Schrdinger equation Timeindependent case Timeindependent Schrdinger equation 1








- Slides: 20

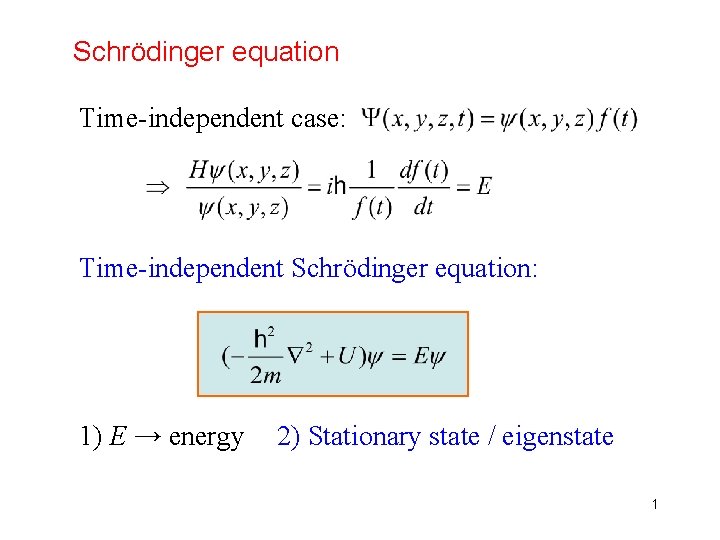
Schrödinger equation Time-independent case: Time-independent Schrödinger equation: 1) E → energy 2) Stationary state / eigenstate 1

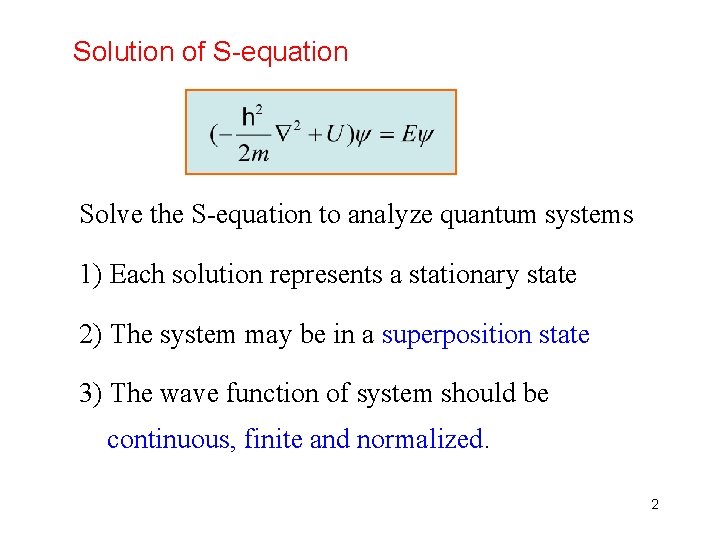
Solution of S-equation Solve the S-equation to analyze quantum systems 1) Each solution represents a stationary state 2) The system may be in a superposition state 3) The wave function of system should be continuous, finite and normalized. 2

Infinitely deep square well potential Particle in an infinitely deep square well potential or “rigid box” Trapped in the well, can’t escape Classical case: Free motion until collision Equal probability at any point 3

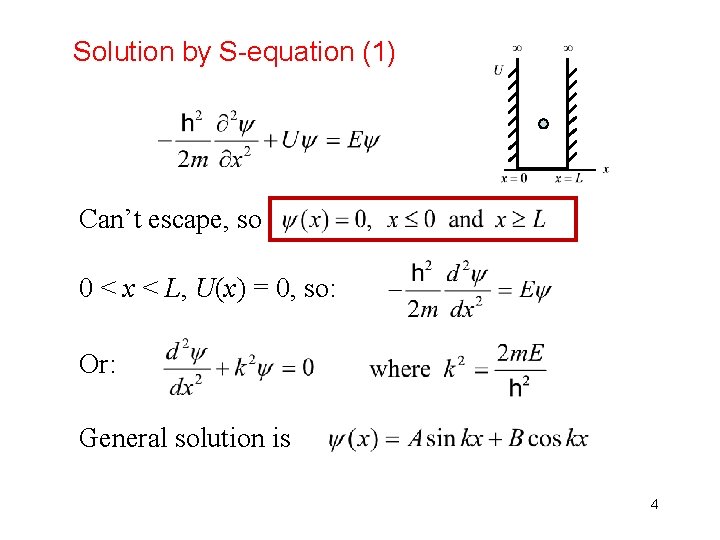
Solution by S-equation (1) Can’t escape, so 0 < x < L, U(x) = 0, so: Or: General solution is 4

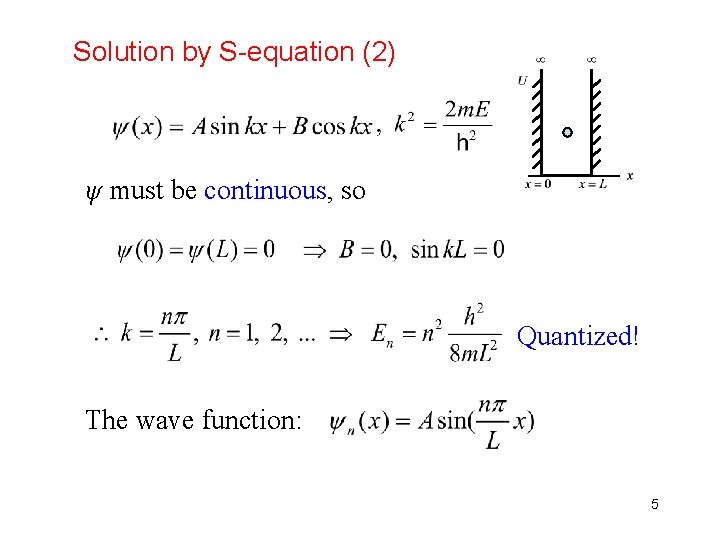
Solution by S-equation (2) ψ must be continuous, so Quantized! The wave function: 5

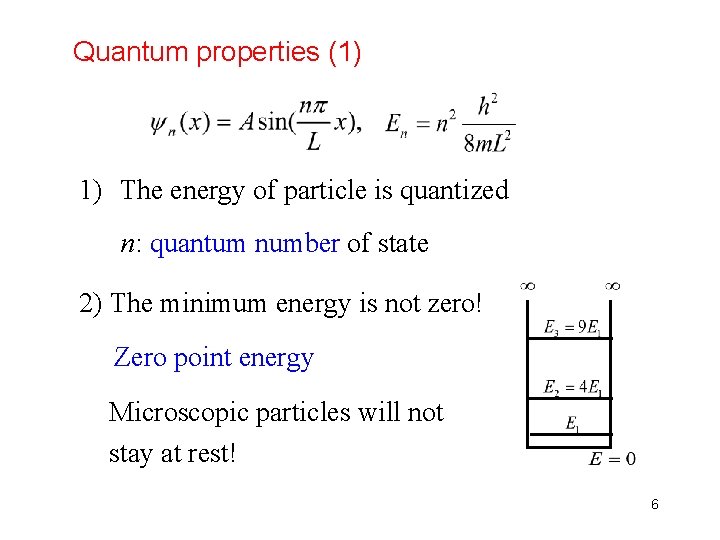
Quantum properties (1) 1) The energy of particle is quantized n: quantum number of state 2) The minimum energy is not zero! Zero point energy Microscopic particles will not stay at rest! 6

Quantum properties (2) 3) Figure of wave function & probability distribution Like a standing wave Notice: The probability is not constant! 7

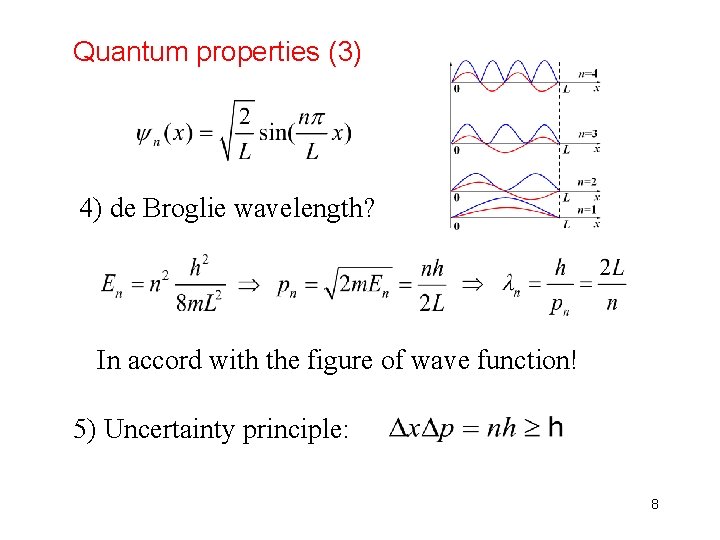
Quantum properties (3) 4) de Broglie wavelength? In accord with the figure of wave function! 5) Uncertainty principle: 8

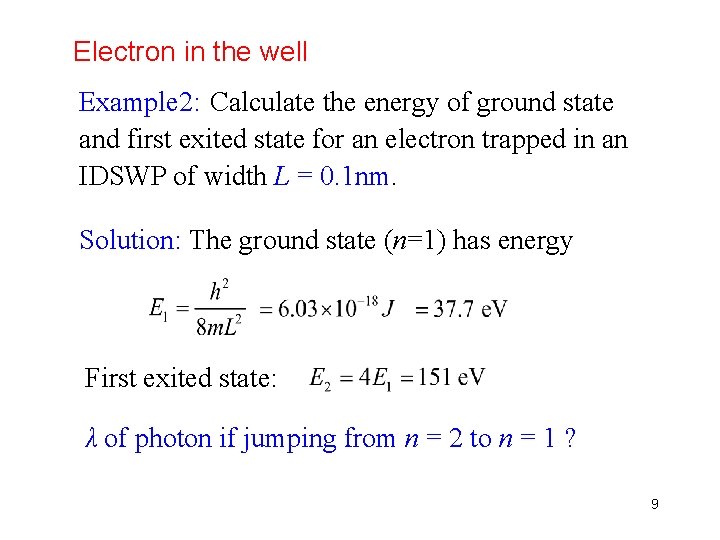
Electron in the well Example 2: Calculate the energy of ground state and first exited state for an electron trapped in an IDSWP of width L = 0. 1 nm. Solution: The ground state (n=1) has energy First exited state: λ of photon if jumping from n = 2 to n = 1 ? 9

Probability in well Example 3: In the state of (a) Where does the particle have maximum probability densities? (b) What is the probability to find the particle in region 0 < x < L/4 ? Solution: (a) So: 10

(b) What is the probability to find the particle in region 0 < x < L/4 ? 11

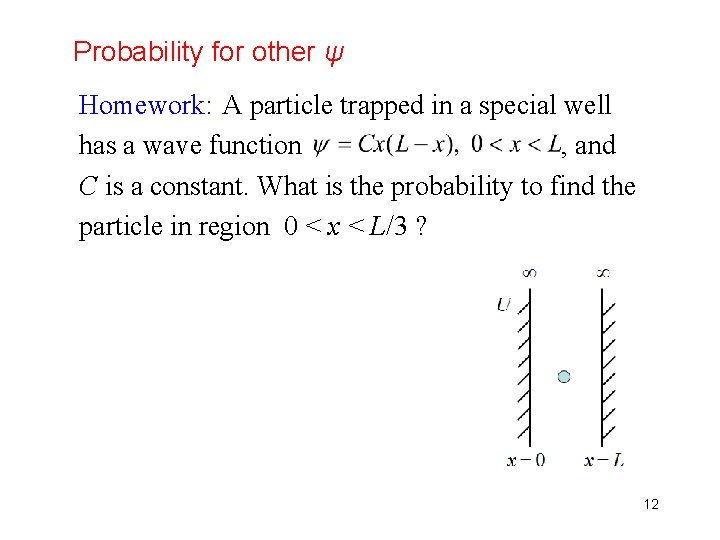
Probability for other ψ Homework: A particle trapped in a special well has a wave function , and C is a constant. What is the probability to find the particle in region 0 < x < L/3 ? 12


Molecular Beam Epitaxy: Man-made potential wells for Quantum mechanical engineering

Molecular Beam Epitaxy: Man-made potential wells for Quantum mechanical engineering

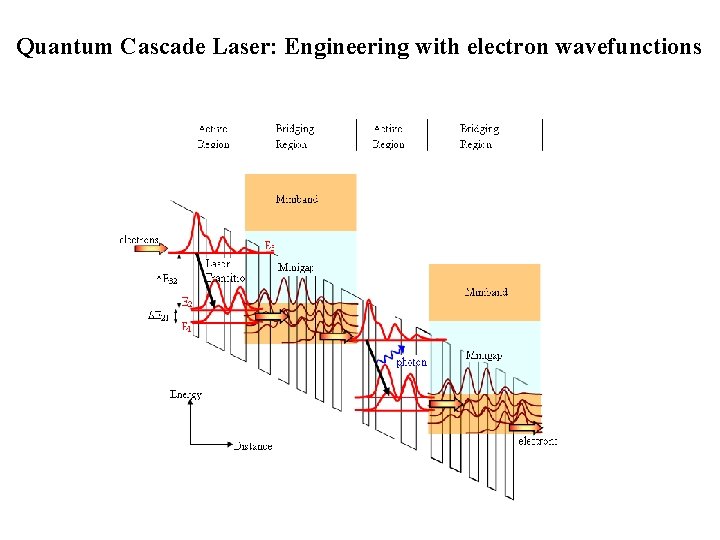
Quantum Cascade Laser: Engineering with electron wavefunctions

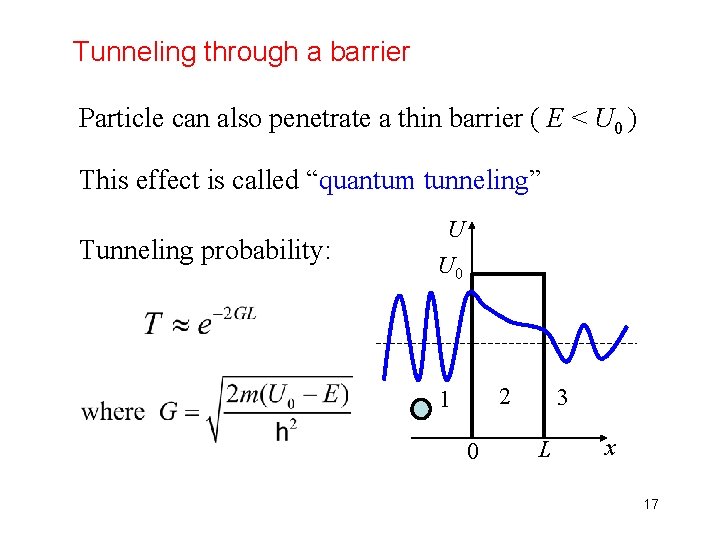
Tunneling through a barrier Particle can also penetrate a thin barrier ( E < U 0 ) This effect is called “quantum tunneling” Tunneling probability: U U 0 2 1 0 3 L x 17

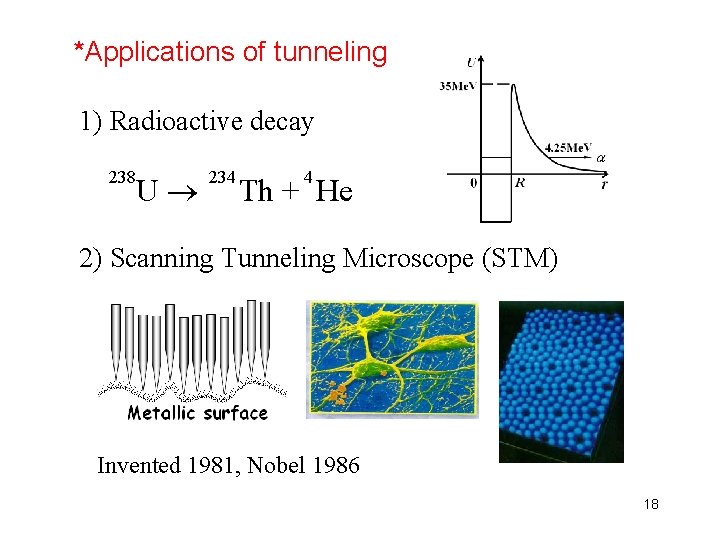
*Applications of tunneling 1) Radioactive decay 238 U 234 4 Th + He 2) Scanning Tunneling Microscope (STM) Invented 1981, Nobel 1986 18

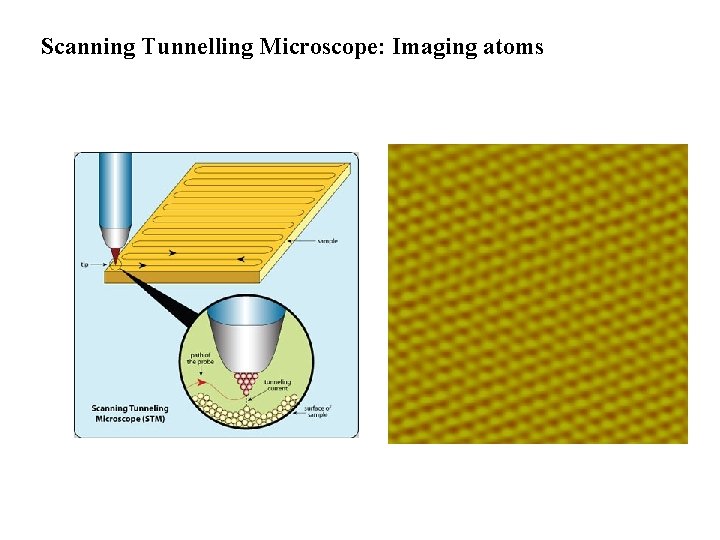
Scanning Tunnelling Microscope: Imaging atoms
