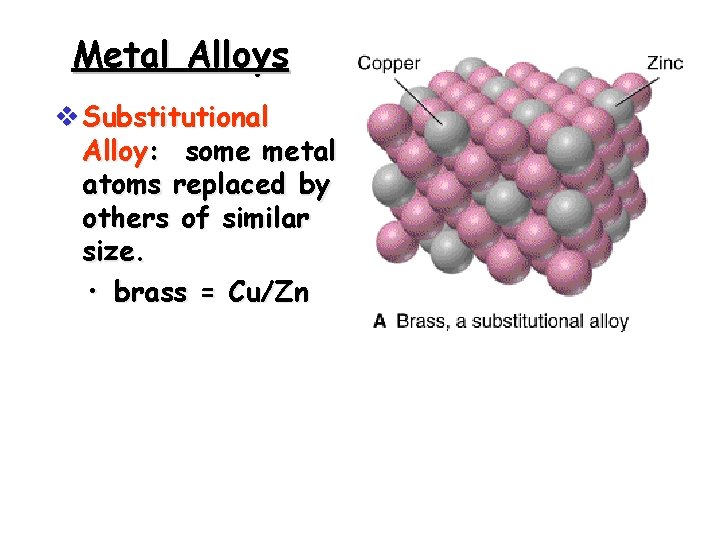
Metal Alloys v Substitutional Alloy: some metal atoms replaced by others of similar size. • brass = Cu/Zn

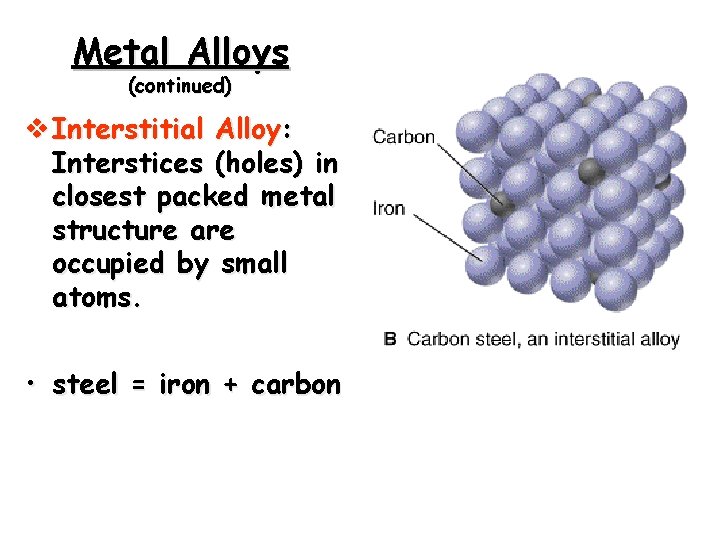
Metal Alloys (continued) v Interstitial Alloy: Interstices (holes) in closest packed metal structure are occupied by small atoms. • steel = iron + carbon

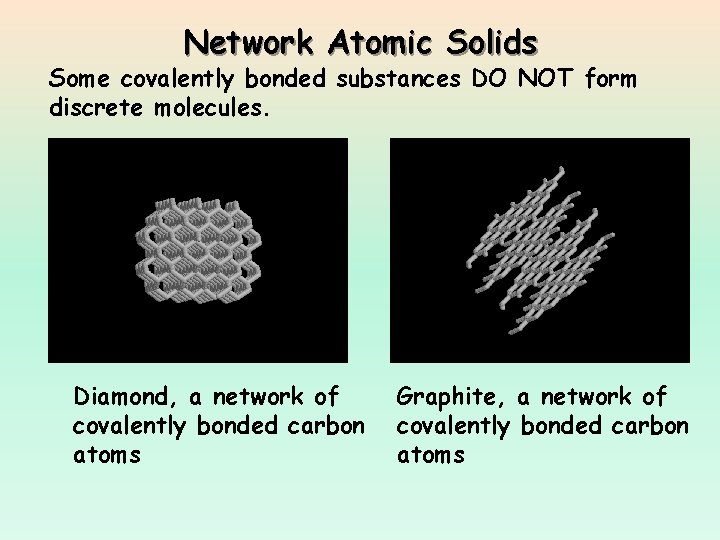
Network Atomic Solids Some covalently bonded substances DO NOT form discrete molecules. Diamond, a network of covalently bonded carbon atoms Graphite, a network of covalently bonded carbon atoms

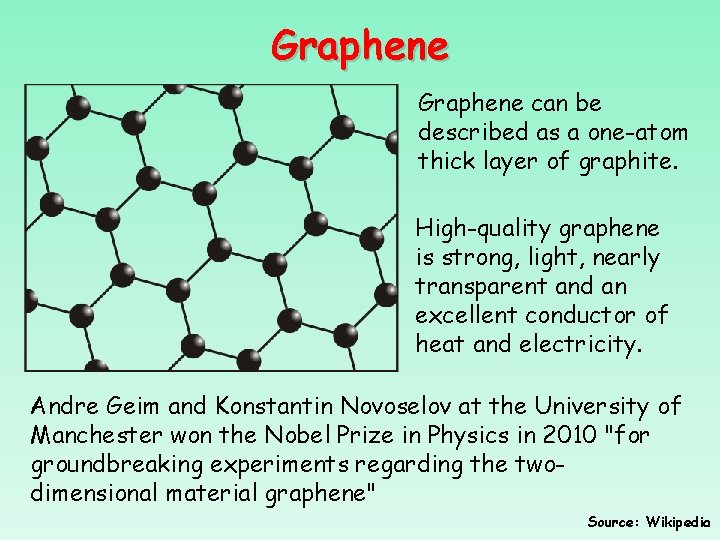
Graphene can be described as a one-atom thick layer of graphite. High-quality graphene is strong, light, nearly transparent and an excellent conductor of heat and electricity. Andre Geim and Konstantin Novoselov at the University of Manchester won the Nobel Prize in Physics in 2010 "for groundbreaking experiments regarding the twodimensional material graphene" Source: Wikipedia

Semiconductors Pure silicon is structurally the same as diamond, but is a semiconductor rather than an insulator. q The conductivity increases at higher temperature. q Conductivity of silicon can be improved by doping with other elements.

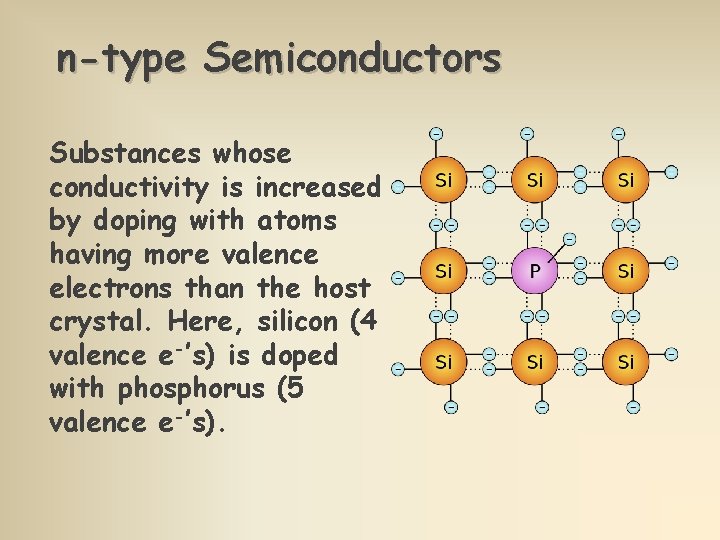
n-type Semiconductors Substances whose conductivity is increased by doping with atoms having more valence electrons than the host crystal. Here, silicon (4 valence e-’s) is doped with phosphorus (5 valence e-’s).

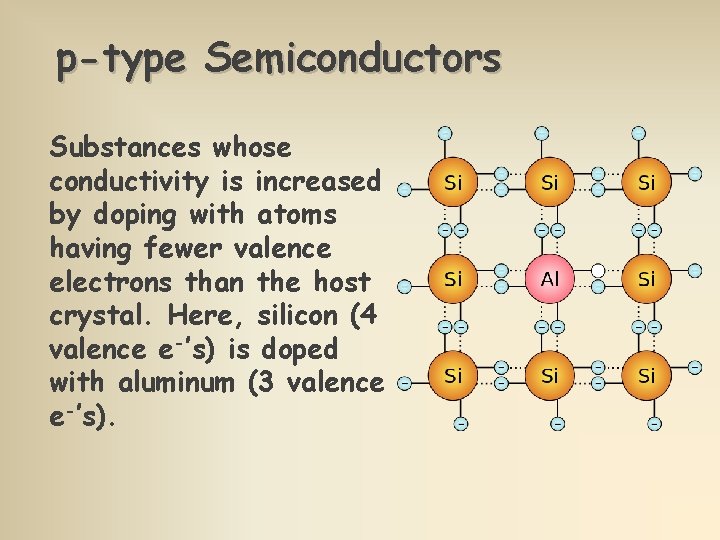
p-type Semiconductors Substances whose conductivity is increased by doping with atoms having fewer valence electrons than the host crystal. Here, silicon (4 valence e-’s) is doped with aluminum (3 valence e-’s).

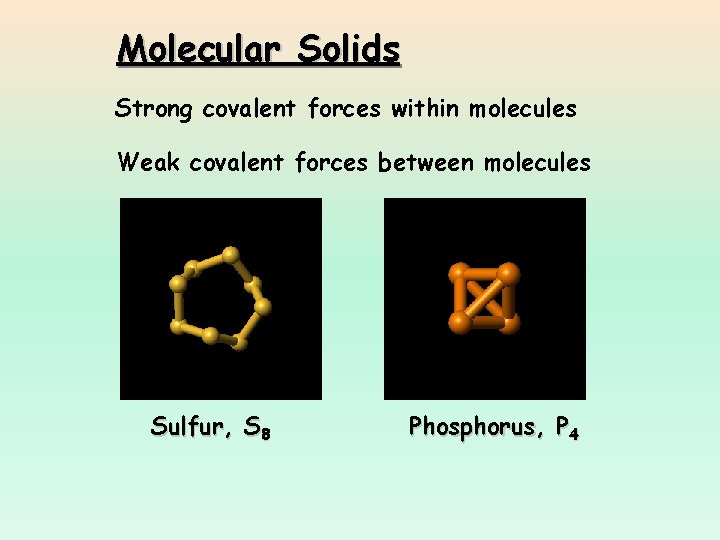
Molecular Solids Strong covalent forces within molecules Weak covalent forces between molecules Sulfur, S 8 Phosphorus, P 4

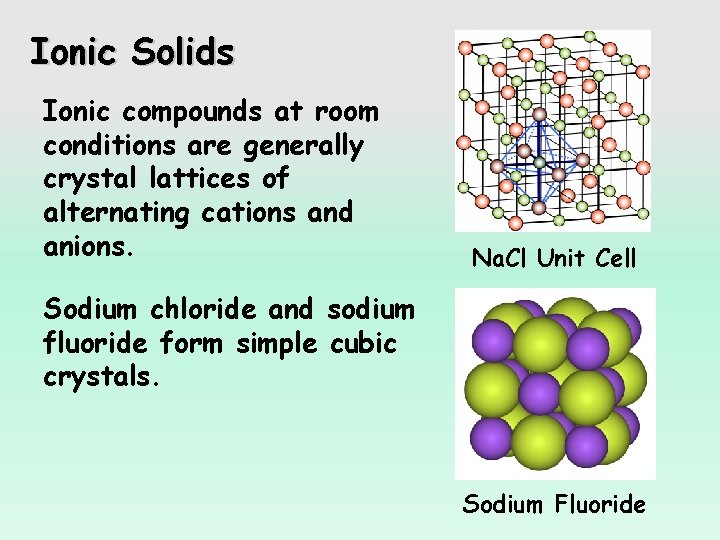
Ionic Solids Ionic compounds at room conditions are generally crystal lattices of alternating cations and anions. Na. Cl Unit Cell Sodium chloride and sodium fluoride form simple cubic crystals. Sodium Fluoride

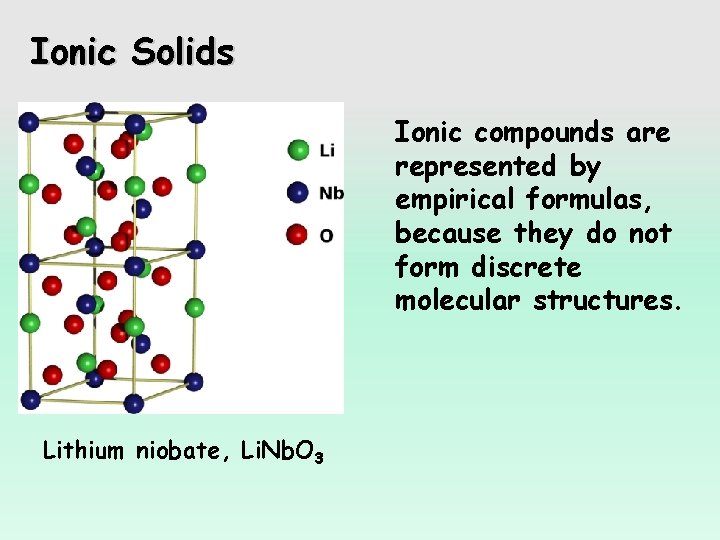
Ionic Solids Ionic compounds are represented by empirical formulas, because they do not form discrete molecular structures. Lithium niobate, Li. Nb. O 3