Medical University of Sofia Faculty of Me Department




























- Slides: 61

Medical University of Sofia, Faculty of Me Department of Pharmacology and Toxi Adverse Drug Reactions (Summary) Assoc. Prof. Iv. Lambev E-mail: itlambev@mail. bg

Adverse reactions (ARs) is any undesirable or unin consequence of drug administration. ARs include all kin of noxious effects – trivial, serious or even fatal. All drugs are capable of producing ARs. Whenever a dru is given a risk is taken. The magnitude of risk has to be considered along with otherapeutic advantages in deciding whether to use or not one drug. ARs may develop promptly or only after prolonged medic even after stoppage of the drug. An incidence of 10– 25% been documented in different clinical studies. So drug AR not rare. They are more common with multiple drug thera in the elderly patients.

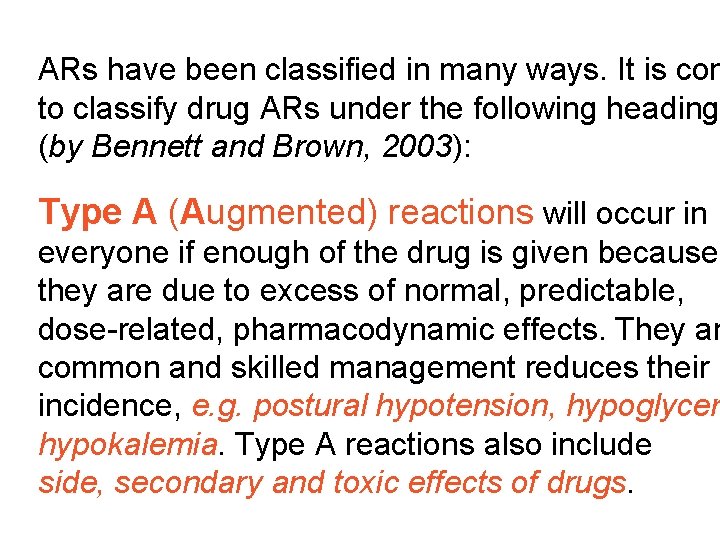
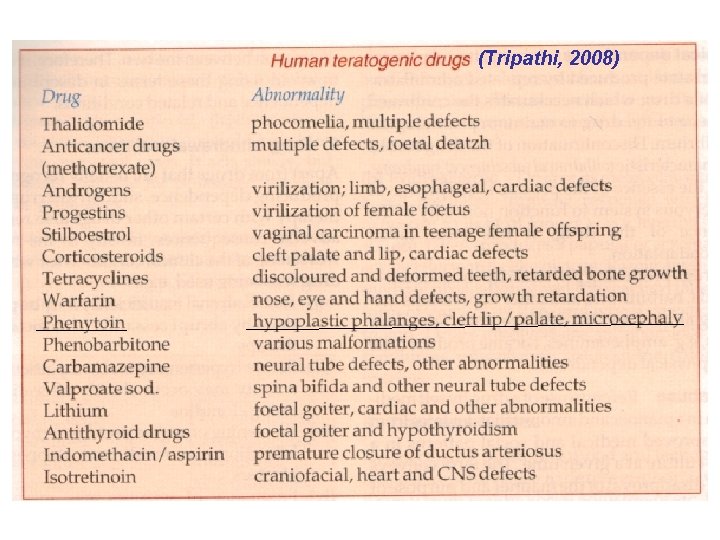
ARs have been classified in many ways. It is con to classify drug ARs under the following heading (by Bennett and Brown, 2003): Type A (Augmented) reactions will occur in everyone if enough of the drug is given because they are due to excess of normal, predictable, dose-related, pharmacodynamic effects. They ar common and skilled management reduces their incidence, e. g. postural hypotension, hypoglycem hypokalemia. Type A reactions also include side, secondary and toxic effects of drugs.

Type B (Bizarre) reactions will occur only in some people. They are not part of the normal pharmacology of the drug, are not dose-related are due to unusual attributes of the patient intera ing with the drug. These effects are predictable where the mechanism is known (though predicti tests may be expensive or impracticable), otherw they are unpredictable for the individual, althoug the incidence may be known. The class includes unwanted effects due to inherited abnormalities (idiosyncrasy) and immunological processes (drug allergy). These account for most drug fata

Type C (Chronic) reactions due to long-term exposure, e. g. analgesic nephropathy, dyskinesi with levodopa. Type D (Delayed) reactions following prolonged exposure, e. g. carcinogenesis or short-term exp at a critical time, e. g. teratogenesis. Type E (Ending of use) reactions, where dicontinuation of chronic therapy is too abrupt, e of adrenal steroid causing rebound adrenocortic insufficiency, of opioid causing the withdrawal syndrome. F (Failures) – unwanted drug interactions.

Causes of drug ARs (1) The patient may be predisposed by age, genetic con tendency to allergy, disease, personality, habits. (2) The drug. Anticancer agents are by their nature cyto Some drugs, e. g. digoxin, have steep dose-response cur small increments of dose are more likely to induce augm (type A) reactions. Other drugs, e. g. antimicrobials, have dency to cause allergy and may lead to bizarre (type B) r Ingredients of a formulation, e. g. coloring, flavoring, sodi tent, rather than the active drug may also cause ARs. (3) The prescriber. ARs may occur because a drug is u for an inappropriately long time (type C), at a critical pha in pregnancy (type D), is abruptly discontinued (type E) o with other drugs (unwanted interactions).

Severity of adverse drug react has been graded as: Minor: No therapy or antidote required. Moderate: Requires change in drug therapy, spe treatment or prolongs hospital stay by at least on Severe: Potentially life threatening, causes perm damage or requires intensive medical treatment. Lethal: Directly or indirectly contributes to death of the patient.

Drug ARs can be minimized (but not altogeth eliminated) by observing the following practices (by Tripathi, 2008): • Avoid all inappropriate use of drugs. • Use appropriate dose, route and frequency of drug adm • Elicit and take into consideration previous history of drug • Elicit history of allergic diseases and exercise caution (d allergy is more common in patients with allergic disease • Rule out possibility of unwanted drug interactions. • Use correct drug administration technique (e. g. i. v. injec aminophyllne must be slow). • Carry out appropriate laboratory monitoring (e. g. prothro and INR with acenocoumarol and warfarin, serum drug l lithium – 0. 4– 1 mmol/L).

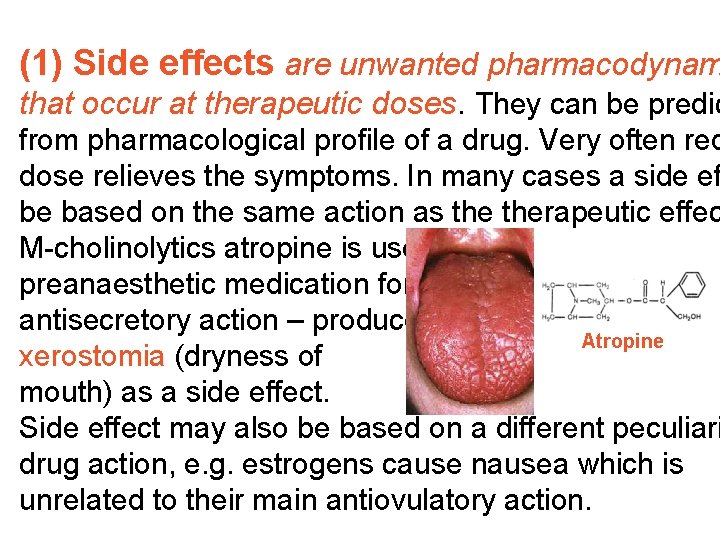
(1) Side effects are unwanted pharmacodynam that occur at therapeutic doses. They can be predic from pharmacological profile of a drug. Very often red dose relieves the symptoms. In many cases a side ef be based on the same action as therapeutic effec M-cholinolytics atropine is used in preanaesthetic medication for its antisecretory action – produces Atropine xerostomia (dryness of mouth) as a side effect. Side effect may also be based on a different peculiari drug action, e. g. estrogens cause nausea which is unrelated to their main antiovulatory action.

(2) Secondary effects are indirect consequen a primary action of the drug, e. g. suppression of b flora by aminopenicillins, cephalosporines, tetracy chloramphenicol, fluoroquinolones or co-trimoxaz results in the development of endogenous superin (3) Toxic effects are the result of excessive ph logical action of the drug due to overdosage or pro use. Overdosage may be absolute (accidental, ho suicidal) or relative (e. g. usual dose of aminoglyco antibiotics in presence of renal failure). Toxic effec predictable and dose related. They result from fun alteration (in high dose atropine causes delirium) induced tissue damage (hepatic paracetamol nec

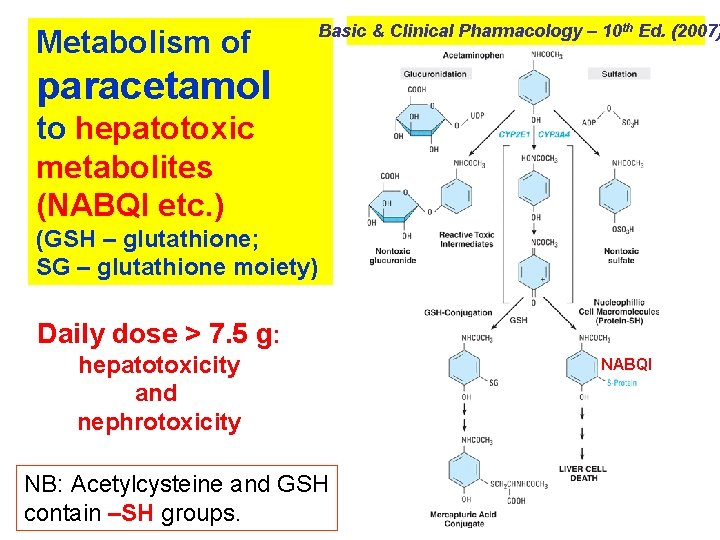
Acute paracetamol poisoning occurs specially in small children who have low hepatic glucoronide conjugating ability. If a large dose (> 150 mg/kg or > 10 g in adult) is taken, serious toxicity can occur. Letal dose is 250 mg/kg. N-acetyl-p-benzoquinoneimine (NABQI) is a highly reactive arylating metbolite of paracetamol which detoxified by conjugation with glutathione. When a very large doses paracetamol are taken, glucuroconjugation capacity is saturated, more NABQI is formed, hepatic glutathione is depleted and NABQI binds covalently to proteins in liver cells (and renal tubules) causing necrosis. In chronic alcoholic even 5– 6 g/d taken for a few days can result in hepatotoxicity because ethanol induces CYP 2 E 2, that metabolizes paracetamol, to NABQI. Treatment needs activated charcoal, given orally or through the tube to prevent GI absorption, and acetylcysteine (150 mg/g by i. v. infusion).

Metabolism of Basic & Clinical Pharmacology – 10 th Ed. (2007) paracetamol to hepatotoxic metabolites (NABQI etc. ) (GSH – glutathione; SG – glutathione moiety) Daily dose > 7. 5 g: hepatotoxicity and nephrotoxicity NB: Acetylcysteine and GSH contain –SH groups. NABQI

Poisoning may result from dosages of drugs. Sp antidotes (receptor antagonists, chelating agents o specific antibodies are available only for few poiso General supportive and symptomatic treatment i • Termination of exposure • Prevention of GI absorbtion of ingested poisons with sus of 20– 40 g of activated charcoal in 200 ml water. • Maintenance of patient airway (artificial respiration, if nee • Maintenance of blood pressure and heart beat by fluid in pressor agents, cardiac stimulants, if needed. • Hastening elimination of poison by inducing diuresis (furo mannitol), altering urinary p. H (alkalinisation for acidic dru acidification for basic drugs), haemodialysis and haemop (passage of blood through a column of charcoal or absor

(4) Intolerance is the appearance of characteristic effects of a drug in a patient at therapeutic doses, only a few doses of carbamazepine may cause ata some people; one tablet of chloroquine (250 mg) m cause vomiting and abdominal pain in some individ Intolerance indicates a low threshold of the individu the action of drugs. (5) Allergic reactions occur only in a small par population exposed to the drug. Prior sensitization needed and a latent period of at least 1– 2 weeks is after the first exposure. The drug or its metabolite antigen (AG) or more commonly hapten (incomple drugs with small molecules which become antigen

after binding with an endogenous protein) and ind production of antibody (AB)/sensitized lymphocyte Chemically related drugs often show cross sensitiv One drug can produce different types of allergic re The course of drug allergy is variable. An individua previously sensitive to a drug may subsequently to without a reaction. There are several types of all actions: humoral (type I–III) and cell mediated Type I (anaphylactic) reactions – immediate hy sensitivity. Reaging antibodies (Ig. E) fix to the ma On exposure to the drug, AG/AB reaction takes pl the mast cell surface releasing mediators (histami

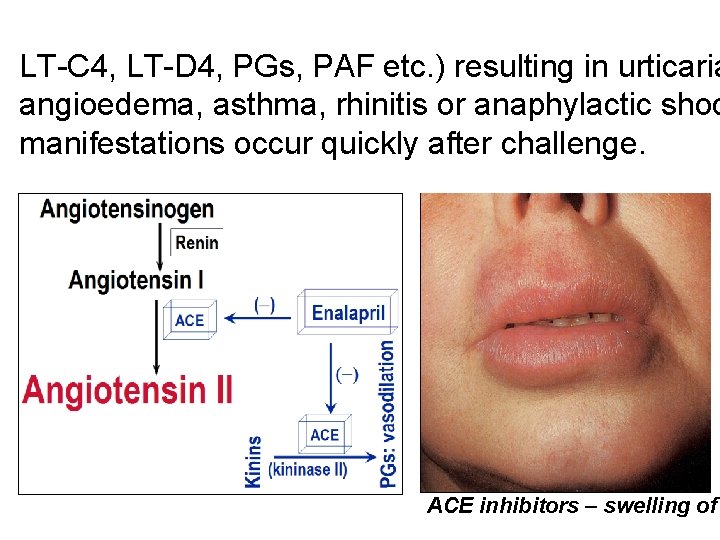
LT-C 4, LT-D 4, PGs, PAF etc. ) resulting in urticaria angioedema, asthma, rhinitis or anaphylactic shoc manifestations occur quickly after challenge. ACE inhibitors – swelling of

Type II (cytolytic) reactions. Drug + compon specific tissue cell act as AG. The resulting antibo (Ig. G, Ig. M) bind to the target cells. On reexposure reaction takes place on the surface of these cells, ment is activated and cytolysis occurs, e. g. thromb penia, agranulocytosis, aplastic anaemia, haemoly organ damage (liver, kidney, muscle), systemic lup erythematosus.

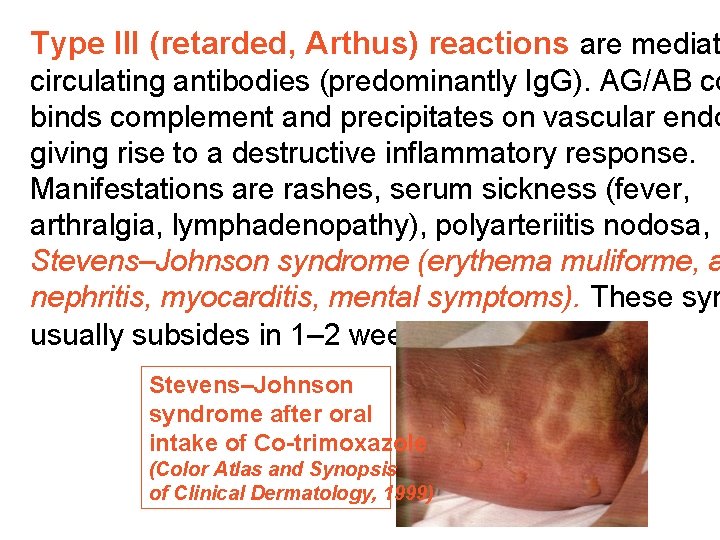
Type III (retarded, Arthus) reactions are mediat circulating antibodies (predominantly Ig. G). AG/AB co binds complement and precipitates on vascular endo giving rise to a destructive inflammatory response. Manifestations are rashes, serum sickness (fever, arthralgia, lymphadenopathy), polyarteriitis nodosa, Stevens–Johnson syndrome (erythema muliforme, a nephritis, myocarditis, mental symptoms). These sym usually subsides in 1– 2 weeks. Stevens–Johnson syndrome after oral intake of Co-trimoxazole (Color Atlas and Synopsis of Clinical Dermatology, 1999)

Type IV (delayed hypersensitivity) reactions. T mediated through production of sensitized T-lymph carrying receptors for the AG. On contact with AG phocytes produce lymphokines which attract gran and generate an inflammatory response, e. g. cont matatitis, some rashes, fever, photosensitization. T type allergic reactions generally take > 12 h to dev Treatment of drug allergy The offending drug must be immediately stopped. mild ARs (some skin rashes) subside without treat Antihistamine (H 1 -blockers desloratadine, levoceti are beneficial in type I reactions (urticaria, rhinitis,

swelling of lips) and some skin rashes. In case of anaphylactic shock or angioedema o a) Put the patients in reclining position, administer and perform cardiopulmonary resuscitation if req b) Inject adrenaline 0. 5 mg/1 ml i. m. (not i. v. ); repe 5 to 10 min if the patient does not improve. This life saving measure. c) Administer a H 1 -blocker (e. g. chlorpheniramine mg i. m. or slow i. v. ). It may adjuvant value. d) In severe/recurrent cases inject slow i. v. methyl solone or betamethasone. It acts slowly but is sp valuable for prolonged reactions and in asthmat

(Tripathi, 2008) Skin test (intradermal, patch) or intranasal test ma forewarn in case Type I hypersensitivity but not in other types.

(6) Idiosyncrasy is genetically determined abn reactivity to a drug and other xenobiotics. Cer of some drugs are largely restricted to individuals a particular genotype. Acetylation is an important route of metabolism for many drugs that possess an amine (-NH 2) gro Most individuals are either rapid or slow acetylato but the proportion of each varies greatly between races. Some 90% of Japanese are rapid acetylato whereas in Western populations the proportion is 50% or less. Isoniazid may cause peripheral neuropathy in slow acetylators on standard doses

and pyridoxine is added to the antituberculosis regimen where there is special risk, e. g. in diab alcoholism, renal failure. Acute hepatocellular necrosis with isoniazid is more common in rapid acetylators, perhaps because they more readily form an hepatotoxic metabolite. Sulphasalazine (used for rheumatoid arthritis) causes ARs more frequently in slow acetylators, probably because of the sulphapyridine, component whic inactivated by acetylation. Bacterial resistance to drugs is genetically determined and is of great clinical importance.

Individuals who are Glucose-6 -phosphate dehydrogenase (G 6 PD) deficient may suffer from acute haemolysis if they are exposed to cert oxidant substances, including drugs (dapsone, methylene blue, nitrofurantoin, primaquine, fluoroquinolone, some sulphonamides). Characteristically there is an acute haemolytic episode 2– 3 days after starting the drug. The haemolysis self-limiting, only older cells with least enzyme being affected. The condition is common in Africa Mediterranean, Middle East and South East Asia The porphyrias comprise a number of rare, gene determined enzyme defects in haem biosynthesis

Porphyrins represent precursors of haem. But people with porphyria the various porphyrins accu Acute porphyrias are characterized by severe atta of neurovisceral dysfunction precipitated principa a wide variety of drugs (and by alcohol and infect Certain peculiarities of an individual (for which no genotype has been described) are included amon idiosyncratic reactions, e. g. phenobarbital causes excitement and mental confusion in some patient

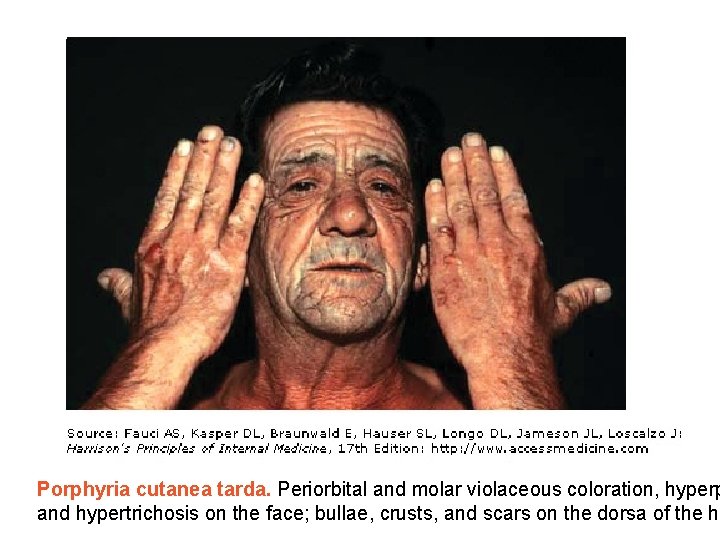
Porphyria cutanea tarda. Periorbital and molar violaceous coloration, hyperp and hypertrichosis on the face; bullae, crusts, and scars on the dorsa of the ha

(7) Drug dependence is a state arising from re periodic or continuous administration of a drug, tha results in harm to the individual and sometimes to (Bennett ad Brown, 2003). The subject feels a des or compulsion to continue using the drug and feels ruptly deprived of it (abstinence or withdrawal synd Drug dependence is characterized by: • Psychological dependence: there is emotional di if the drug is withdrawn. • Physical dependence: there is a physical illness drug is withdrawn. • Tolerance.

Psychological dependence develops when ind believe the optimal state of wellbeing is achieved through the action of drugs. It may start as liking drug effects and may progress to compulsive dru in some individuals. The intensity of psychologica dependence may vary from desire to craving. Reinforcement is the ability of drug to produce ef that make the user whish to take it again. Opioids cocaine and amphetamine are strong reinforcers while benzodiazepines are week reinforcers.

Physical dependence is an altered physiologica produced by repeated administration of a drug wh necessitates the continued presence of the drug t maintain physiological equilibrium. Discontinuatio drug results in a characteristic withdrawal (abstine syndrome. Drugs producing physical dependence are depres of CNS: opioids (morphinomimetics), barbiturates benzodiazepines, alcohol. Central nervous stimulants such as amphetamine cocaine produce little or no physical dependence

Drug abuse means the use of psychotropic substa in a way that would “constitute a public health and problem”. For regulatory agency “drug abuse” refe any use of an illicit drug. Drug addiction is a pattern of compulsive drug us characterized by overwhelming involvement with th use of a drug. Amphetamines, cocaine, cannabis, are drugs which produce addiction but little or no p dependence. Drug habituation is less intensive involvement wi drug, so that its withdrawal produces only mild disc without physical dependence (e. g. tea, coffee, toba

Types of drug dependence (Bennett, Brown Morphine-type: — psychological dependence severe — physical dependence severe; develops quickly — tolerance marked — cross-tolerance with related drugs — naloxone induces abstinence syndrome. Barbiturate-type: — psychological dependence severe — physical dependence very severe; develops slowly at high doses — tolerance less marked than with morphine — cross-tolerance with alcohol and benzodiazepine

Amphetamine-type: — psychological dependence severe — physical dependence slight: psychoses occur during use — tolerance occurs. Cannabis-type: — psychological dependence — physical dependence dubious (no characteris abstinence syndrome) — tolerance occurs. Cocaine-type: — psychological dependence severe — physical dependence slight — tolerance slight (to some actions).

Alcohol-type: — psychological dependence severe — physical dependence with prolonged heavy us — cross-tolerance with other sedatives. Tobacco-type: — psychological dependence — physical dependence. Drug mixtures: Barbiturate-amphetamine mixtur induce a characteristic alteration of mood that do not occur with either drug alone — psychological dependence strong — physical dependence occurs — tolerance occurs. Heroin-cocaine mixtures: similar characteristics

(8) Drug withdrawal syndrome – sudden inte of therapy with certain drugs (glucocorticoids, anti drugs, CV drugs etc. ) usually results in ARs, most form of worsening of the clinical condition for whic drug was being used. Examples: • Frequency of seizures may increase on sudden withdra of an antiepileptic drug. • Worsening of angina pectoris of acute myocardial infarc may result from stoppage of beta-blockers of nitrovasod • Severe hypertension and sympathetic overactivity may shortly after discontinuing clonidine. In all these cases is very important to keep pat compliance and/or to stop drug gradually.

(9) Effects of prolonged administrat Chronic organ toxicity (types C and D reac Eye. Toxic cataract can be due to chloroquine and related drugs, adrenal steroids (topical and systemic), phenothiazines and alkylating agents Corneal opacities occur with phenothiazines an chloroquine. Retinal injury occurs with thioridaz (particularly of the antipsychotics), chloroquine indomethacin. Nervous system. Tardive dyskinesias occur w neuroleptics; polyneuritis with metronidazole; optic neuritis with ethambutol.

Pulmotoxicty. Amiodarone may cause pulmonary Sulphasalazine is associated with fibrosing alveolit Nephrotoxicity: Aminoglycosides, polymyxines, g Hepatotoxicity: Methotrexate, paracetamol, halot Carcinogenesis. The principal mechanisms are: • Alteration of DNA (genotoxicity, mutagenicity). • Immunosuppression. A wide range of cancers dev in immunosuppressed patients, e. g. after organ transplantation and cancer chemotherapy. • Hormonal. Long-term use of estrogen replacemen postmenopausal women induces endometrial can

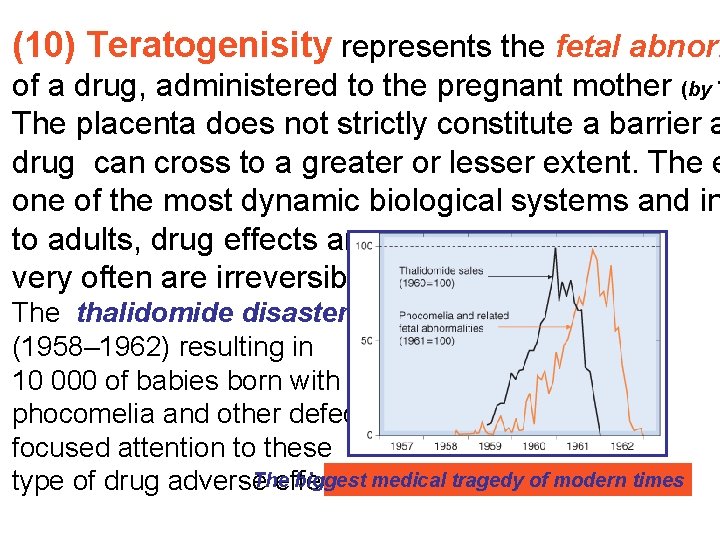
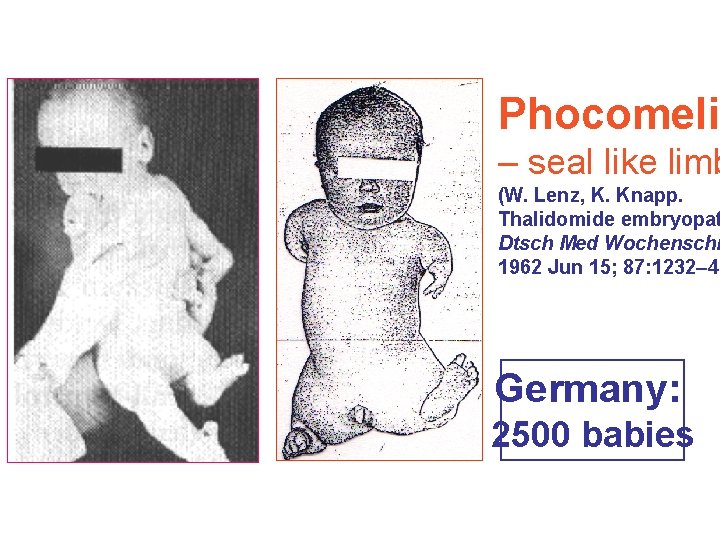
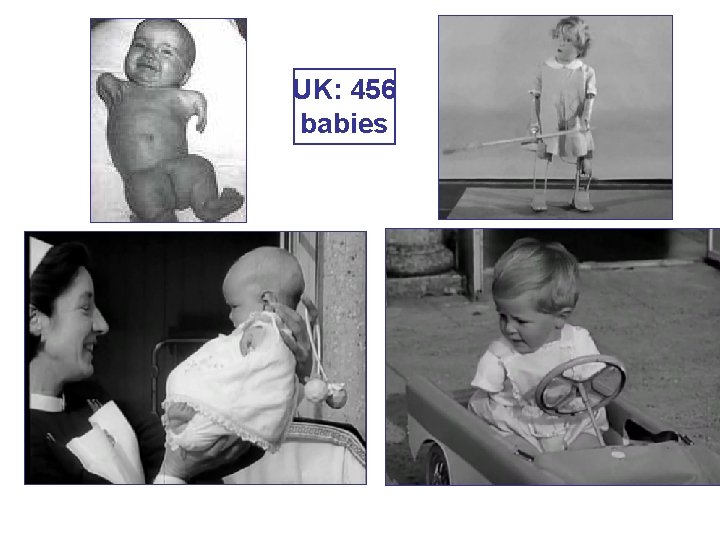
(10) Teratogenisity represents the fetal abnorm of a drug, administered to the pregnant mother (by T The placenta does not strictly constitute a barrier a drug can cross to a greater or lesser extent. The e one of the most dynamic biological systems and in to adults, drug effects are very often are irreversible. The thalidomide disaster (1958– 1962) resulting in 10 000 of babies born with phocomelia and other defects focused attention to these Тhe biggest medical tragedy of modern times type of drug adverse effects.

The Australian obstetrician William Mc. Bridge and the German ediatrician Widukind Lenz suspecte a link between birth defects and the drug, and this was proved by Lenz in 1961. Mc. Bride was later awarded a number of honours ncluding a medal and prize money b the L'Institut de la Vie in Paris.

Phocomelia – seal like limb (W. Lenz, K. Knapp. Thalidomide embryopat Dtsch Med Wochenschr 1962 Jun 15; 87: 1232– 42 Germany: 2500 babies

UK: 456 babies

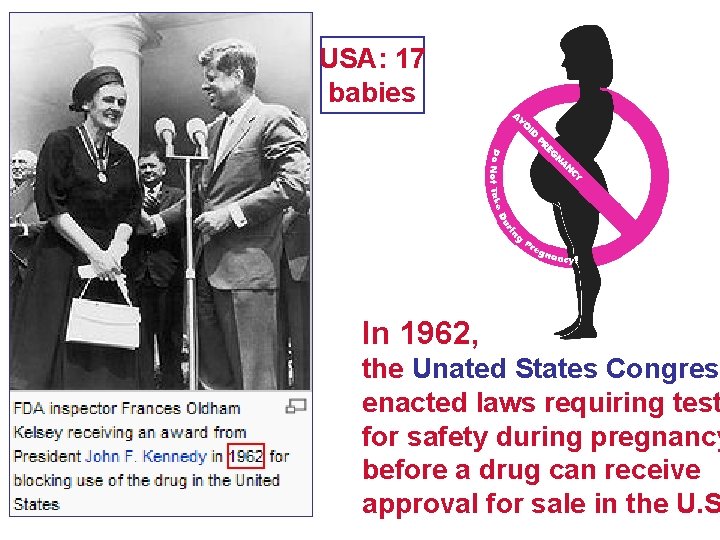
USA: 17 babies In 1962, the Unated States Congress enacted laws requiring test for safety during pregnancy before a drug can receive approval for sale in the U. S


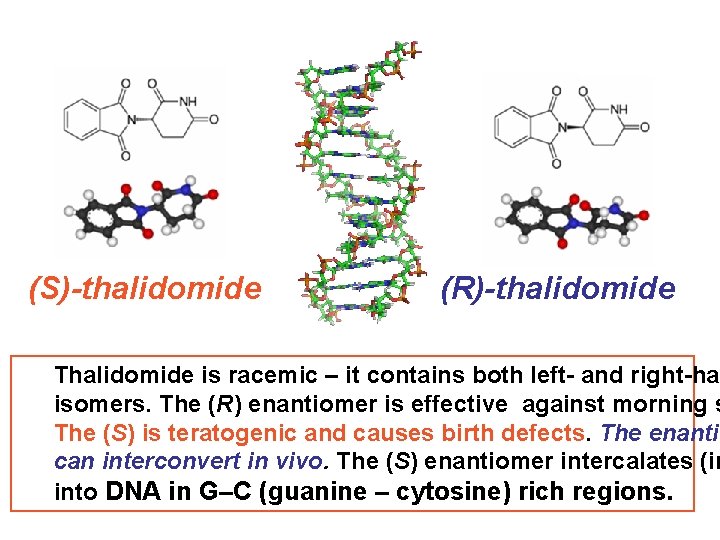
(S)-thalidomide (R)-thalidomide Thalidomide is racemic – it contains both left- and right-han isomers. The (R) enantiomer is effective against morning s The (S) is teratogenic and causes birth defects. The enantio can interconvert in vivo. The (S) enantiomer intercalates (in into DNA in G–C (guanine – cytosine) rich regions.

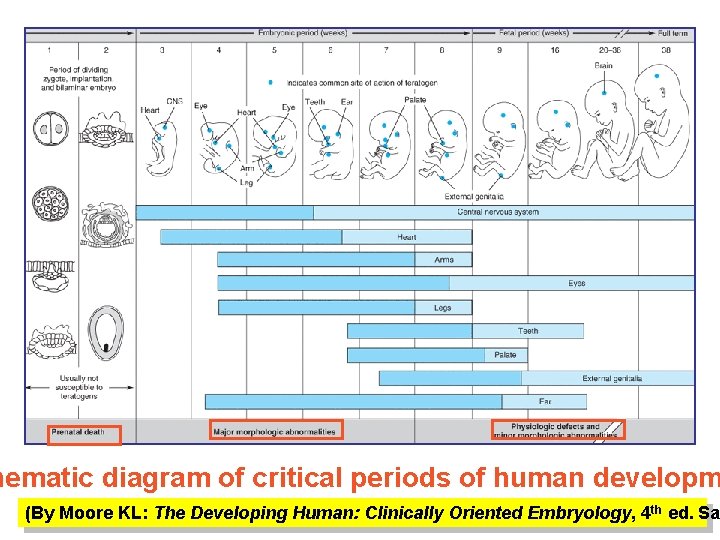
Drugs can affect the fetus at follow stages • Fertilization and implantation – from conception days: failure of pregnancy (which often goes unno • Embryogenesis (organogenesis) – between 15– days of gestation: most vulnerable period, deformi are produced. • Fetogenesis (growth and development) – from 56 gestation to birth: developmental and functional ab ties can occurs, e. g. aminosides can cause ototox ACE inhibitors – hypoplasia of cranium, lungs and NSAIDs may induce premature closure of d. arteri The type of malformation depends on the drug as stage of exposure to the teratogen.

Embryogenes is Dorland’s Illustrated Medical Dictionary (2003, 2004)

hematic diagram of critical periods of human developm (By Moore KL: The Developing Human: Clinically Oriented Embryology, 4 th ed. Sa

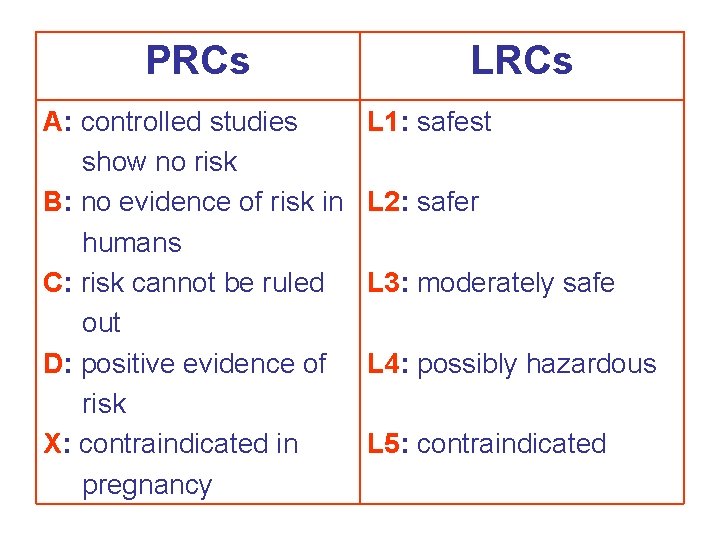
Pregnancy Risk Categories (PRCs) – FDA A B C The FDA (USA) has established 5 categories to indicate the potential of systematically absorbed drug for causing birth defects. Th key differentiation among the categories rests upon the reliability of documentation and the risk: benefit ratio (Lacy et al. , 1998). D X

PRC A: Controlled studies in pregnant women f demonstrate a risk to the fetus in the first trimest no evidence of risk in later trimesters. The possib fetal harm appears remote. Examples: Folic acid, T 4, Magnesium sulfate (in PRC B: The animal-reproduction studies have n demonstrated a fetal risk but there are no contro studies in pregnant women, or animal-reproduct studies have shown an ARs (other than a decrea fertility) that was not confirmed in controlled stud women in the first trimester and there is no evide of later trimesters. Examples: penicillins, erythro paracetamol, lidocaine.

PRC C: The studies in animals have revealed AR on the fetus (teratogenic, embryocidal or other eff and there are no controlled studies in women, or in women are not available. Drug should be given if the potential benefits justify the potential risk to Examples: atropine, adrenaline, thiopental, bisop PRC D: There is positive evidence of human feta but the benefits from use in pregnant women may acceptable despite the risk (e. g. if the drug is nee a life-threatening situation or for a serious disease which safer drugs cannot be used are ineffective. Examples: phenytoin, valproate, diazepam, loraz

PRC X: Studies in animals or human beings have demonstrated fetal abnormalities or there is evide of fetal risk based on human experience, or both, the risk of the use of the drug in pregnant women outweighs any possible benefit. The drug is contr cated in women who are or may become pregnan Examples: thalidomide, estrogens, isotretionoin, ergometrine.

(Tripathi, 2008)

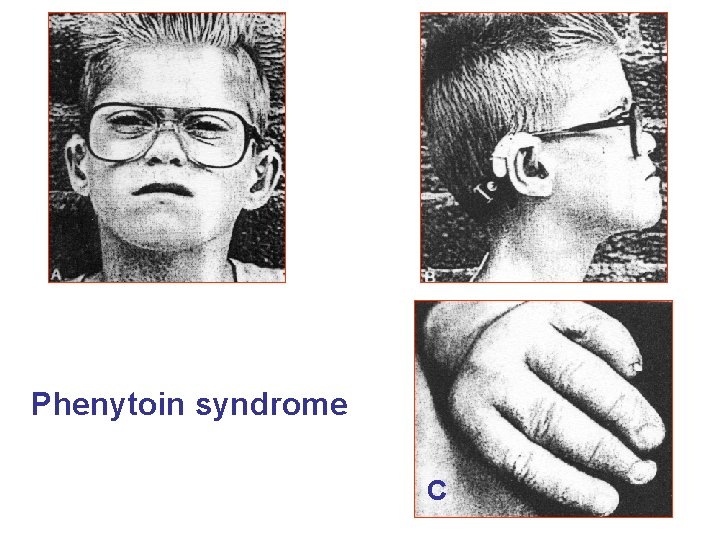
Phenytoin syndrome С

11) Lactation Risk Categories – LRCs (adapted by T. Hale, 2004; 2008 – Medications and Mothers’ Milk) L 1: Safest Drug which has been taken by a large numbe of breastfeeding mothers without any observ increase in adverse effects in the infant. Controlled studies in breastfeeding women fa demonstrate a risk to the infant and the possi of harm to the breastfeeding infant is remote; or the product is not orally bioavailable in an Examples: Paracetamol, Ibuprofen, Epinephri

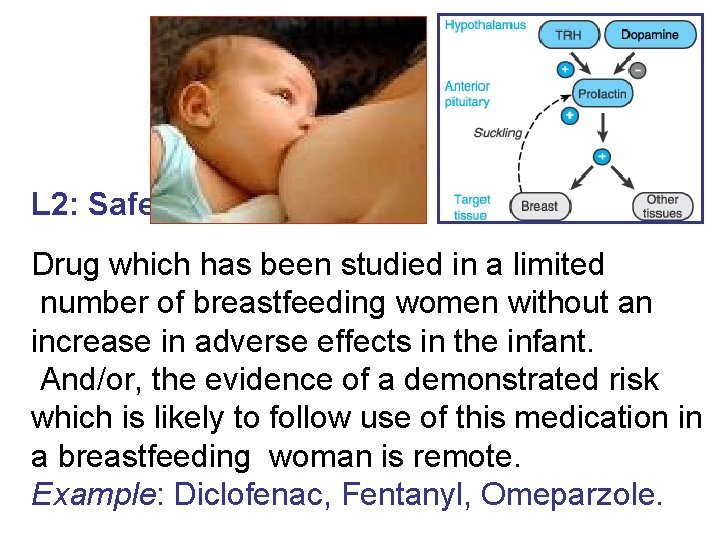
L 2: Safer Drug which has been studied in a limited number of breastfeeding women without an increase in adverse effects in the infant. And/or, the evidence of a demonstrated risk which is likely to follow use of this medication in a breastfeeding woman is remote. Example: Diclofenac, Fentanyl, Omeparzole.

L 3: Moderately Safe There are no controlled studies in breastfeeding women, however the risk of untoward effects to a breastfed infant is possible; or, controlled studies show only minimal non-threatening adverse effects. Drugs should be given only if the potential benefi justifies the potential risk to the infant. Examples: Acarbose, Aspirin, Indometacin, Morp

L 4: Possibly Hazardous There is positive evidence of risk to a breastfed or to breastmilk production, but the benefits from in breastfeeding mothers may be acceptable de the risk to the infant. (e. g. if the drug is needed i life-threatening situation or for a serious disease which safer drugs cannot be used or are ineffec Examples: Colchicine, Diazepam (in chronic use Lithium

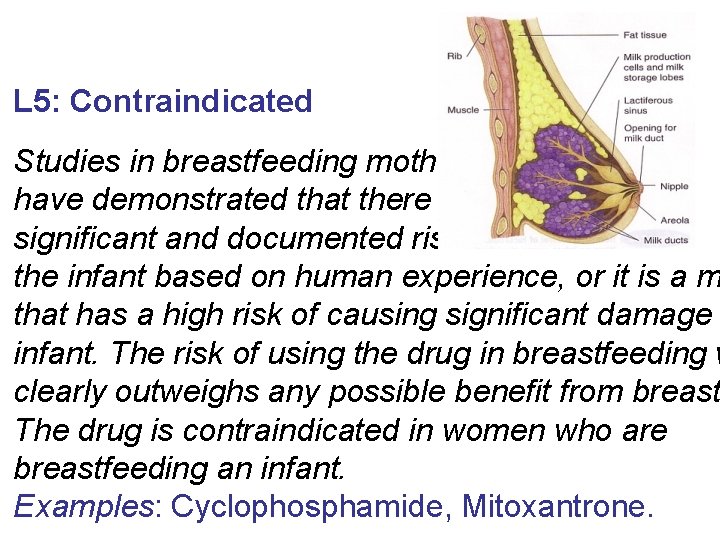
L 5: Contraindicated Studies in breastfeeding mothers have demonstrated that there is significant and documented risk to the infant based on human experience, or it is a m that has a high risk of causing significant damage infant. The risk of using the drug in breastfeeding w clearly outweighs any possible benefit from breast The drug is contraindicated in women who are breastfeeding an infant. Examples: Cyclophosphamide, Mitoxantrone.

PRCs A: controlled studies show no risk B: no evidence of risk in humans C: risk cannot be ruled out D: positive evidence of risk X: contraindicated in pregnancy LRCs L 1: safest L 2: safer L 3: moderately safe L 4: possibly hazardous L 5: contraindicated

(12) Cancerogenesity and mutagenesity re capacity of a drug to cause cancer and genetic de respectively. Oxidation of the drug may result in th production of reactive intermediate compounds wh affect genesis and can case structural changes in chromosomes. Chemically cancerogenesis usually 10– 40 years to develop. Chemical compounds imp in these ARs include some anticancer drugs, radio estrogens, nicotine (tobacco) etc.

(13) Drug induced diseases are also called iatrogenic (physician induced) diseases. They re functional disturbances (diseases) caused by drug persist even after the offending remedy has been withdrawn and largely eliminated. Examples: • Parkinsonism by phenothiazine and other neuro • Hepatitis by isoniazid • Peptic ulcer by salicylates, glucocorticoids or res • Aplastic anaemia by chloramphenicol etc.

Selected References Bennett PN, Brown MJ. Clinical pharmacology. 9 th Edition Churchill Livingstone, 2003. Color Atlas and Synopsis of Clinical Dermatology. 3 rd Ed. B. Fitzpatrick (ed. ). Mc Grow-Hill International, Ney Yo Katzung BG (Editor). Basic & Clinical Pharmacology. 10 th The Ma. Graw-Hill Companies, Inc. (Lange Medical Boo New York, 2007. Lacy CF et al. (eds). Drug Information Handbook. 6 th Edit Lexi-Comp Inc. Hudson (Cleveland), APh. A, 1998 -99. Rang HP et al. Pharmacology. 6 th Edition. London, Chirch Livingstone, 2007. Tripathi KD. Essentials of Medical Pharmacology. 6 st Edit Jaypee Brothers. New Delhi, 2008.
 Masaryk university medical faculty
Masaryk university medical faculty Masaryk university medical faculty
Masaryk university medical faculty Keralastec
Keralastec Elearn st kliment
Elearn st kliment University of chemical technology and metallurgy sofia
University of chemical technology and metallurgy sofia Technical university sofia
Technical university sofia Technical university of sofia
Technical university of sofia Medical faculty in novi sad dean
Medical faculty in novi sad dean Herszon kherson maritime college of merchant marine fleet
Herszon kherson maritime college of merchant marine fleet University of bridgeport engineering
University of bridgeport engineering University of bridgeport engineering
University of bridgeport engineering Hubert kairuki memorial university faculty of medicine
Hubert kairuki memorial university faculty of medicine Semmelweis university faculty of medicine
Semmelweis university faculty of medicine King abdulaziz university faculty of medicine
King abdulaziz university faculty of medicine Fsu computer science
Fsu computer science Faculty of business and economics mendel university in brno
Faculty of business and economics mendel university in brno Singularity executive program
Singularity executive program Territorial matrix vs interterritorial matrix
Territorial matrix vs interterritorial matrix Faculty of engineering university of porto
Faculty of engineering university of porto Webkredit cuni
Webkredit cuni Faculty of veterinary medicine cairo university logo
Faculty of veterinary medicine cairo university logo Faculty of law of the university of zagreb
Faculty of law of the university of zagreb University of montenegro faculty of law
University of montenegro faculty of law University of kragujevac faculty of technical sciences
University of kragujevac faculty of technical sciences University of cologne faculty of management
University of cologne faculty of management Leading university founder
Leading university founder Hacettepe university faculty of medicine
Hacettepe university faculty of medicine Brown university computer science faculty
Brown university computer science faculty Auricle of heart vs atrium
Auricle of heart vs atrium Semmelweis university faculty of medicine
Semmelweis university faculty of medicine Lebanese university roumieh
Lebanese university roumieh York university my file
York university my file University of debrecen faculty of economics and business
University of debrecen faculty of economics and business Clemson electrical engineering
Clemson electrical engineering Faculty of veterinary medicine cairo university
Faculty of veterinary medicine cairo university Faculty of mechanical engineering thammasat university
Faculty of mechanical engineering thammasat university Faculty of business and economics mendel university in brno
Faculty of business and economics mendel university in brno Mahidol university public health
Mahidol university public health Facultad de ciencias de la salud uma
Facultad de ciencias de la salud uma Faculty of education khon kaen university
Faculty of education khon kaen university Shawhong ser
Shawhong ser Cairo university faculty of veterinary medicine
Cairo university faculty of veterinary medicine Tracer card in medical records
Tracer card in medical records Medical education and drugs department
Medical education and drugs department Department of law university of jammu
Department of law university of jammu Department of geology university of dhaka
Department of geology university of dhaka Mechanicistic
Mechanicistic University of bridgeport it department
University of bridgeport it department University of iowa math department
University of iowa math department Sputonik v
Sputonik v Texas state university psychology
Texas state university psychology Department of information engineering university of padova
Department of information engineering university of padova Department of information engineering university of padova
Department of information engineering university of padova Manipal university chemistry department
Manipal university chemistry department Syracuse university pool
Syracuse university pool Jackson state university finance department
Jackson state university finance department Computer science department columbia
Computer science department columbia Msu physics and astronomy
Msu physics and astronomy Columbia university cs department
Columbia university cs department University of sargodha engineering department
University of sargodha engineering department Stanford university philosophy department
Stanford university philosophy department California medical license application
California medical license application