Neoplasia lecture 8 Dr Heyam Awad FRCPath Fourth




















- Slides: 34

Neoplasia lecture 8 Dr Heyam Awad FRCPath

Fourth hallmark • Evasion of cell death by evading apoptosis or autophagy

apoptosis • Apoptosis: programmed cell death in which cells activate enzymes that degrade the cells’ own nuclear DNA and nuclear and cytoplasmic proteins • So the cells commit suicide! • The cells fragment and the fragments are phagocytosed without eliciting inflammatory response

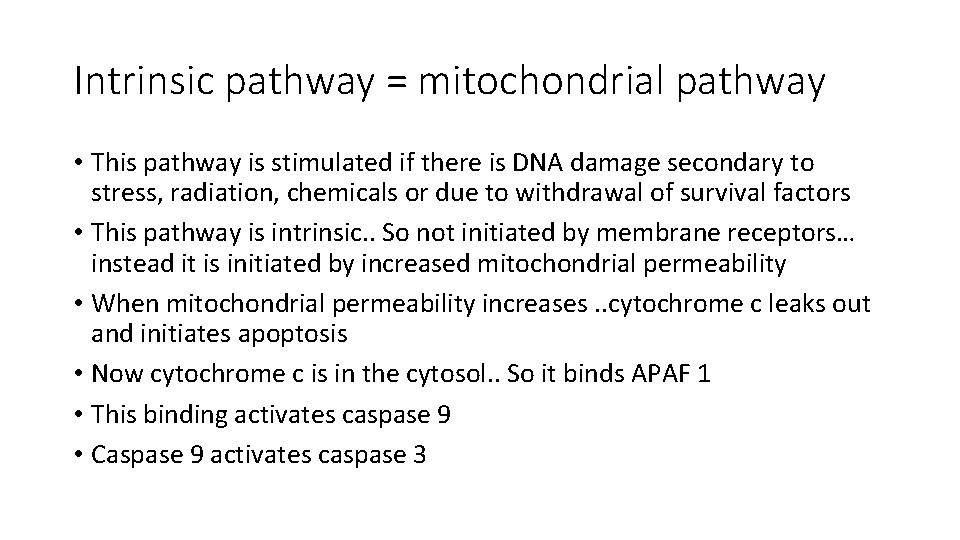
extrinsic pathway • Trigger that starts apoptosis is outside the cells. • The pathway starts when Fas ligand binds to Fas receptor • Upon this the receptor is activated; it trimerizes and its cytoplasmic part (death domain) is activated. • Activation of the receptor attracts a cytoplasmic protein= FADD • FADD recruits procaspase 8 • Procaspase cleaved to active caspase 8 (initiation caspase) • Caspase 8 activates caspase 3 (executioner) which cleaves DNA and cellular protein

Extrinsic pathway • Fas ligand • Fas receptor • FADD • Caspase 8 • Caspase 3 • Decrease any of the above…. . Evasion of cell death

Extrinsic pathway • FLIP is a caspase 8 antagonist • So if FLIP is increased cells can evade apoptosis • FLIP-similar proteins are produced by some viruses. . Helping them to keep infected cells alive.

Intrinsic pathway = mitochondrial pathway • This pathway is stimulated if there is DNA damage secondary to stress, radiation, chemicals or due to withdrawal of survival factors • This pathway is intrinsic. . So not initiated by membrane receptors… instead it is initiated by increased mitochondrial permeability • When mitochondrial permeability increases. . cytochrome c leaks out and initiates apoptosis • Now cytochrome c is in the cytosol. . So it binds APAF 1 • This binding activates caspase 9 • Caspase 9 activates caspase 3

Intrinsic pathway Internal stresses within cells Increase mitochondrial permeability Cytochrome c leaks outside the mitochondria Cytochrome c binds to APAF 1 Caspase 9 activated Caspase 3 activated Again: decrease any of these and the cell can avoid apoptosis

note • IAP= inhibitor of apoptotic protein , inhibits caspase 9 • So increase IAP and apoptosis can be avoided.

apoptosis

Mitochondrial permeability • Mitochondrial permeability is controlled by BH 3 proteins (BAD, BID, PUMA) • When BH 3 proteins sense internal stress. . Stimulate proapoptotic proteins and inhibit antiapoptotic ones • Proapoptotic: BAX, BAK • Antiapoptotic: BCL 2, BCL- Xl • So decrease BAD, BID, PUMA, BAX, BAK… NO APOPTOSIS • Increase BCL 2 AND BCL-Xl…. No apoptosis

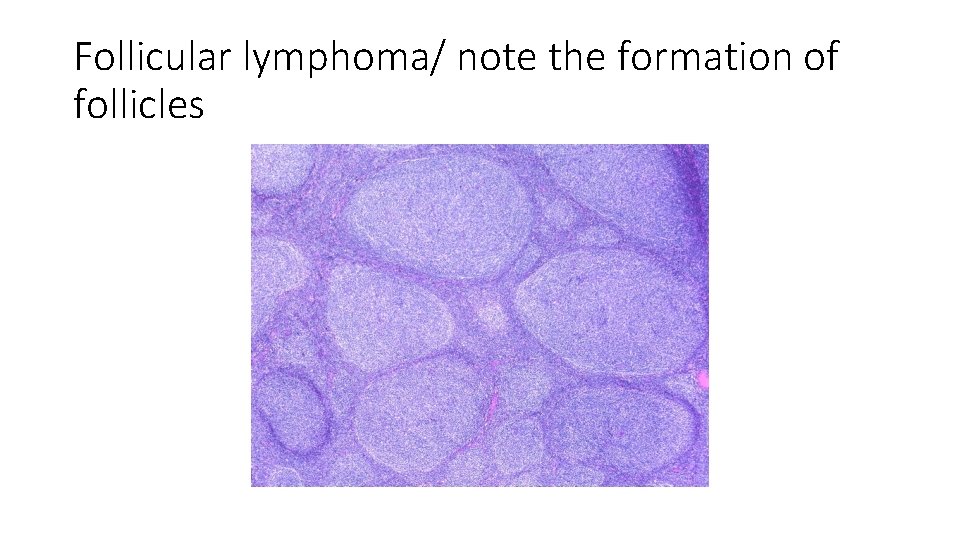
bcl 2 • Follicular lymphomas are slow growing (indolent) tumors that have a translocation causing increased bcl 2 • T (14; 18) …. Bcl 2 translocated and overexpressed • In lymphocytes having this mutation… apoptosis is decreased • These lymphocytes live longer…. Rather than being transformed… that’s why this type of lymphoma ( follicular lymphoma) is indolent

Follicular lymphoma/ note the formation of follicles

• P 53 is important in regulating apoptosis • P 53 induces apoptosis when there is DNA damage or increased expression of oncogenes • So if p 53 is normal , cells that have increased oncogenes or have damaged DNA will die and no tumor will occur • But if p 53 is inhibited. . Transformation can happen in such cells

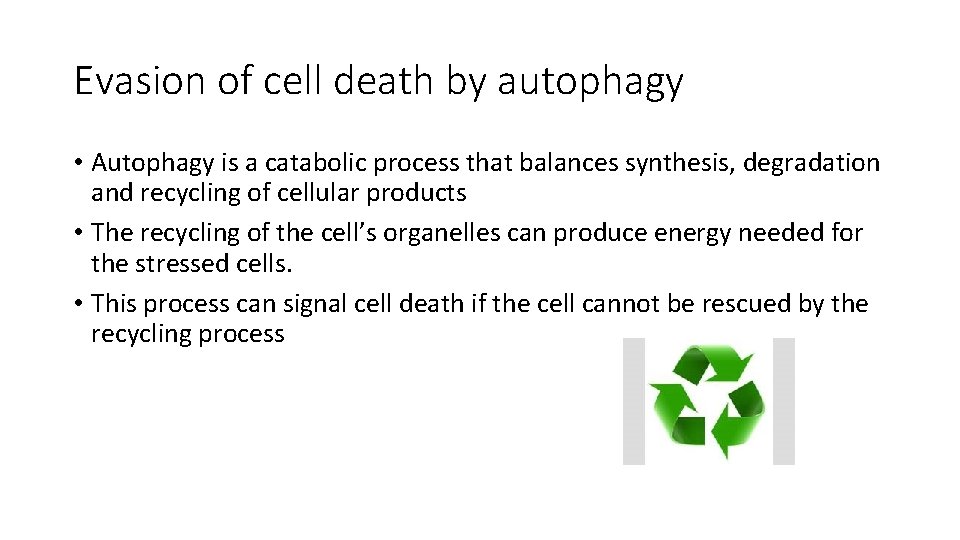
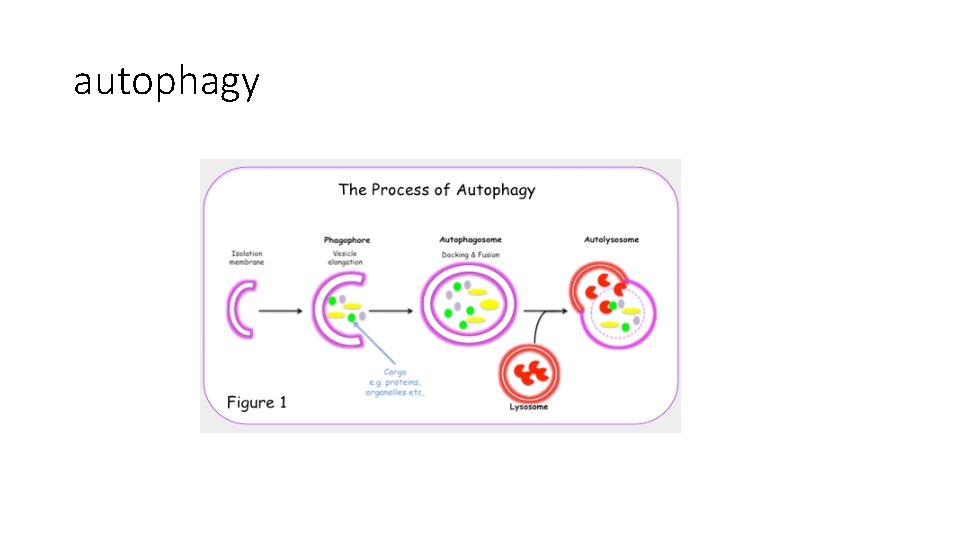
Evasion of cell death by autophagy • Autophagy is a catabolic process that balances synthesis, degradation and recycling of cellular products • The recycling of the cell’s organelles can produce energy needed for the stressed cells. • This process can signal cell death if the cell cannot be rescued by the recycling process

• Autophagy is regulated by mechanisms and proteins that overlap with apoptosis • The main stimulus for autophagy is Beclin 1 which is a member of the BH 3 Family. • So: internal stresses can stimulate cell death by apoptosis or autophagy. • Decreased autophagy… helps in tumorogenesis

note • Although autophagy is an anti-tumor process…. . Later on if there is a tumor mass formed, autophagy can help the tumor to survive if it’s used to recycle organelles to be used as an energy source. • Autophagy can help tumor cells to survive during unfriendly climates: for example during chemotherapy treatment.

autophagy

Fifth hallmark: sustained angiogenesis • Tumors cannot grow for more than 1 -2 mm without blood supply • This 1 -2 mm zone is the maximum direct diffusion distance. Angiogenesis important for tumors to: 1. supply oxygen and nutrients 2. Get rid of waste products 3. gain access to host blood vessels which is important for invasion and metastasis. • 4. the endothelial cells in these vessels secrete growth factors that can help tumor growth • •

angiogenesis • Two processes: • 1. neoangiogensis: new vessels formed from preexisting host capillaries • 2. vasculogenesis: completely new vessels are formed by recruiting endothelial cells from bone marrow.

note • Tumor blood vessels are abnormal : they are leaky, dilated and have haphazard pattern of connections

angiogenesis • Angiogenesis is accomplished by factors secreted from the parenchymal tumor cells as well as the stroma. Also inflammatory cells surrounding the tumor can produce angiogenic factors. • the balance between pro-angiogenic and anti-angiogenic factors controls formation of new blood vessels • Main pro-angiogenic: VEGF= vascular endothelial growth factor • Main anti-angiogenic: TSP 1= thrombospondin 1

• Tumors usually stay in situ or small for several years… at this stage there is no angiogenesis • Angiogenesis switch happens when VEGF ( and other proangiogenic factors) increases and TSP 1 ( or other antiangiogenic factors) decreases.

Angiogenic switch • VEGF produced from tumor cells or macrophages • Protease (secreted from tumor cells or stromal cells) can release FGF (an angiogenic agnt) from ECM • TSP 1 is produced from fibroblasts in response to tumor cells…. TSP is anti angiogenic • Normal P 53 induces synthesis of TSP 1. . So if p 53 is deleted. . Decreased TSP 1

What causes the angiogenic switch • Hypoxia is an important factor that favours angiogenesis • Hypoxia. . Stimulates production of hypoxia –inducible factor 1 alpha (HIF 1 alpha) • HIF is a transcription factor which will stimulate production of VEGF • HIF is destructed by VHL (von Hipple- Lindau )protein • Hypoxia prevents VHL from recognizing HIF … no destruction. . more angiogenesis

• VEGF. . Stimulates Notch pathway which regulates the branching and density of vessels.

Von Hippel- Lindau syndrome • VHL gene is a tumor suppressor gene ( because it decreases angiogenesis) • Rarely some pole inherit defective VHL gene… they develop tumors like renal cell carcinoma, pheochromocytoma. .

Sixth hallmark: reprogramming of energy metabolism Normal cells obtain energy by: • Oxidative phosphorylation if oxygen is available. In this process each glucose molecule used produces 36 ATP molecules. • Anaerobic respiration if oxygen levels are low. In this process glucose is converted to lactic acid and for each glucose molecule used only 2 ATP molecules are produced.

Reprogramming of energy metabolism • Cancer cells have a third way! • They convert glucose to lactic acid even in the presence of adequate oxygen • This process is called : aerobic glycolysis or Warburg effect.

Warburg effect • Although less ATP is produced… the Warburg effect ensures that carbon atoms in glucose ( which is converted to Pyruvate) are used for synthesis of organic compounds like lipids and proteins which are important in building new cells in the highly proliferative tumor. • Oncogenes ( myc, ras) and tumor suppressor genes that favor cell growth ( TP 53 can affect ) and upregulate this process.

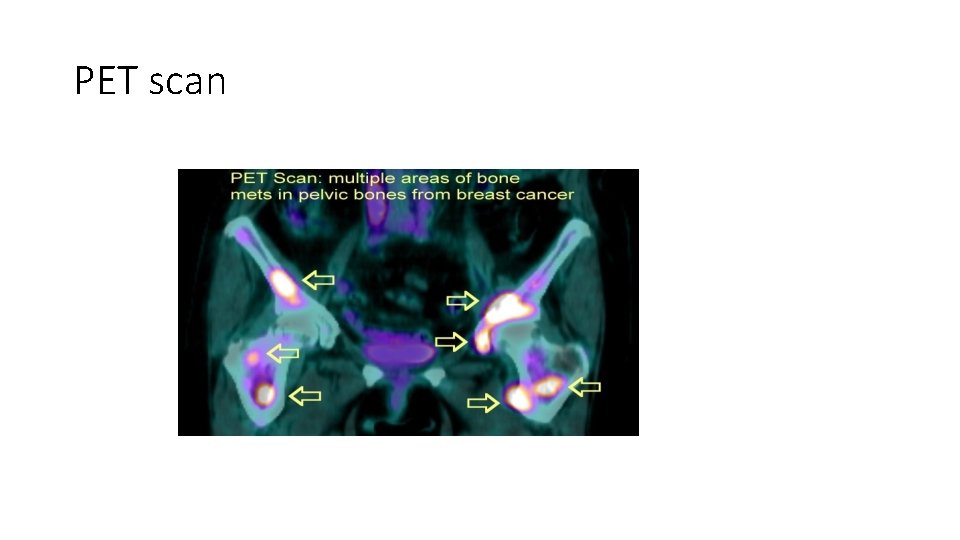
PET scan • Tumor cells are “glucose hungry” and this property is used in PET scans • PET: positron emission tomography • Patient is injected with a glucose derivative. . Tumor cells take this derivative more than normal cells and as such detected with the scan • The more proliferative the tumor is… more uptake and more positivity with PET scan

PET scan

PET scan

PET scan
 Awad mataria
Awad mataria Dr khaled awad
Dr khaled awad Exemplum speech
Exemplum speech Frcpath chemical pathology
Frcpath chemical pathology Frcpath exam dates 2020
Frcpath exam dates 2020 Frcpath part 1 microbiology
Frcpath part 1 microbiology Willis definition of neoplasia
Willis definition of neoplasia Enfermedades de la vulva
Enfermedades de la vulva Burkitt lymphoma
Burkitt lymphoma Neoplasia literally means
Neoplasia literally means Vaginal neoplasia
Vaginal neoplasia Neoplasia
Neoplasia Hnpcc
Hnpcc Neoplasia testicular
Neoplasia testicular Displasia tanatofórica
Displasia tanatofórica Multiple endocrine neoplasia type 2
Multiple endocrine neoplasia type 2 Dysplastic squamous cell
Dysplastic squamous cell Vaginal neoplasia
Vaginal neoplasia Mamria
Mamria Clinical features of neoplasia
Clinical features of neoplasia Que es una neoplasia
Que es una neoplasia Md frcpc definition
Md frcpc definition 01:640:244 lecture notes - lecture 15: plat, idah, farad
01:640:244 lecture notes - lecture 15: plat, idah, farad Fourth step in essay writing
Fourth step in essay writing Fourth root of 162
Fourth root of 162 Fourth district pta
Fourth district pta Fourth assessment report
Fourth assessment report One two three four five
One two three four five Triple derivative
Triple derivative Fourth normal form example
Fourth normal form example Cefoxit
Cefoxit 2 generation cephalosporins
2 generation cephalosporins Ethics in information technology fourth edition
Ethics in information technology fourth edition Nursing diagnosis as evidenced by
Nursing diagnosis as evidenced by Fourth and fifth commandment
Fourth and fifth commandment