Molecular structure The Schrdinger Equation for molecules The

- Slides: 21

Molecular structure The Schrödinger Equation for molecules The Born-Oppenheimer approximation 4. 1 Molecular orbital theory 4. 2. 1 The hydrogen molecule-ion 4. 2. 2 The structure of diatomic molecules 4. 2. 3 Heteronuclear diatomic molecules 4. 2. 4 Energy in the LCAO approach 4. 2. Molecular orbitals for polyatomic systems 4. 3. 1 The Hückel approximation 4. 3. 2 The band theory of solids

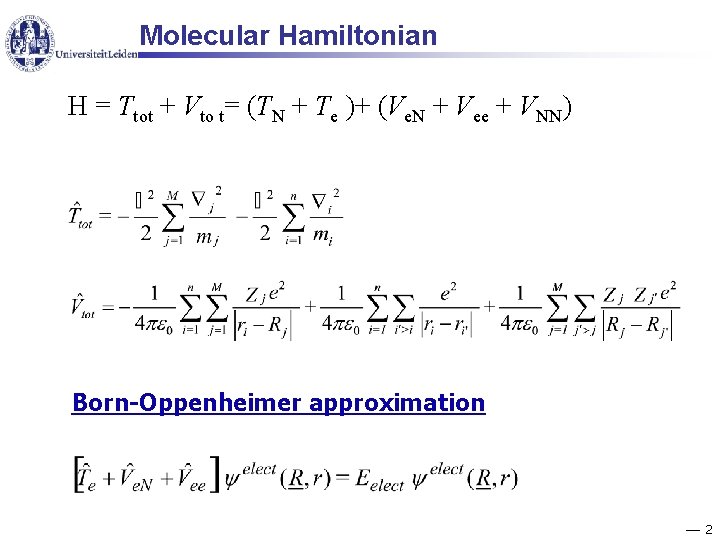
Molecular Hamiltonian H = Ttot + Vto t= (TN + Te )+ (Ve. N + Vee + VNN) Born-Oppenheimer approximation 2

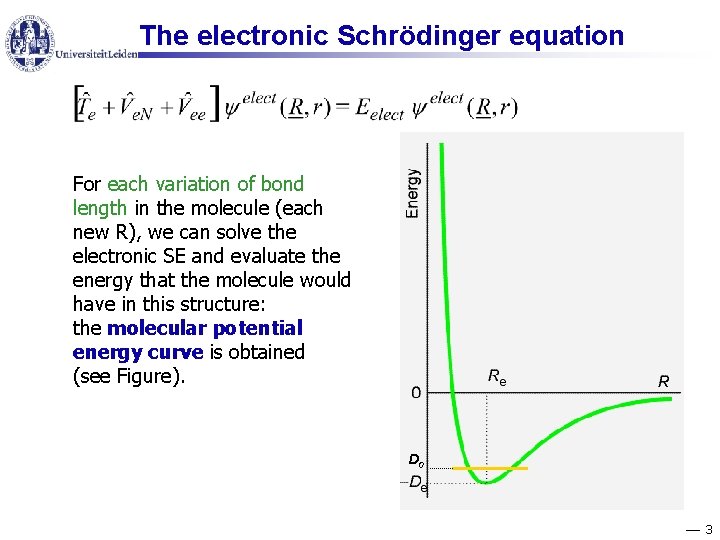
The electronic Schrödinger equation For each variation of bond length in the molecule (each new R), we can solve the electronic SE and evaluate the energy that the molecule would have in this structure: the molecular potential energy curve is obtained (see Figure). D 0 3

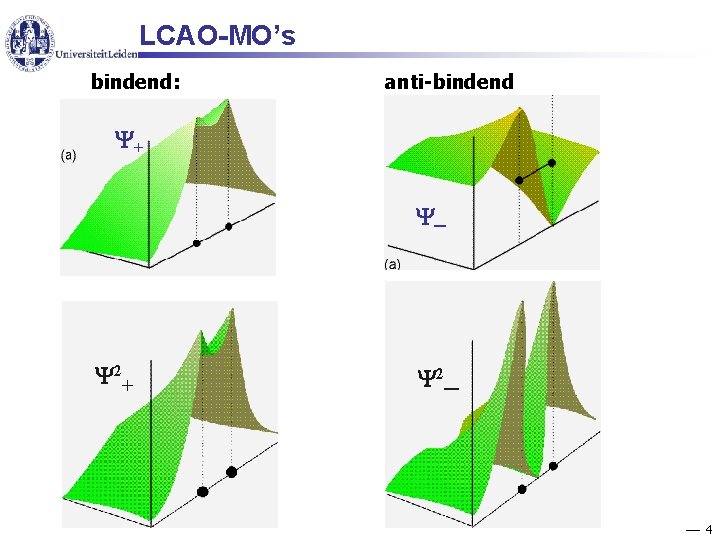
LCAO-MO’s bindend: anti-bindend + ─ 2+ 2─ 4

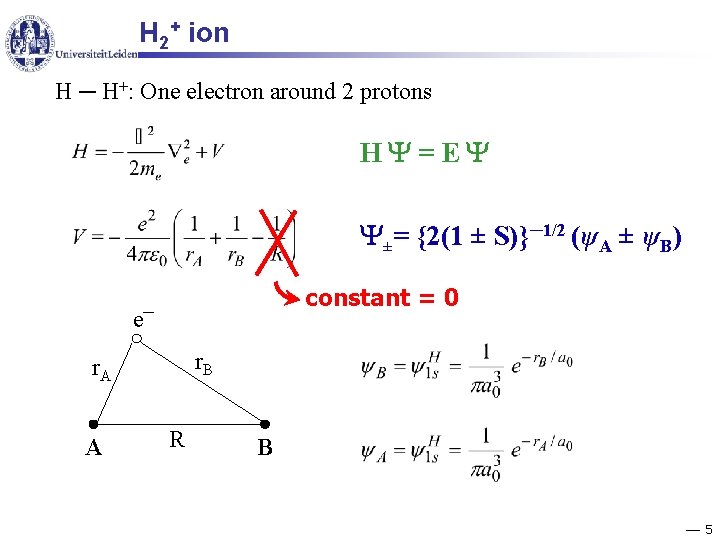
H 2+ ion H ─ H+: One electron around 2 protons H =E ±= {2(1 ± S)}─1/2 (ψA ± ψB) e constant = 0 ─ r. B r. A A R B 5

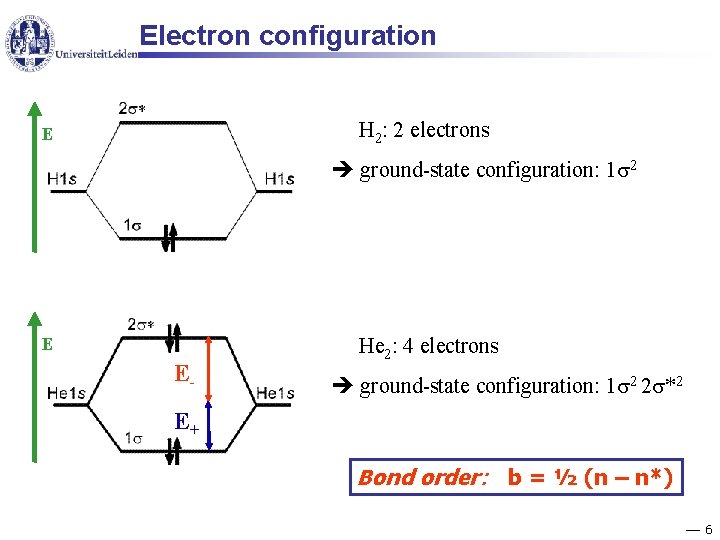
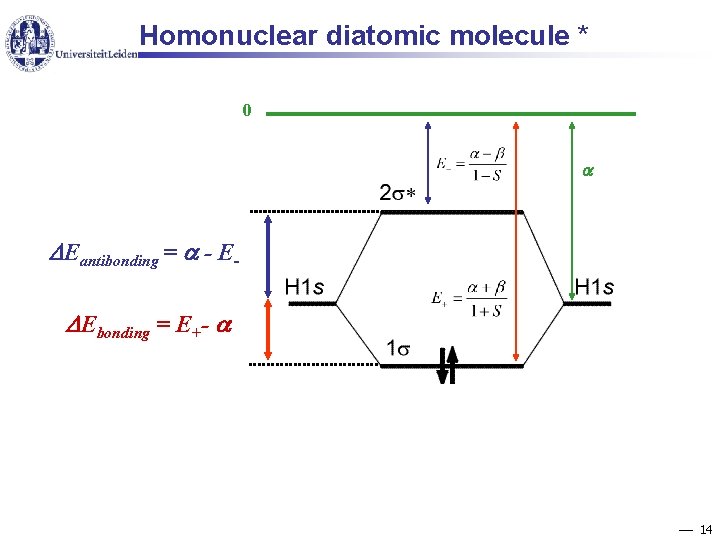
Electron configuration H 2: 2 electrons E ground-state configuration: 1 2 E E- He 2: 4 electrons ground-state configuration: 1 2 2 *2 E+ Bond order: b = ½ (n – n*) 6

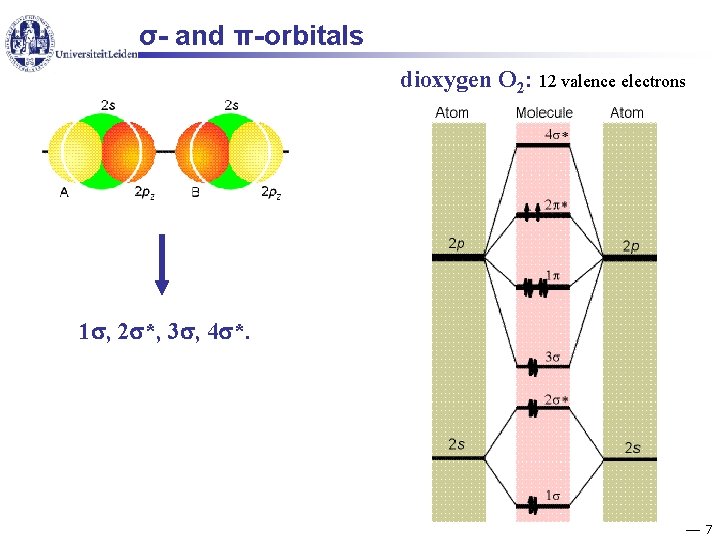
σ- and π-orbitals dioxygen O 2: 12 valence electrons 1 , 2 *, 3 , 4 *. 7

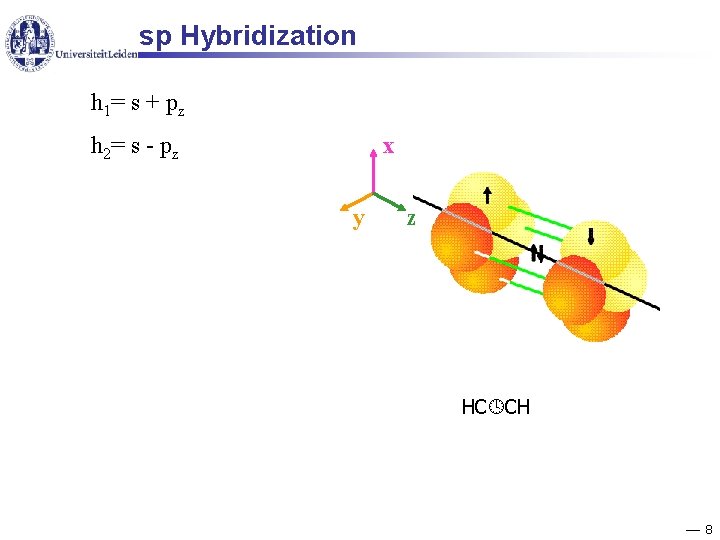
sp Hybridization h 1= s + pz x h 2= s - pz y z HC CH 8

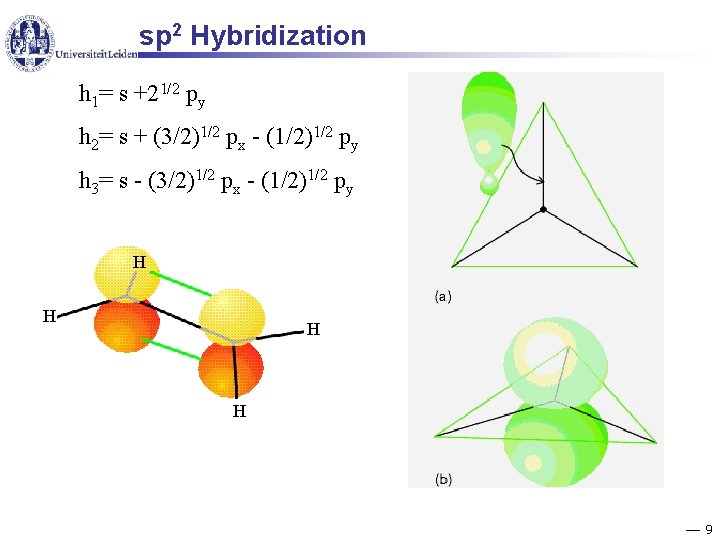
sp 2 Hybridization h 1= s +21/2 py h 2= s + (3/2)1/2 px - (1/2)1/2 py h 3= s - (3/2)1/2 px - (1/2)1/2 py H H 9

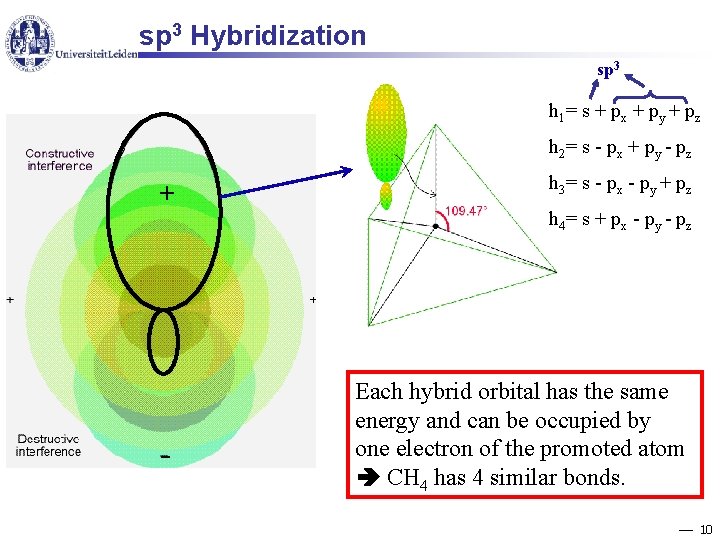
sp 3 Hybridization sp 3 h 1= s + px + py + pz h 2= s - px + py - pz + - h 3= s - px - py + pz h 4= s + px - py - pz Each hybrid orbital has the same energy and can be occupied by one electron of the promoted atom CH 4 has 4 similar bonds. 10

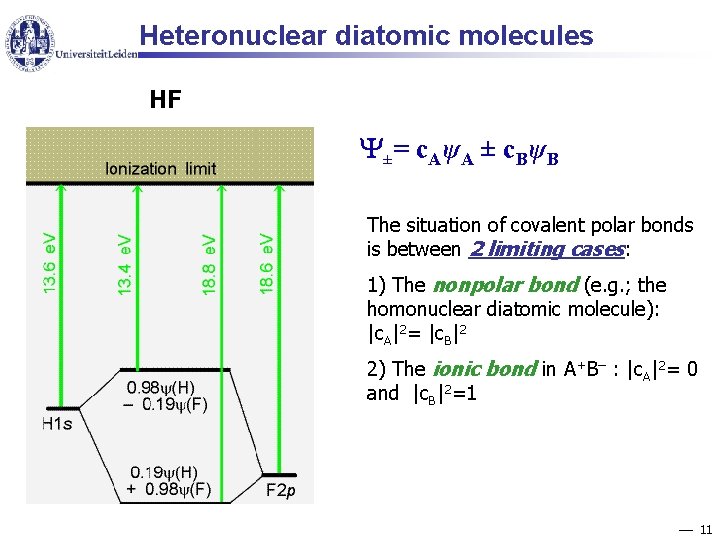
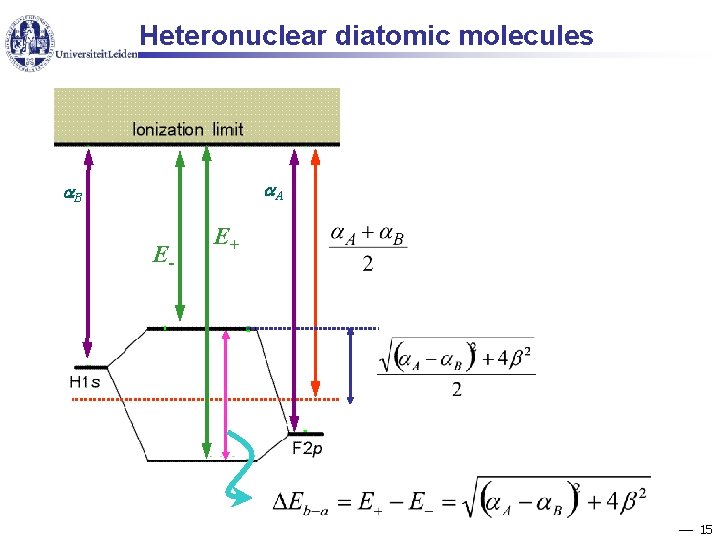
Heteronuclear diatomic molecules HF ± = c Aψ A ± c B ψ B The situation of covalent polar bonds is between 2 limiting cases: 1) The nonpolar bond (e. g. ; the homonuclear diatomic molecule): |c. A|2= |c. B|2 2) The ionic bond in A+B– : |c. A|2= 0 and |c. B|2=1 11

Variation principle If an arbitrary wavefunction is used to calculate the energy, the value calculated is never less than the true energy. 12

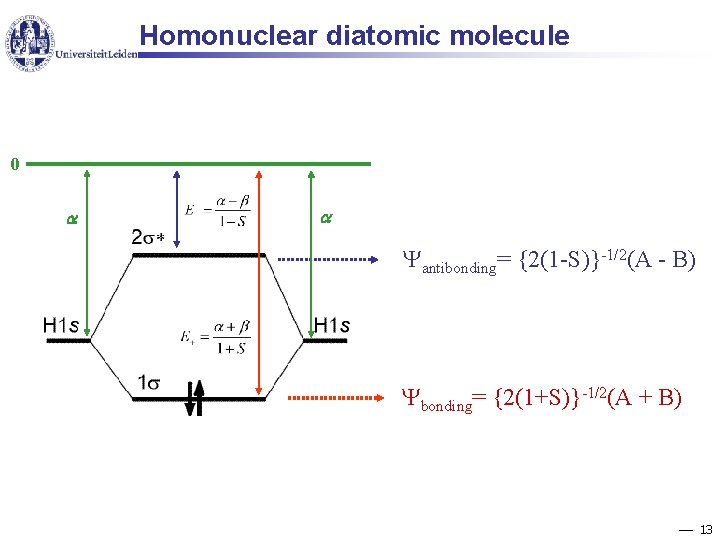
Homonuclear diatomic molecule 0 antibonding= {2(1 -S)}-1/2(A - B) bonding= {2(1+S)}-1/2(A + B) 13

Homonuclear diatomic molecule * 0 Eantibonding = - E Ebonding = E+- 14

Heteronuclear diatomic molecules A B E- E+ 15

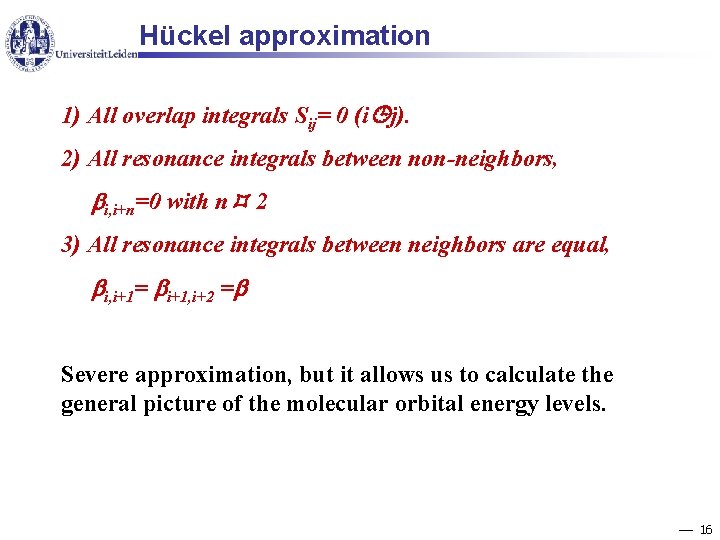
Hückel approximation 1) All overlap integrals Sij= 0 (i j). 2) All resonance integrals between non-neighbors, i, i+n=0 with n 2 3) All resonance integrals between neighbors are equal, i, i+1= i+1, i+2 = Severe approximation, but it allows us to calculate the general picture of the molecular orbital energy levels. 16

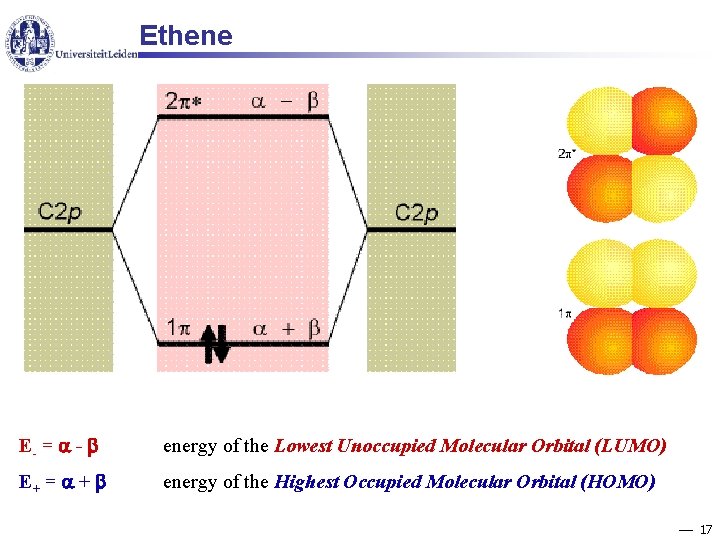
Ethene E- = - energy of the Lowest Unoccupied Molecular Orbital (LUMO) E+ = + energy of the Highest Occupied Molecular Orbital (HOMO) 17

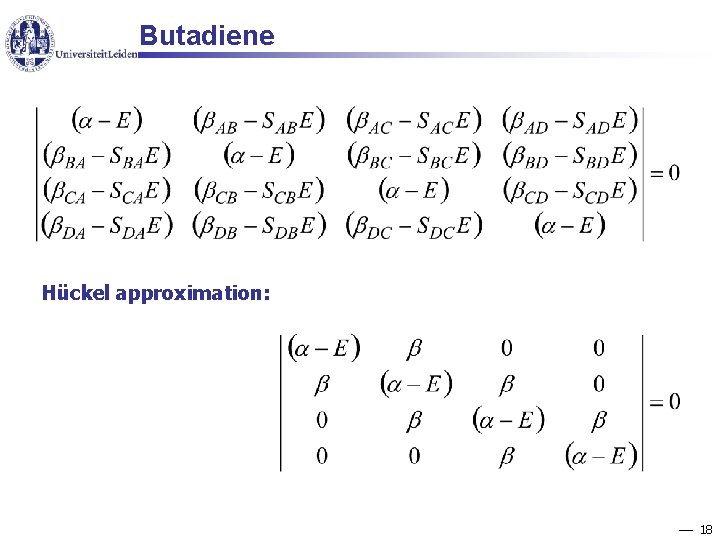
Butadiene Hückel approximation: 18

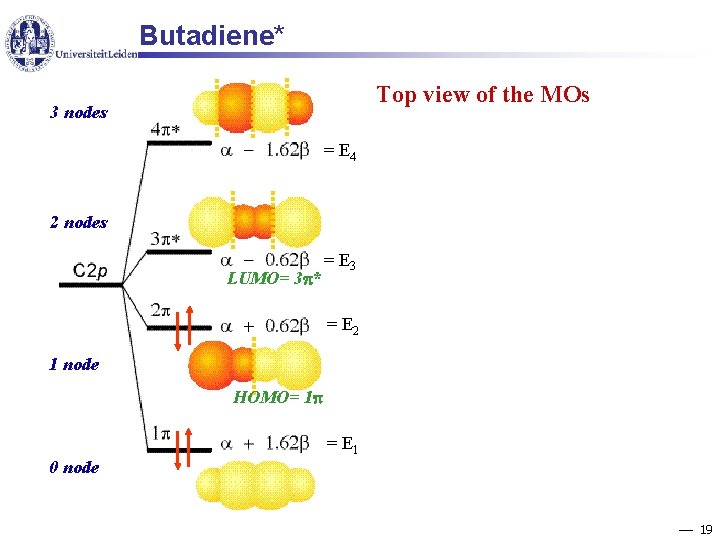
Butadiene* Top view of the MOs 3 nodes = E 4 2 nodes LUMO= 3 * = E 3 = E 2 1 node HOMO= 1 = E 1 0 node 19

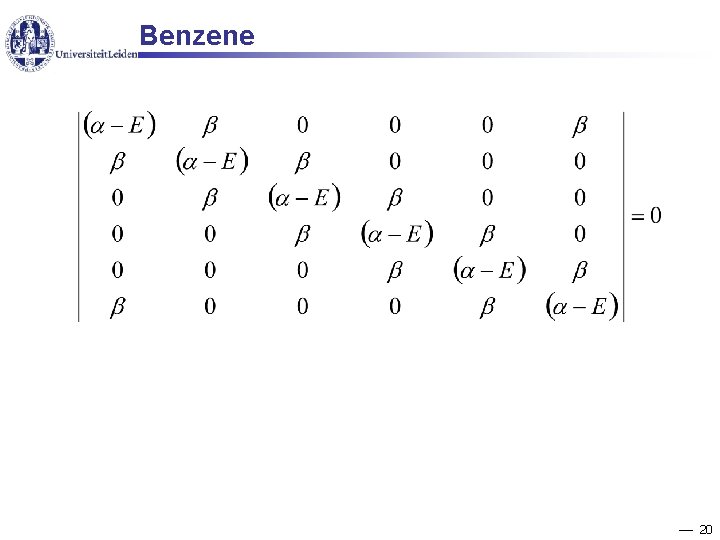
Benzene 20

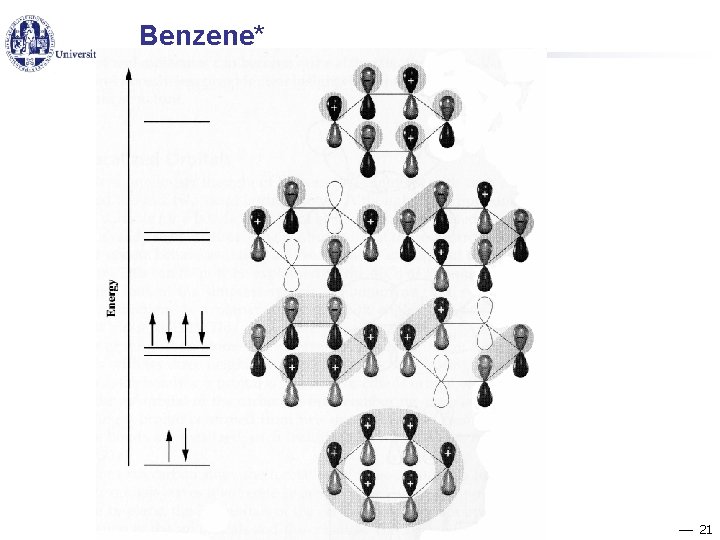
Benzene* 21