Matter and the Atom Matter is anything that









- Slides: 15

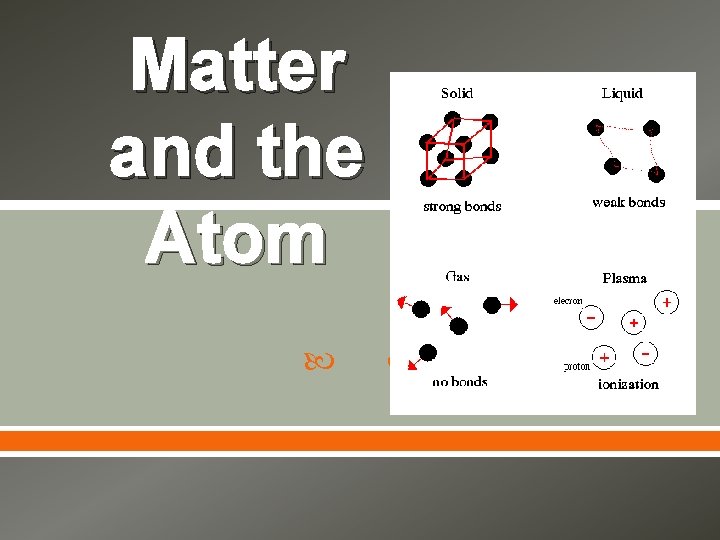
Matter and the Atom

Matter is anything that has mass and takes up space. The amount of space taken up, or occupied, by an object is its volume. One object cannot share with another object the same space at the same time

Matter and Mass is the amount of matter in an object. The mass of an object is the same no matter where in the universe the object is located. The only way to change an object’s mass is to change the amount of matter that makes up the object.

A little bit of atomic history All matter is made up of different kinds of atoms The study of atoms goes all the way back to 440 BC! Scientists realized that all matter is made of tiny particles called atoms • Atoms are the smallest particles that an element can be divided into and still be the same substance • In other words, you can’t get any smaller and have a stable substance

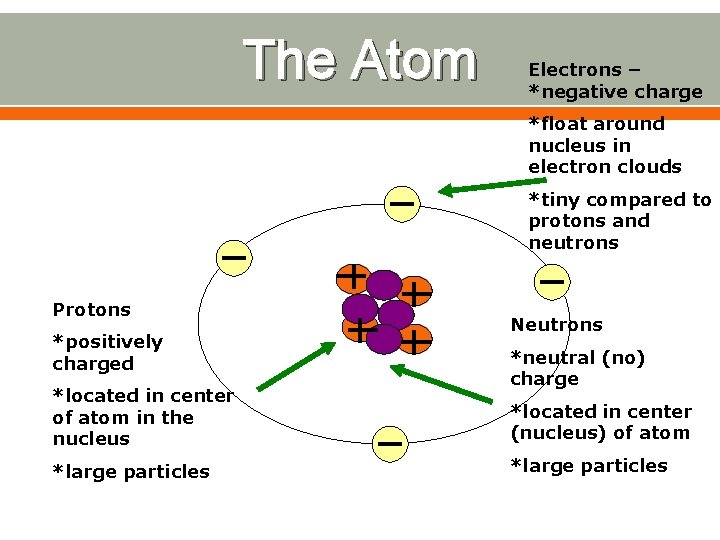
The history lesson continues Numerous scientists continued to study and do research on the atom o They discovered that there is a negatively charged part of the atom, which we now call electrons o Later on, it was suggested that the center of the atom has the positively charged nucleus • The negatively charged electrons float around the center

The Atom Electrons – *negative charge *float around nucleus in electron clouds *tiny compared to protons and neutrons Protons *positively charged *located in center of atom in the nucleus *large particles Neutrons *neutral (no) charge *located in center (nucleus) of atom *large particles

Weight – its just a number The parts of an atom are so small that scientists had to come up with a special way to measure their mass o Atoms and parts of atoms are measured in amus • Atomic mass units o Protons and neutrons have masses of approximately 1 amu • They provide almost all of the mass of an atom o Electrons are tiny particles • They are so small that their mass is seen as being 0 amus

Charge!! What is the charge on the nucleus? o Positive Why? o Protons have a positive charge and neutrons are not charged What is the charge on electrons? o Negative So what is the overall charge on an atom? o The positive nuclues and the negative electrons are opposites and therefore cancel each other out o Neutral – no charge

Why do we care about neutrons? As we’ve talked about charges on atoms, we’ve only been concerned with protons and electrons– why is this (why have we ignored neutrons)? o Because neutrons have no charge so they do not change the charge of an atom So when do we care about neutrons? o When we are talking about the mass of an atom

Weight – it’s a heavy subject Each atom has a specific atomic number o This number is equal to the number of protons found in its nucleus The atomic mass number of each atom is equal to the sum of the number of protons and the number of neutrons in an atom’s nucleus o Why is it just the protons and neutrons? • Electrons are so small that their mass does not matter, only their charge o Ex: if an atom has 8 protons and 9 neutrons, its mass number would be 17 amu

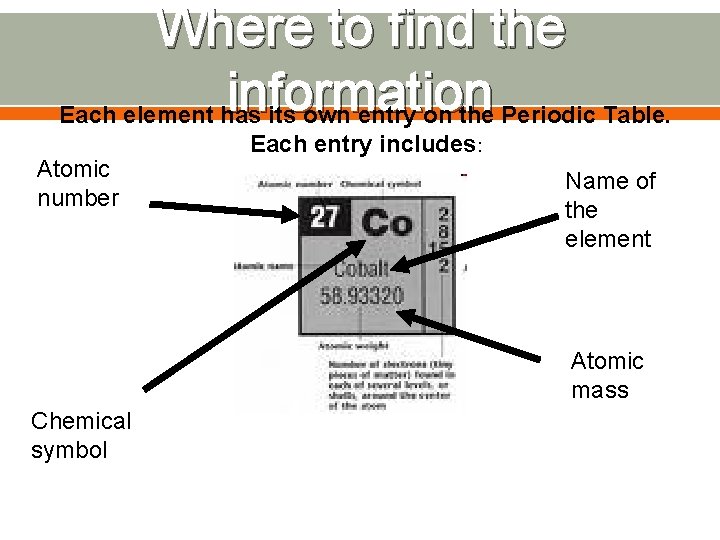
Where to find the information Each element has its own entry on the Periodic Table. Atomic number Each entry includes: Name of the element Atomic mass Chemical symbol

The mass of atoms is written as a decimal because its an average of all types of each element o Each element may have several isotopes • The same element with the same number of protons and electrons, but different numbers of neutrons • Ex: There are 15 isotopes of the element Carbon • The most abundant Carbon has 6 protons, electrons and neutrons • So the atomic number is 12 • There is also Carbon 14 which still has 6 protons and electrons, but 8 neutrons • The atomic mass is now 14 because of the extra neutrons

Its not just neutrons…. The number of neutrons is not the only thing that can change in an atom o You can also have different numbers of electrons o Atoms that have either gained or lost electrons are called ions • If an atom gains electrons, there will be more electrons than protons • These atoms will now be negatively charged • If an atom loses electrons, there will be less electrons than protons • These atoms will now be positively charged

Its ELEMENTary my dear Watson An element is a pure substance that cannot be separated into simpler substances o A pure substance is one in which there is only one type of particle, in this case atoms o Elements are the most basic substance on the planet

Arranging the elements All known elements are arranged in order of increasing atomic number in the periodic table of elements