NearInfrared Spectroscopy LipidRich Plaque Study Update Ron Waksman








- Slides: 21

Near-Infrared Spectroscopy: Lipid-Rich Plaque Study Update Ron Waksman, MD Professor of Medicine, (Cardiology) Georgetown University Director, Cardiovascular Research Advanced Education, Med. Star Heart & Vascular Institute, Washington DC

Disclosure Statement of Financial Interest Within the past 12 months, I or my spouse/partner have had a financial interest/arrangement or affiliation with the organization(s) listed below. Affiliation/Financial Relationship • Grant/Research Support • Infraredx (for this study) • Consulting Fees/Honoraria Company • • • Boston Scientific Biotronik Biosensors Astra Zeneca Medtronic Vascular Abbott Vascular Symetis Med Alliance Life. Tech Amgen

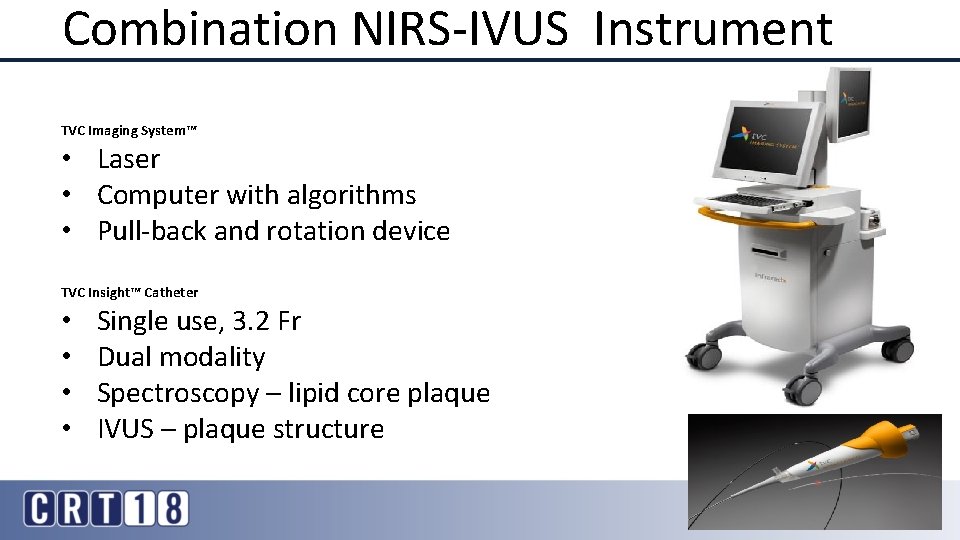
Combination NIRS-IVUS Instrument TVC Imaging System™ • Laser • Computer with algorithms • Pull-back and rotation device TVC Insight™ Catheter • • Single use, 3. 2 Fr Dual modality Spectroscopy – lipid core plaque IVUS – plaque structure 3

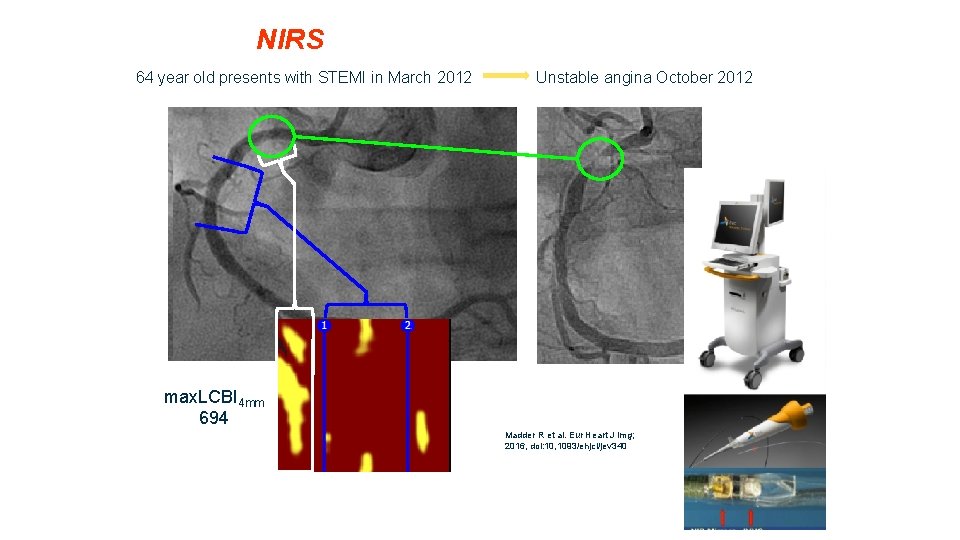
NIRS 64 year old presents with STEMI in March 2012 Unstable angina October 2012 max. LCBI 4 mm 694 Madder R et al. Eur Heart J Img; 2016, doi: 10, 1093/ehjci/jev 340

Cardiac Arrest in 5 K Race associated with LAD Stenosis and a Circular Lipid Core Plaque Ventricular Fibrillation Courtesy Dr. Madder et al, JIC, In press. R

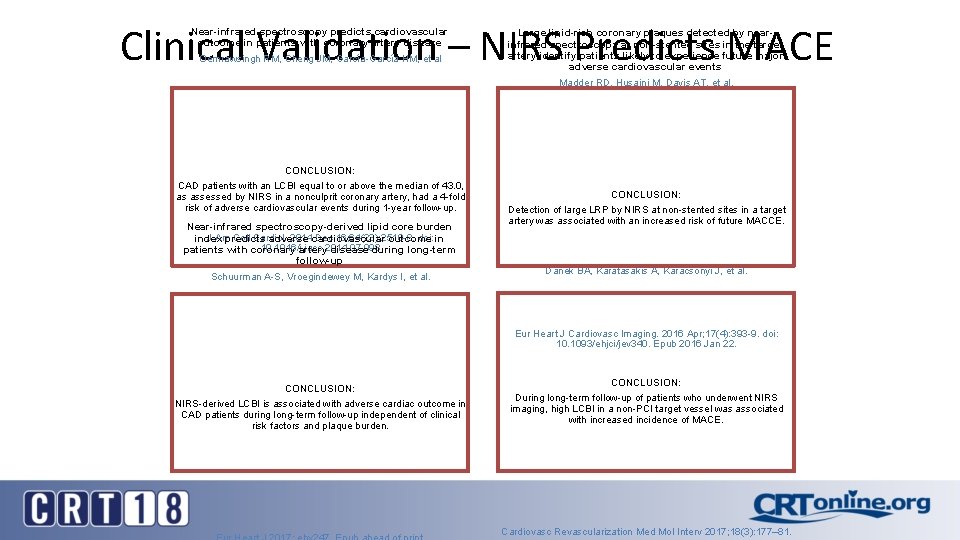
Clinical Validation – NIRS Predicts MACE Near-infrared spectroscopy predicts cardiovascular outcome in patients with coronary artery disease Oemrawsingh RM, Cheng JM, García-García HM, et al CONCLUSION: CAD patients with an LCBI equal to or above the median of 43. 0, as assessed by NIRS in a nonculprit coronary artery, had a 4 -fold risk of adverse cardiovascular events during 1 -year follow-up. Near-infrared spectroscopy-derived lipid core burden J Am Coll Cardiol. 2014 Dec 16; 64(23): 2510 -8. doi: index predicts adverse cardiovascular outcome in 10. 1016/j. jacc. 2014. 07. 998 patients with coronary artery disease during long-term follow-up Schuurman A-S, Vroegindewey M, Kardys I, et al. Large lipid-rich coronary plaques detected by nearinfrared spectroscopy at non-stented sites in the target artery identify patients likely to experience future major adverse cardiovascular events Madder RD, Husaini M, Davis AT, et al. CONCLUSION: Detection of large LRP by NIRS at non-stented sites in a target artery was associated with an increased risk of future MACCE. Long-term follow-up after near-infrared spectroscopy coronary imaging: Insights from the lipid c. ORe plaque association with CLinical events (ORACLE-NIRS) registry Danek BA, Karatasakis A, Karacsonyi J, et al. Eur Heart J Cardiovasc Imaging. 2016 Apr; 17(4): 393 -9. doi: 10. 1093/ehjci/jev 340. Epub 2016 Jan 22. CONCLUSION: NIRS-derived LCBI is associated with adverse cardiac outcome in CAD patients during long-term follow-up independent of clinical risk factors and plaque burden. CONCLUSION: During long-term follow-up of patients who underwent NIRS imaging, high LCBI in a non-PCI target vessel was associated with increased incidence of MACE. Cardiovasc Revascularization Med Mol Interv 2017; 18(3): 177– 81.

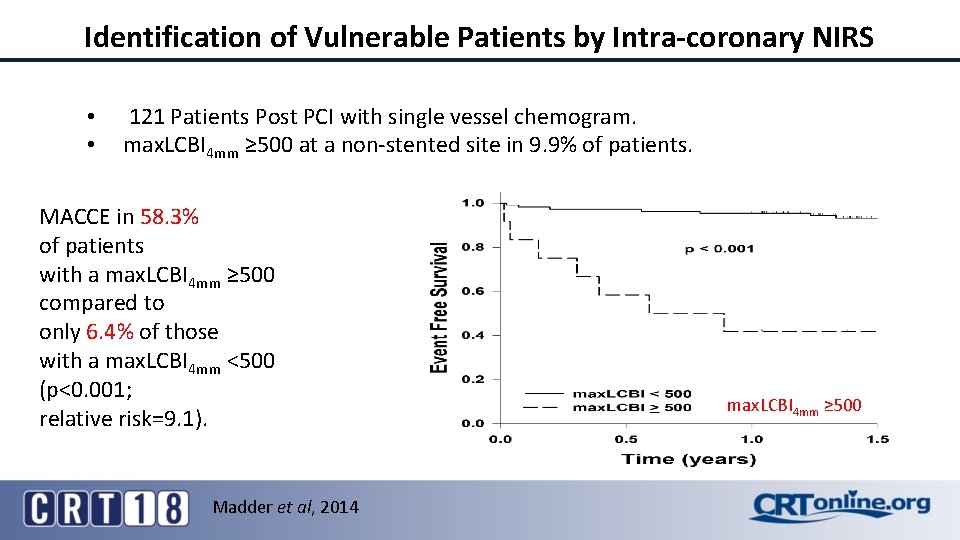
Identification of Vulnerable Patients by Intra-coronary NIRS • • 121 Patients Post PCI with single vessel chemogram. max. LCBI 4 mm ≥ 500 at a non-stented site in 9. 9% of patients. MACCE in 58. 3% of patients with a max. LCBI 4 mm ≥ 500 compared to only 6. 4% of those with a max. LCBI 4 mm <500 (p<0. 001; relative risk=9. 1). Madder et al, 2014 max. LCBI 4 mm ≥ 500

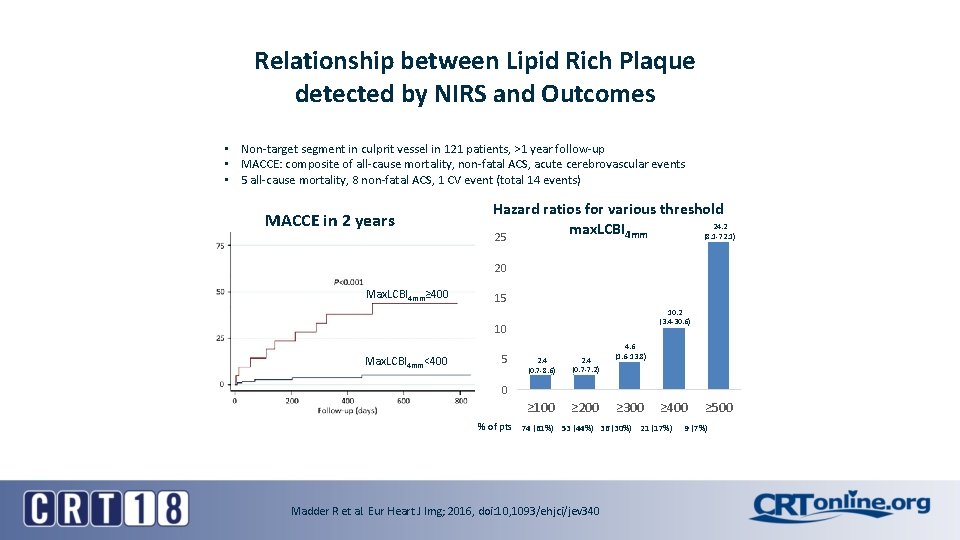
Relationship between Lipid Rich Plaque detected by NIRS and Outcomes • Non-target segment in culprit vessel in 121 patients, >1 year follow-up • MACCE: composite of all-cause mortality, non-fatal ACS, acute cerebrovascular events • 5 all-cause mortality, 8 non-fatal ACS, 1 CV event (total 14 events) MACCE in 2 years Hazard ratios for various threshold 24. 2 max. LCBI 4 mm (8. 1 -72. 1) 25 20 Max. LCBI 4 mm≥ 400 15 10. 2 (3. 4 -30. 6) 10 Max. LCBI 4 mm<400 5 2. 4 (0. 7 -8. 6) 2. 4 (0. 7 -7. 2) ≥ 100 ≥ 200 4. 6 (1. 6 -13. 8) 0 ≥ 300 ≥ 400 % of pts 74 (61%) 53 (44%) 36 (30%) 21 (17%) Madder R et al. Eur Heart J Img; 2016, doi: 10, 1093/ehjci/jev 340 ≥ 500 9 (7%)

LCBI in Non-culprit Artery Identifies: Vulnerable Patients • Prospective Single Center Study, 206 patients (ACS 47%) • Primary Endpoint: Composite of all-cause mortality, non-fatal ACS, stroke and unplanned PCI, >40 mm non culprit segment of NIRS LCBI in Non-culprit Artery Above the Median LCBI = 43 Rohit M. Oemrawsingh, MD, ET AL. JACC VOL. 6 4 , NO. 2 3 , 2014



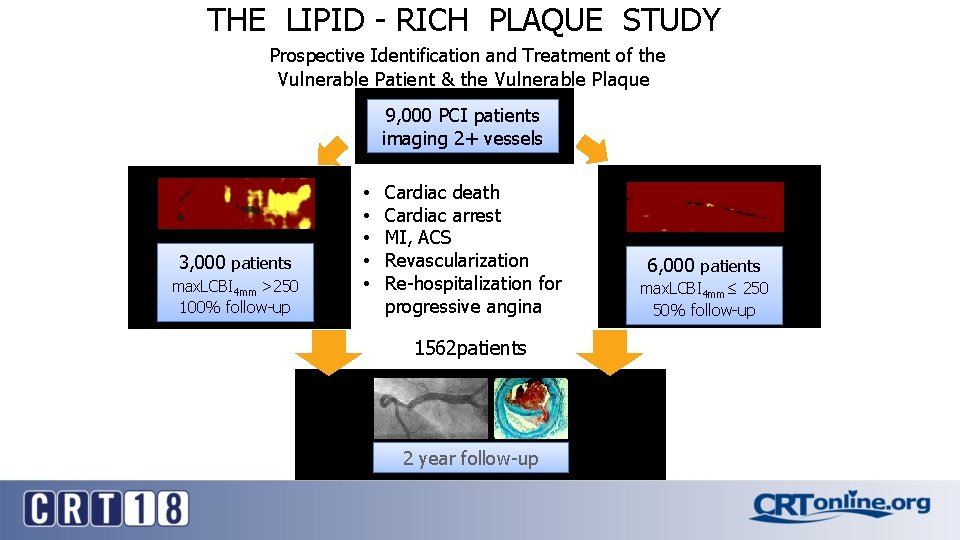
THE LIPID - RICH PLAQUE STUDY Prospective Identification and Treatment of the Vulnerable Patient & the Vulnerable Plaque 9, 000 PCI patients imaging 2+ vessels 3, 000 patients max. LCBI 4 mm >250 100% follow-up • • • Cardiac death Cardiac arrest MI, ACS Revascularization Re-hospitalization for progressive angina 1562 patients 2 year follow-up 6, 000 patients max. LCBI 4 mm≤ 250 50% follow-up

Active Sites and Research Staff • • • • • Washington Hospital Center: Dr. Waksman, J. Lancaster Crittenton Hospital: Dr. Kazziha, M. Cribbs St. John Hospital, Detroit: Dr. Lalonde, T. Jacobson Charleston Area Medical Center: Dr. Lewis, J. Hogan Columbia University Medical Center: Dr. Ali, L. Jaquez Central Baptist Hospital: Dr. Skinner, J. Chapman St. John’s Hospital, Springfield: Dr. Goswami, S. Smith, J. Davis Emory University Hospital: Dr. Samady, E. Rasoul-Arzrumly Methodist Hospital: Dr. Artis, K. Armstrong Medical University of South Carolina: Dr. Powers, L. Carson Community Heart and Vascular Hospital: Dr. Dube, J. Greene-Nashold St. Luke’s Hospital: Dr. Walton, G. De. Freitas Davis Medical Center: Dr. Kim, E. Mansell Mc. Laren Macomb Hospital: Dr. Zainea, T. Gardner-Mosley Palm Beach Gardens Medical Center: Dr. Villa, E. Wettermann University of Minnesota Medical Center: Dr. Raveendran, E. Caldwell Amsterdam Medical Center: Dr. Wykrzykowska, R. Kraak Mc. Laren Bay Region: Dr. Lee, C. Quart Alexian Brothers Heart & Vascular Institute: Dr. Pop, E. Enger Northshore-LIJ Health System: Dr. Singh, P. Chu, G. Chan, M. Hyland • • • • • • Florida Hospital Orlando: Dr. Arias, K. Mink Latvian Centre of Cardiology: Dr. Erglis, S. Jegere University of California Los Angeles Medical Center : Dr. Tobis, L. Douangvila ACRC Cardiology/JFK Medical Center: Dr. Lovitz, J. Mitten Hillcrest Oklahoma Heart Institute: Dr. Leimbach, J. Durham Palmetto General Hospital: Dr. Diaz, R. Perez Delray Medical Center: Dr. Carida, P. Bech Emory Midtown: Dr. Liberman, T. Sanders Royal Brompton Hospital: Dr. Di Mario, D. Dempster New York Presbyterian Hospital – Cornell: Dr. Wong, H. Piscitell University of Texas Galveston: Dr. Fujise, S. Ronald Radboud University Medical Centre: Dr. ten Cate, I. Vereussel SUSCCH, Slovakia: Dr. Hudec, Zeleznikova Maasstad Ziekenhuis: Dr. van der Ent, C. van Vliet Erasmus Medical Center: Dr. Regar, E. Huijskens University of Edinburgh: Dr. Newby, L. Flint Metrohealth Hospital: Dr. Hodgson, J. Nichols Memorial West: Dr. Tami, M. Abdurrahman Heart Hospital Plano: Dr. Potluri, J. Mc. Cracken Golden Jubilee National Hospital: Dr. Mc. Entegart, E. Boyd San Giovanni Hospital: Dr. Prati, Dr. Imola Continued

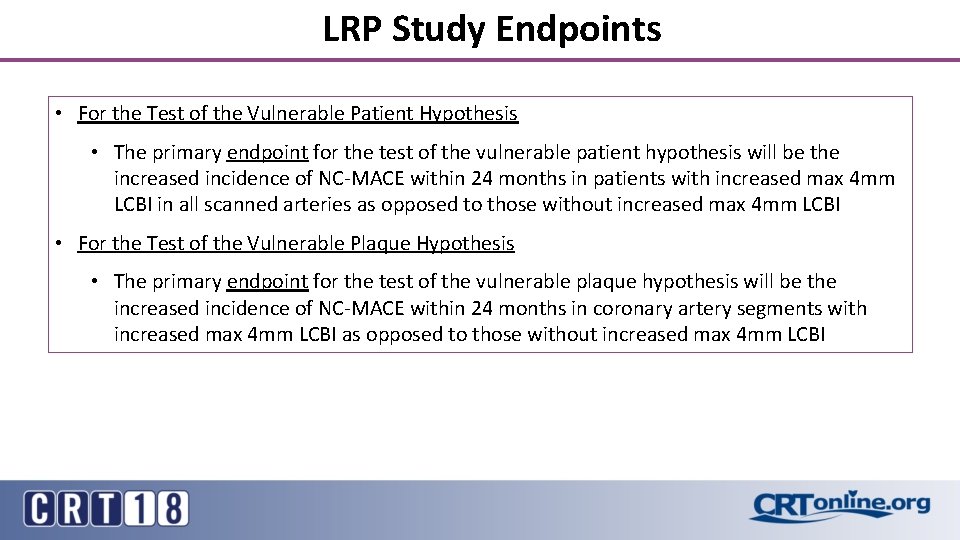
LRP Study Endpoints • For the Test of the Vulnerable Patient Hypothesis • The primary endpoint for the test of the vulnerable patient hypothesis will be the increased incidence of NC-MACE within 24 months in patients with increased max 4 mm LCBI in all scanned arteries as opposed to those without increased max 4 mm LCBI • For the Test of the Vulnerable Plaque Hypothesis • The primary endpoint for the test of the vulnerable plaque hypothesis will be the increased incidence of NC-MACE within 24 months in coronary artery segments with increased max 4 mm LCBI as opposed to those without increased max 4 mm LCBI

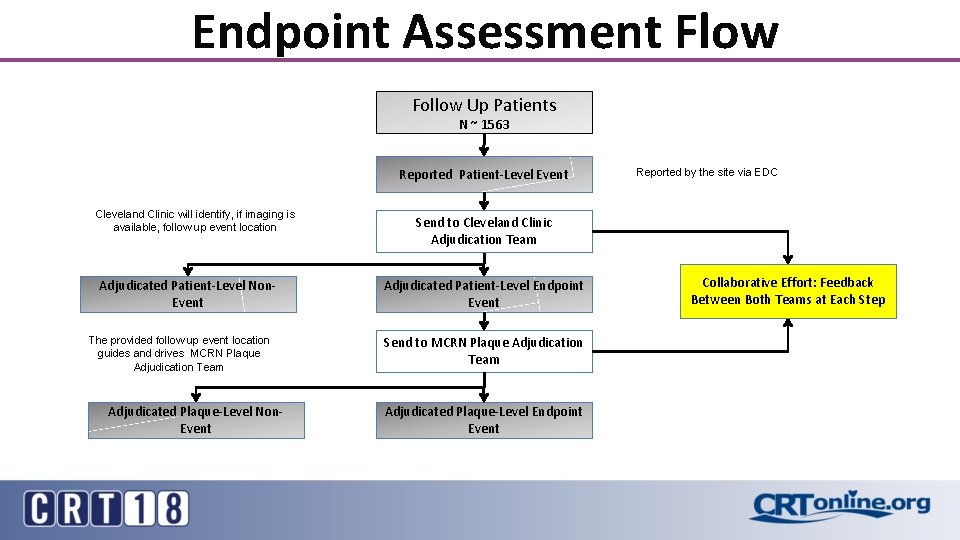
Endpoint Assessment Flow Follow Up Patients N ~ 1563 Reported Patient-Level Event Cleveland Clinic will identify, if imaging is available, follow up event location Send to Cleveland Clinic Adjudication Team Adjudicated Patient-Level Non. Event Adjudicated Patient-Level Endpoint Event The provided follow up event location guides and drives MCRN Plaque Adjudication Team Send to MCRN Plaque Adjudication Team Adjudicated Plaque-Level Non. Event Reported by the site via EDC Adjudicated Plaque-Level Endpoint Event Collaborative Effort: Feedback Between Both Teams at Each Step

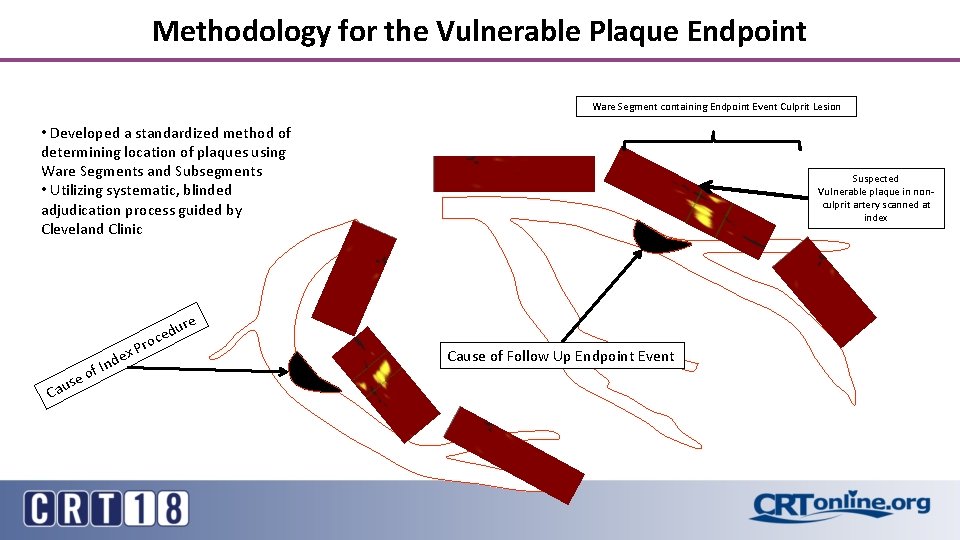
Methodology for the Vulnerable Plaque Endpoint Ware Segment containing Endpoint Event Culprit Lesion • Developed a standardized method of determining location of plaques using Ware Segments and Subsegments • Utilizing systematic, blinded adjudication process guided by Cleveland Clinic Cau se nd of I ex re u d ce Pro Suspected Vulnerable plaque in nonculprit artery scanned at index Cause of Follow Up Endpoint Event

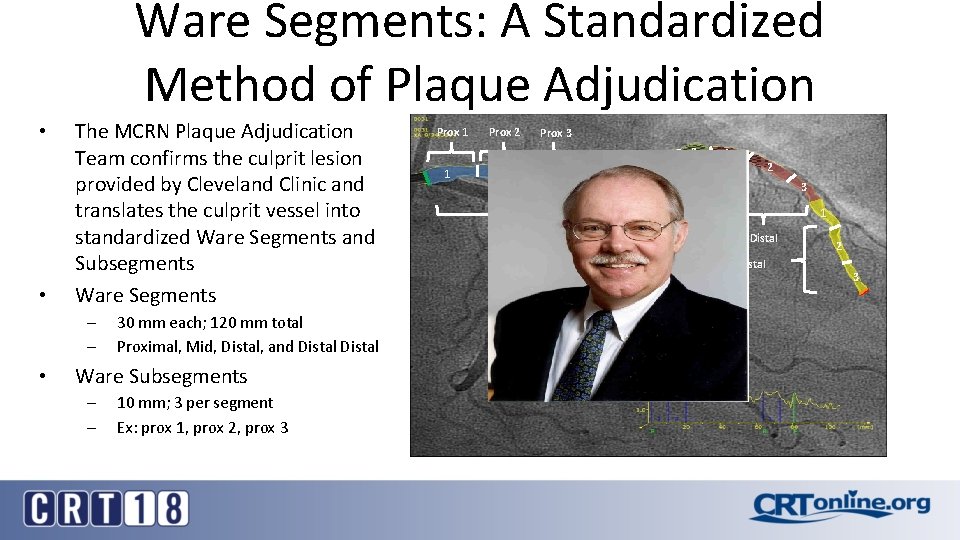
Ware Segments: A Standardized Method of Plaque Adjudication • • The MCRN Plaque Adjudication Team confirms the culprit lesion provided by Cleveland Clinic and translates the culprit vessel into standardized Ware Segments and Subsegments Ware Segments – – • 30 mm each; 120 mm total Proximal, Mid, Distal, and Distal Ware Subsegments – – 10 mm; 3 per segment Ex: prox 1, prox 2, prox 3 Prox 1 1 Prox 2 2 Prox 3 3 1 2 3 1 Proximal Mid Distal 2 3

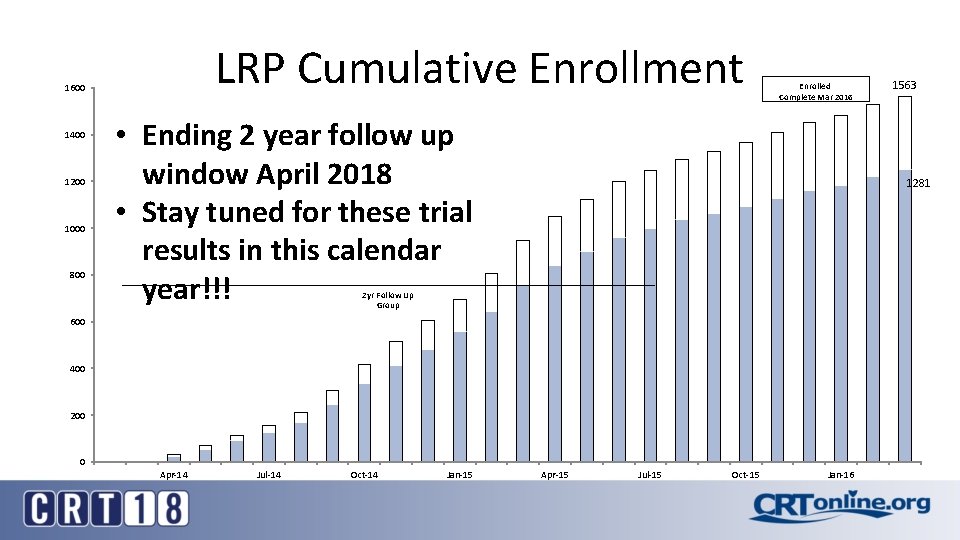
LRP Cumulative Enrollment 1600 1400 1200 1000 800 Enrolled Complete Mar 2016 • Ending 2 year follow up window April 2018 • Stay tuned for these trial results in this calendar year!!! 1281 2 yr Follow Up Group 600 400 200 0 Apr-14 Jul-14 Oct-14 Jan-15 1563 Apr-15 Jul-15 Oct-15 Jan-16

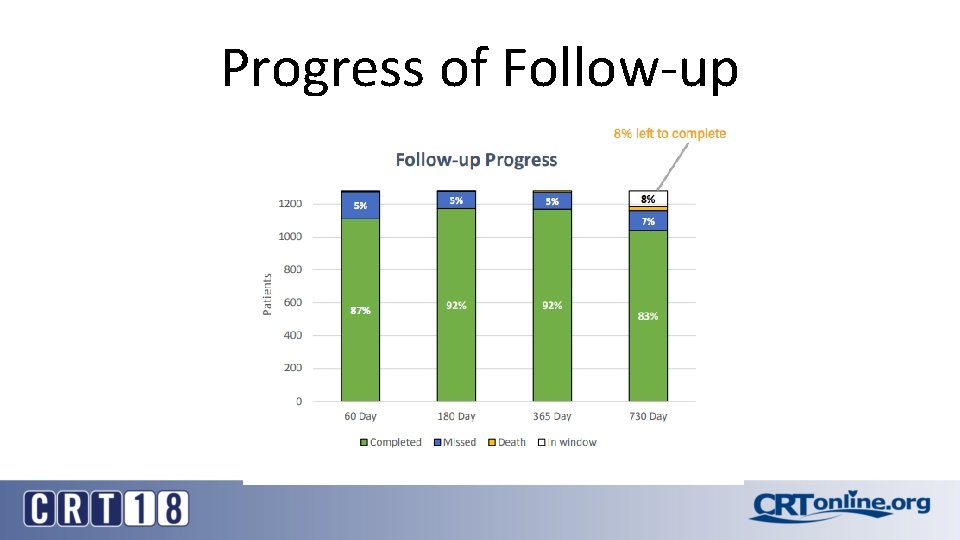
Progress of Follow-up

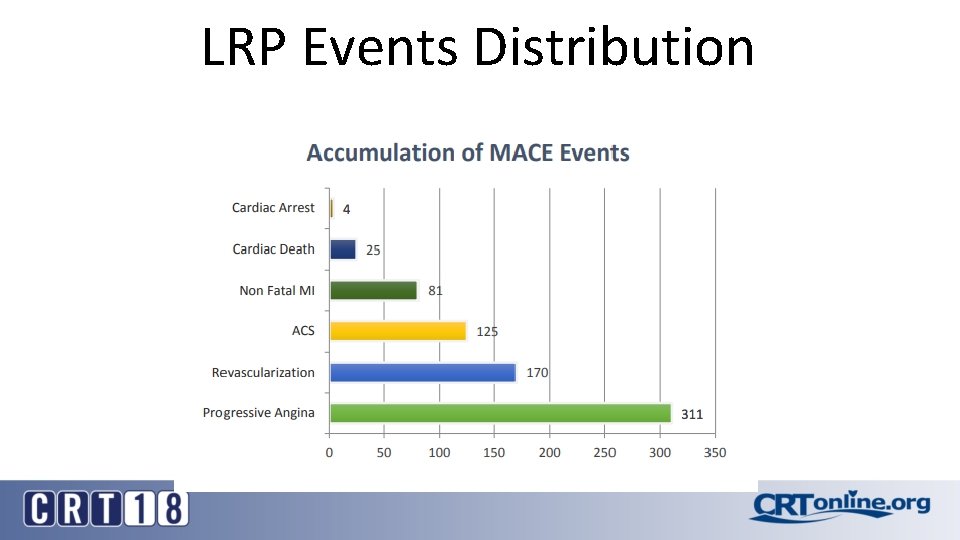
LRP Events Distribution

Planned to report Study Results in the fall of 2018 Stay Tuned
 Adam waksman
Adam waksman Which is an alternative of log based recovery
Which is an alternative of log based recovery Germe didermique
Germe didermique Vis plaque de judet
Vis plaque de judet Plaque continentale et océanique
Plaque continentale et océanique Normal ranges of vital signs
Normal ranges of vital signs Foam cells definition
Foam cells definition Plaque signalétique moteur asynchrone triphasé
Plaque signalétique moteur asynchrone triphasé Leiomyocytes
Leiomyocytes Kontraindikasi absolut fibrinolitik
Kontraindikasi absolut fibrinolitik Plaque index scoring criteria
Plaque index scoring criteria Corn cob structure in plaque
Corn cob structure in plaque Materia alba definition
Materia alba definition Dmft index calculation
Dmft index calculation Plaque signalétique moteur asynchrone triphasé
Plaque signalétique moteur asynchrone triphasé La plaque motrice
La plaque motrice Plaque labdec
Plaque labdec Critères de mcdonald 2017 pdf
Critères de mcdonald 2017 pdf Classification de castaing radius
Classification de castaing radius La plaque motrice
La plaque motrice Echangeur a plaque
Echangeur a plaque Atherosclerotic plaque
Atherosclerotic plaque