MANUFACTURING FACILITIES OF STERILE PRODUCTS N SANTHI PRIYA





























- Slides: 34

MANUFACTURING FACILITIES OF STERILE PRODUCTS N. SANTHI PRIYA, Mpharm ASSISTANT PROFESSOR CHALAPATHI INSTITUTE OF PHARMACEUTICAL SCIENCES 1

INTRODUCTION Ø Sterile products are dosage forms of therapeutic agents that are free of viable microorganisms. Ø These include : Parenteral preparations and Ophthalmic preparations. Ø Sterile products are more frequently solutions or suspensions and may even be solid pellets for tissue implantation. Ø All components and process involved in the preparation of these products must be selected and designed to eliminate as much as possible, contamination of all types. 2

INTRODUCTION • Pareteral products are injected through the skin or mucous membranes into the internal body compartments. • They may be given by various routes: intravenous, intramuscular, intraspinal, subcutaneous and intradermal. • Ophthalmic preparations are not introduced into the internal body cavities but are placed in contact with tissues that are very sensitive to contamination. 3

DEFINITIONS v ASEPTIC: Area or process obtained by sterilization. v A condition in which every reasonable means have been used to destroy or eliminate viable microorganisms. v ASEPTIC PROCESSING: Those operations performed between the sterilization of an object and the final sealing of its package carried out in the complete absence of microorganisms. v This process is filling of product into individual containers. v This process is used for the products that cannot be terminally sterilized. 4

Terminal sterilization Aseptic processing §Product containers are filled §Drug product, container, and closure and sealed under high-quality are subject to sterilization separately, environmental conditions designed and then brought together. to minimize contamination, but not to guarantee sterility. §Because there is no process to sterilize §Product in its final container is the product in its final container, it is subject to a sterilization process critical that containers be filled and such as heat or irradiation. sealed in an extremely high –quality environment. 5

CLEAN AREA CLASSIFICATION UNITED STATES CLASSIFICATI ON INTERNATION AL SOCIETY OF PHARMA. ENG. DESCRIPTION MAX. NO. OF PARTICLES PER METER CUBE >/=0. 5µm MAX. NO. OF PARTICLES PER METER CUBE >/=5µm A 100 Critical 3, 500 0 B 1000 Clean 3, 5000 0 C 10, 000 Controlled 350, 000 2000 D 1, 000 pharmaceutical 3, 500, 000 20, 000 EUROPEAN GRADE 6

REQUIREMENTS FOR ASEPTIC PROCESSING q Facilities q Environmental control q Traffic control q House keeping q Surface disinfection q Air control q Personnel 7

FACILITIES The prevention of contamination must be the primary objective in the design of these facilities. The ceiling, walls and floors should be constructed of material that is easy to clean and non porous, to prevent the accumulation of debris and moisture. Best finishes for rigid surfaces is the “ spray - on - tail”. This is a ceramic epoxy finish applied by spraying or painting to form a continuous smooth , seal coating on the ceiling and walls. Ceramic – plastic cement is applied as thick coat on the existing rigid surface to form a continuous sealed surface. 8

FACILITIES In areas of less heavy traffic flouring material used is vinyl sheet. Glass is used in partitions to permit supervisory view of the operation. Furniture should be of made of stainless steel and counters should be suspended from the wall. Equipment that are difficult to sterilize should be kept outside the aseptic area. The water, electrical, gas, air ventilation and other utility service lines must be flour above, space beneath , or a corridor along side. 9

TRAFFIC CONTROL § Unauthorized personnel should never be permitted to enter the aseptic area. § Access by personnel to the aseptic corridor and compounding is only through an air lock. § Personnel should be permitted to enter aseptic area after removing their street clothing, washing their hands and wearing gowns, hats shoes, facemasks, gloves and other prescribed attire. § Once they entered into the area they should not be allowed to move in and out of the area without regowning. 10

HOUSE KEEPING & SURFACE DISINFECTION o All equipment and surrounding work area must be cleaned thoroughly at the end of working day. o All cleaning equipment should be selected for its effectiveness and free from lint - producing tendencies. o It should be reserved for use in the aseptic area only. o After cleaning, all surfaces should be disinfected an effective liquid disinfectant should be sprayed or wiped on all surfaces. 11

AIR FLOW v Air is primary source of contaminants in clean rooms. v Fresh outside air or recycled air must be filtered to remove gross particulate matter. v A spun glass, cloth or shredded polyethylene filter may be used for this preliminary cleaning operation. v Blowers should be installed in the air ventilation system upstream to the filters so that all dirt producing devices are ahead of the filters. v The clean aseptic area must be distributed in such a manner that it flows at a greatest volume flow rate. v The air velocity employed should be 100+/- 20 ft/min. 12

AIR FLOW v The equipments that maintain air flow and controlled environment are: Laminar air flow chambers HEPA filters LAMINAR AIR FLOW CHAMBERS : These are also known as uni directional air flow equipments. The unidirectional air flow procedure was developed in 1961. In this device the entire air with in a confined area moves with in one direction with uniform velocity along parallel flow lines. 13

AIR FLOW The velocity of air used in these devices is 90 fpm+/-20%. There are three types of laminar air flow chambers. They are : Horizontal laminar air flow chamber Vertical laminar air flow chamber Curvilinear laminar air flow chamber. Ø In horizontal laminar air flow chamber , air moment is in horizontal direction and wash organisms from the operators hand. Ø But the disadvantage is that any air borne particulate matter generated in the units is blown directly against the working personnel. 14

AIR FLOW HEPA FILTER: § High efficiency particulate air filter. § This removes finer debris down to the submicron range including microorganisms. § It is defined as at least 99. 97% efficient in removing particles of 0. 3µm size and larger. § The first HEPA filters were developed in the 1940's by the USA Atomic Energy Commission to fulfill a top-secret need for an efficient, effective way to filter radioactive particulate contaminants. 15

AIR FLOW Ø The most common designs are a box filter cell and a cylindrical filter cell. In a box cell the pleated media is placed in a rigid, square frame constructed of wood or metal. The air flows from the front to the back of the filter. Ø The media in a cylindrical filter cell is supported by inner and outer wire frameworks. A metal cap seals the media at one end. Air flows from the outside to the inside of the filter. 16

CLASSIFICATION OF HEPA FILTERS Ø Based on efficiency HEPA filters are classified into two types. They are : Ø Type 1: 99. 99% efficient filters. Ø These are used for laminar flow and ultra low penetration air (ULPA). Ø Type 2: 99. 97% efficient filters. Ø They are used in industrial, non critical, nuclear containment, toxic, nuclear, and biohazard containment. 17

MECHANISM OF FILTRATION Ø Filtration by interception : § Direct interception works on particles in the mid-range size that are not quite large enough to have inertia and not small enough to diffuse within the flow stream. These mid-sized particles follow the flow stream as it bends through the fiber spaces. Particles are intercepted or captured when they touch a fiber. 18

MECHANISM OF FILTRATION Ø Filtration by Brownian diffusion : § Very fine particles in the air stream will collide with gas molecules and create a random path through the media. The smaller the particle the longer the particle will zigzag around. This random motion increases the probability of the particle contacting a fiber. 19

MECHANISM OF FILTRATION Ø Filtration by sieving : § Sieving, the most common mechanism in filtration. Sieving stops large particles that are just too big to fit through the open areas of the filter. This includes all particles above 5 μm in size and larger. 20

HEPA FILTER TESTING v FILTER LEAK TESTING : • Any aerosol used for challenging a HEPA filter should meet specifications for critical physicochemical attributes such as viscosity. • Dioctylphthalate (DOP) and poly-alpha-olefin (PAO) are examples of appropriate leak testing aerosols. • The challenge involves use of a polydispersed aerosol usually composed of particles with a light-scattering mean droplet diameter in the submicron size range, 9 including a sufficient number of particles at approximately 0. 3 μm. • A single probe reading equivalent to 0. 01 percent of the upstream challenge would be considered as indicative of a significant leak and calls for replacement of the HEPA filter. 21

PERSONNEL The people who produce sterile products are generally non professional persons. They must be neat and alert. All employees should in good health and should be subjected to periodic physical examination. The attire worn by the personnel consists of sterile coverall, face masks, shoe covers, caps and rubber gloves. Coverall is made of material dacron. It is made of a continuous fiber, which makes it essentially lint – free. Hats and masks are made of special parchment paper and are discarded after use. 22

FILLING • Aseptic fill is defined as a process of filling sterile containers with sterile products under controlled conditions but without terminal sterilization of the product. • A liquid can be subdivided from bulk container to individual dose containers more easily and uniformly than a solid. • Subdivision of mobile, low viscous liquids can be achieved with light duty machinery but viscous sticky liquids require machines that can with stand pressure required to fill the containers. 23

FILLING EQUIPMENT FOR LIQUID Containers are filled by repetitively forcing a measured volume of the liquid through the orifice of a delivery tube into the opening of the container. The tube must freely enter the neck of the container. The delivery of the small volume of liquids is obtained from the stroke of the plunger of a syringe. The stroke of the syringe forces the liquid through a two way valve that provides for an alternate filling of the syringe from the reservoir and deliver to a container. 24

FILLING EQUIPMENT FOR LIQUID Ø The parts of the filling machines should be designed in a way that they can be demounted easily for cleaning and for sterilization. Ø These parts should be constructed of stainless steel or borosilicate glass. Ø Bottles of solution are filled by gravity, pressure or vacuum filling machines. v GRAVITY FILLER : • Gravity filling is relatively slow but is a simple manner. • The liquid reservoir is positioned above the filling line with a hose connection from the reservoir to a shut off device at the filling line. • The shut off device is hand operated and the bottles are filled to graduations. 25

FILLING EQUIPMENT FOR LIQUID v PRESSURE PUMP FILLER: § The pressure pump is operated semiautomatically and the liquid is under pressure. § It is equipped with an overflow tube connected to a reservoir to prevent excess filling of the container. v VACUUM FILLER: o Vacuum filling is used for large liquid volumes. o A vacuum is created in the bottle when the nozzle gasket makes a seal against the lip of the bottle, vacuum draws the liquid from the reservoir into the bottle. o When the liquid reaches seal is loosened mechanically and the vacuum is released. 26

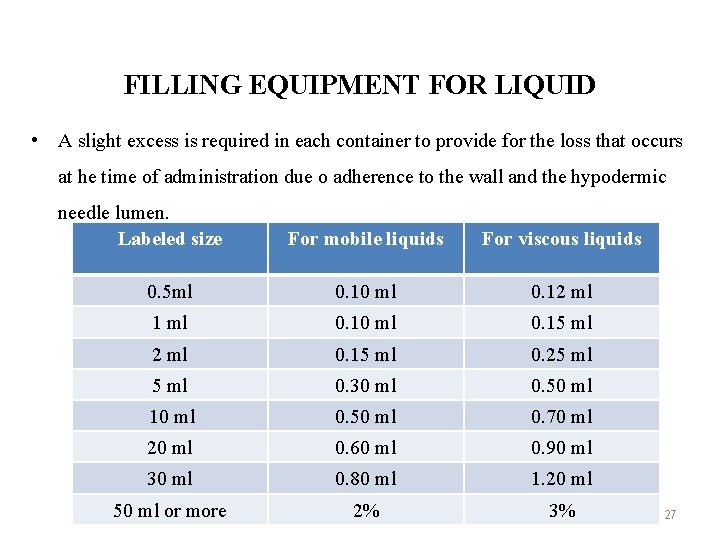
FILLING EQUIPMENT FOR LIQUID • A slight excess is required in each container to provide for the loss that occurs at he time of administration due o adherence to the wall and the hypodermic needle lumen. Labeled size For mobile liquids For viscous liquids 0. 5 ml 0. 10 ml 0. 12 ml 1 ml 0. 10 ml 0. 15 ml 2 ml 0. 15 ml 0. 25 ml 0. 30 ml 0. 50 ml 10 ml 0. 50 ml 0. 70 ml 20 ml 0. 60 ml 0. 90 ml 30 ml 0. 80 ml 1. 20 ml 50 ml or more 2% 3% 27

FILLING EQUIPMENT FOR SOLID • The rate of flow of solid material tends to be slow and irregular, particularly if finely powdered. • Containers with a relatively large opening must be used , though the filing rate is slow and the risk of spillage is present. • Sterile solids can be subdivided into containers by individual weighing, but this is a slow process. • For the solids which are relatively in free flowing form, machine methods of filling can be employed. • In machine methods volume of the solid material that should be delivered is calibrated in terms of weight desired. 28

FILLING EQUIPMENT FOR SOLID § Major problems in using machines are : Ø Stratification of particles due to vary in sizes. Ø Development of electrostatic charges with in the mass of dry solid particles. Ø Formation of air pockets. Ø uneven flow due to clumping of particles. § The problems can be minimized by attaining uniform sized particles and a small electric current is used to neutralize the developing charges. 29

SEALING • Containers should be sealed in the aseptic are immediately adjacent to the filling machine. • Sealing of containers assures the user that it has not been opened. Ø SEALING AMPULES : § Ampoules can be closed by melting a portion of the glass of the neck to form either bead seals (tip – seals) or pull seals. § Tip seals are made by melting sufficient glass at the tip of the ampoule neck to form a bead of glass and close the opening. § Pull seals are made by heating the neck of a rotating ampoule below the tip, then pulling the tip away to form a small , twisted capillary just prior to being melted closed. 30

SEALING • Powder ampoules and other types having wide opening must be sealed by pull sealing. • Excessive heating of air and gases in the neck causes expansion against the soft glass with the formation of fragile bubbles at the point if seal. • Fracture of the neck of the ampoule often occurs during sealing if wetting had occurred at the time of filling. • It is necessary to displace the air in the space with in the ampoule above the product to prevent decomposition. • This is done by introducing a stream of inert gas such as nitrogen or carbondioxide and then immediately sealed. 31

SEALING Ø SEALING BOTTLES AND VIALS : • Rubber closures must fit the opening of the container snugly enough to produce a seal. • They can be inserted by hand , using sterile forceps. • A faster hand method involves picking up the closure and inserting it into a vial by means of a tool connected to a vacuum line. • When closures are to be inserted by means of a machine , the surface of the closure is usually halogenated or coated with silicone to reduce the friction. • Aluminium caps are used to hold rubber closures in place. 32

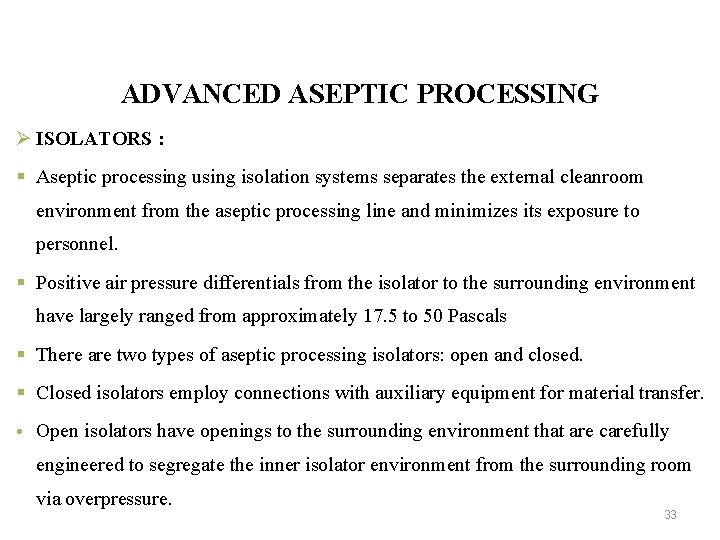
ADVANCED ASEPTIC PROCESSING Ø ISOLATORS : § Aseptic processing using isolation systems separates the external cleanroom environment from the aseptic processing line and minimizes its exposure to personnel. § Positive air pressure differentials from the isolator to the surrounding environment have largely ranged from approximately 17. 5 to 50 Pascals § There are two types of aseptic processing isolators: open and closed. § Closed isolators employ connections with auxiliary equipment for material transfer. • Open isolators have openings to the surrounding environment that are carefully engineered to segregate the inner isolator environment from the surrounding room via overpressure. 33

ADVANCED ASEPTIC PROCESSING v BLOW-FILL- SEAL TECHNOLOGY : • Blow-fill-seal (BFS) technology is an automated process by which containers are formed, filled, and sealed in a continuous operation. • This manufacturing technology includes economies in container closure processing and reduced human intervention and is often used for filling and packaging ophthalmics, respiratory care products, and, less frequently, injectables. • Particles generated during the plastic extrusion, cutting, and sealing processes should be controlled. 34
 Principle of autoclave
Principle of autoclave Non sterile compounding examples
Non sterile compounding examples Santhi soundararajan
Santhi soundararajan Aseptic technique pharmacy
Aseptic technique pharmacy Sterile products and aseptic techniques
Sterile products and aseptic techniques Gmp sterile pharmaceutical products
Gmp sterile pharmaceutical products Gmp sterile pharmaceutical products
Gmp sterile pharmaceutical products Sterile products and aseptic techniques
Sterile products and aseptic techniques Cost concept and classification
Cost concept and classification Manufacturing cost vs non manufacturing cost
Manufacturing cost vs non manufacturing cost Manufacturing cost vs non manufacturing cost
Manufacturing cost vs non manufacturing cost Additive manufacturing vs subtractive manufacturing
Additive manufacturing vs subtractive manufacturing Job costing vs process costing examples
Job costing vs process costing examples Sphoorti joglekar
Sphoorti joglekar Panchayati raj institutions accounting software (priasoft)
Panchayati raj institutions accounting software (priasoft) Priya yesu nirminchitivi
Priya yesu nirminchitivi Priya malik tanu malik
Priya malik tanu malik Priya sekhon
Priya sekhon Priya narayanan naac
Priya narayanan naac Priasoft payment voucher
Priasoft payment voucher Ninne preminthunu song in english
Ninne preminthunu song in english Priya raghubir
Priya raghubir Purchasing power parity
Purchasing power parity Priya dehedkar
Priya dehedkar Priya ramaswami
Priya ramaswami Hetu viparita chikitsa
Hetu viparita chikitsa Nee priya prabhuni sevakai
Nee priya prabhuni sevakai Sindhu priya ks
Sindhu priya ks Priya yesu nirminchithivi song
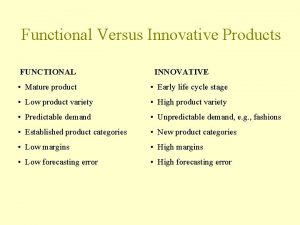
Priya yesu nirminchithivi song Functional vs innovative products
Functional vs innovative products Pepsi 4ps marketing mix
Pepsi 4ps marketing mix Sterile pyuria definition
Sterile pyuria definition Sterile semisolid preparations for ophthalmic use only are
Sterile semisolid preparations for ophthalmic use only are Sterile technique cell culture
Sterile technique cell culture What part of the surgical gown is considered sterile
What part of the surgical gown is considered sterile