STERILIZATION OF PARENTERALS 1 N SANTHI PRIYA Mpharm














![TEMPATURE [°C] HOLDING TIME (min) FOR STERILZATION MOIST HEAT 121 126 134 15 10 TEMPATURE [°C] HOLDING TIME (min) FOR STERILZATION MOIST HEAT 121 126 134 15 10](https://slidetodoc.com/presentation_image_h/2e07e27fac58f502bb2e6b7f4be2e860/image-20.jpg)




























- Slides: 62

STERILIZATION OF PARENTERALS 1 N. SANTHI PRIYA, Mpharm ASSISTANT PROFESSOR CHALAPATHI INSTITUTE OF PHARMACEUTICAL SCIENCES

PARENTERAL The term parenteral Derived from the Greek words: para (outside) and enteron (intestine) Denotes routes of administration other than oral route. 2

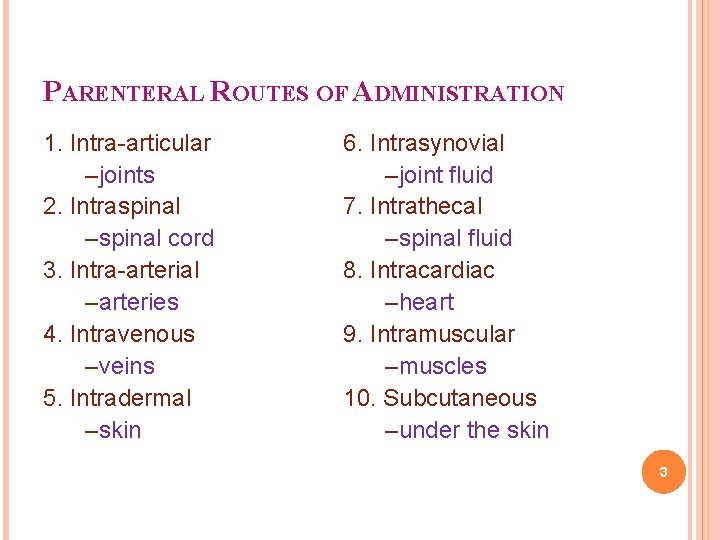
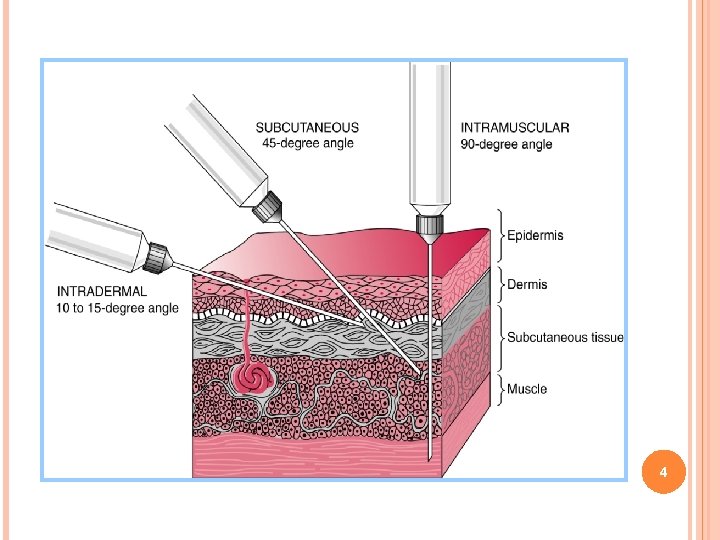
PARENTERAL ROUTES OF ADMINISTRATION 1. Intra-articular –joints 2. Intraspinal –spinal cord 3. Intra-arterial –arteries 4. Intravenous –veins 5. Intradermal –skin 6. Intrasynovial –joint fluid 7. Intrathecal –spinal fluid 8. Intracardiac –heart 9. Intramuscular –muscles 10. Subcutaneous –under the skin 3

4

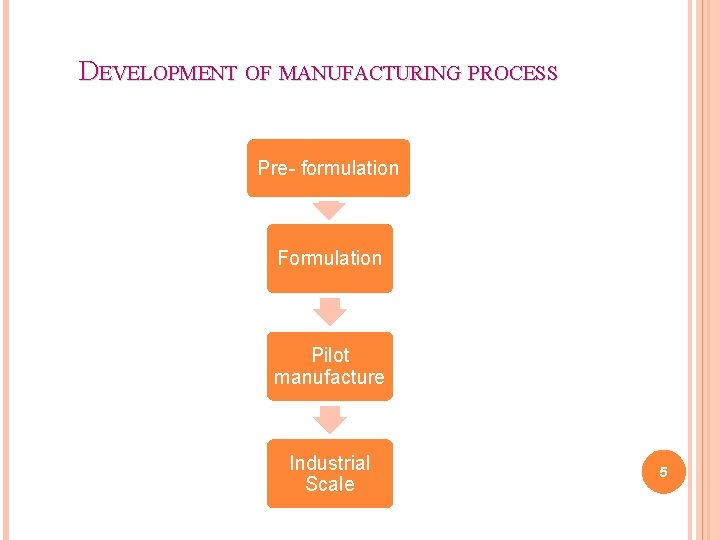
DEVELOPMENT OF MANUFACTURING PROCESS Pre- formulation Formulation Pilot manufacture Industrial Scale 5

STERILIZATION 6

TERMINOLOGY Aseptic: areas / practices where the product is to be sterile Aseptic processing : those operations performed between the sterilization of preparation and final sealing of its package Bacteriocide: any agent that destroys the microorganism 7

Terminology Bacteriostat: any agent that arrests or retard the growth of microorganism Bioburden: the number of viable microorganism present prior to sterilization usually expressed in colony forming units Terminology Disinfection: a process that decreases the probability of infection by destroying vegetative microorganisms, but not ordinary bacterial spores Sanitization: a process that reduces the level of bioburden to a safe level 8

Sterile: absolute absence of viable microorganism. Sterility assurance level: an estimate of the effectiveness of sterilization process. It is usually expresses interms of negative power of 10 { 1 in million = 10 -6 } Terminal sterilization: a process used to render products sterile to a preferred SAL Validation: the act of verifying that a procedure is capable of producing the intended result under prescribed circumstances and challenges to predefined specifications 9

STERILIZATION The term sterilization , is a process by which all viable microorganisms are removed or destroyed, based on the probability function 10

STERILIZATION METHODS I. Steam sterilization II. Dry heat sterilization III. Filtration sterilization IV. Gas sterilization V. Sterilization by Ionizing radiation 11

PHYSICAL PROCESSES OF STERILIZATION Thermal Method Microorganisms are killed by heat by coagulation of the protein of a living cell. The lethal effectiveness of heat is dependent on: 1. The degree of heat 2. The exposure period 3. The moisture present 12

STEAM STERILIZATION Steam is an effective sterilant for two reasons: 1. saturated steam is an extremely effective “carrier” of thermal energy. It is many times more effective in conveying this type of energy to the item than is hot (dry) air. 2. steam is an effective sterilant because any resistant, protective outer layer of the microorganisms can be softened by the steam, allowing coagulation of the sensitive inner portions of the microorganism 13

Steam sterilization requires four conditions: Adequate contact Sufficiently high temperature Time Sufficient moisture 14

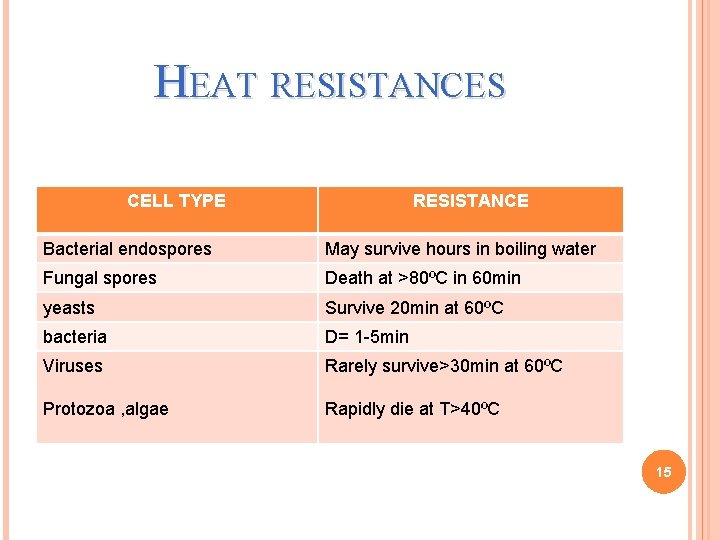
HEAT RESISTANCES CELL TYPE RESISTANCE Bacterial endospores May survive hours in boiling water Fungal spores Death at >80ºC in 60 min yeasts Survive 20 min at 60ºC bacteria D= 1 -5 min Viruses Rarely survive>30 min at 60ºC Protozoa , algae Rapidly die at T>40ºC 15

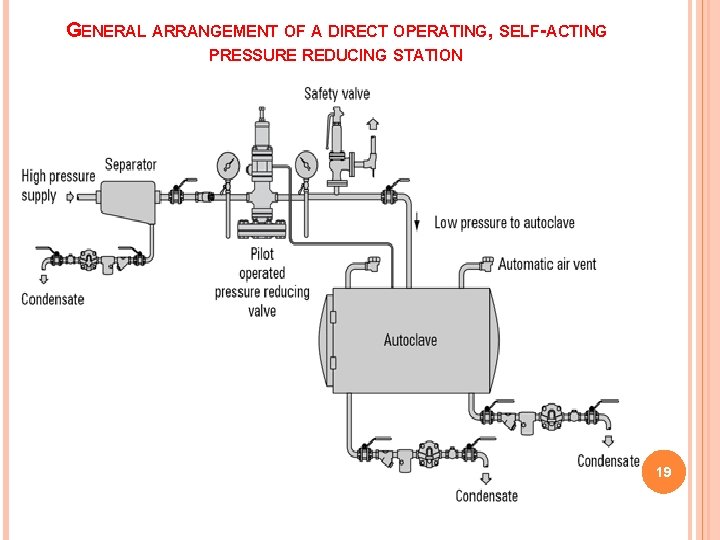
CONDITION FOR AN ACCURATE STERILIZATION It is a perfect and total air release from the sterilization chamber. The air needs to be totally replaced by steam to create a 100% steam atmosphere. Only this ensures an efficient steam sterilization and a homogenous temperature distribution. Remaining air in the chamber results even at sterilization temperature in a few folds less sterilization efficiency 16

The usual steam pressures, the temperatures obtainable under these pressures, and the approximate length of time required after the system reaches the indicated temperatures are as follows: 1. 10 pounds pressure (115°C), for 30 min. 2. 15 pounds pressure (121°C), for 20 min. 3. 20 pounds pressure (126°C), for 15 min 17

18

GENERAL ARRANGEMENT OF A DIRECT OPERATING, SELF-ACTING PRESSURE REDUCING STATION 19
![TEMPATURE C HOLDING TIME min FOR STERILZATION MOIST HEAT 121 126 134 15 10 TEMPATURE [°C] HOLDING TIME (min) FOR STERILZATION MOIST HEAT 121 126 134 15 10](https://slidetodoc.com/presentation_image_h/2e07e27fac58f502bb2e6b7f4be2e860/image-20.jpg)
TEMPATURE [°C] HOLDING TIME (min) FOR STERILZATION MOIST HEAT 121 126 134 15 10 3 DRY HEAT 160 170 18 120 60 3 PROCESS 20

DRY HEAT STERILIZATION Usually carried out in ovens and are generally thermostatically controlled Due to the high temperatures required for dry heat sterilization can only be used for thermostable, moisture sensitive or moisture impermeable pharmaceutical and medicinal. These include products like; v Dry powdered drugs v Oily injections, implants, ophthalmic ointments, ointment base 21

DRY HEAT STERILIZATION The Dry-Heat sterilization process is accomplished by conduction; that is where heat is absorbed by the exterior surface of an item and then passed inward to the next layer. Eventually, the entire item reaches the proper temperature needed to achieve sterilization. proper time and temperature for Dry-Heat sterilization is 160°C (320°F) for 2 hours or 170°C (340°F) for 1 hour Dry-heat destroys microorganisms by causing coagulation of proteins. 22

There are two types of Hot-Air convection (Convection refers to the circulation of heated air within the chamber of the oven) sterilizers: I. II. Natural convection Mechanical convection 23

NATURAL CONVECTION PROCESS As air is heated, it expands and possesses less density (weight per unit volume) than cooler air. Therefore, the heated air rises and displaces the cooler air (the cooler air descends). The method of Dry-heat gravity convection produces inconsistent temperatures within the chamber and has a very slow turn over. 24

MECHANICAL CONVECTION PROCESS A mechanical convection oven contains a blower that actively forces heated air throughout all areas of the chamber. The flow created by the blower ensures uniform temperatures and the equal transfer of heat throughout the load. For this reason, the mechanical convection oven is the more efficient of the two processes. 25

Natual convection Forced convection 26

STERILIZATION BY FILTRATION Reduces microbial population or sterilizes solutions of heat -sensitive materials by removing microorganisms Depth filters – thick fibrous or granular filters that remove microorganisms by physical screening, entrapment, and/or adsorption Membrane filters – porous membranes with defined pore sizes that remove microorganisms primarily by physical screening 27

Filtration process does not destroy but removes the microorganisms. It is used for both the clarification and sterilization of liquids and gases as it is capable of preventing the passage of both viable and non viable particles. The major mechanisms of filtration are Ø sieving Ø adsorption Ø trapping within the matrix of the filter material Sterilizing grade filters are used in the treatment of heat sensitive injections and ophthalmic solutions, biological products. 28

APPLICATION OF FILTRATION FOR STERILIZATION HEPA (High efficiency particulate air) filters can remove up to 99. 97% of particles >0. 3 micrometer in diameter. Air is first passed through prefilters to remove larger particles and then passed through HEPA filters. The performance of HEPA filter is monitored by pressure differential and airflow rate measurements 29

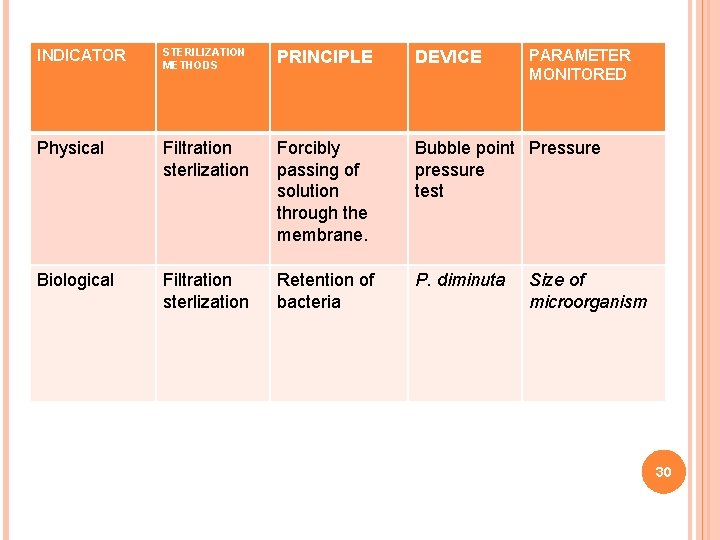
INDICATOR STERILIZATION METHODS PRINCIPLE DEVICE Physical Filtration sterlization Forcibly passing of solution through the membrane. Bubble point Pressure pressure test Biological Filtration sterlization Retention of bacteria P. diminuta PARAMETER MONITORED Size of microorganism 30

GAS STERILIZATION Some heat sensitive and moisture sensitive materials can be sterilized much better by exposure gases like ethylene oxide or propylene dioxide These gases are highly flammable when mixed with air but can be employed safely when properly diluted with an inert gas such as carbon dioxide or a suitable flourinated hydrocarbon 31

ETHYLENE OXIDE It is used to sterilize heat-sensitive materials, microbicidal and sporicidal 2. Boiling point 10. 7°C, Very good penetration 3. Used with CO 2, argon etc. to avoid explosions, > 3% ethylene oxide in air is explosive. Mechanism: ethylene oxide alkylates hydroxyl, carbonyl and sulfhydryl amino groups of enzymes Ø Effect depends on v gas pressure v exposure time v temperature v type of microorganism or spore v moisture content (optimum 28 -33%) 1. 32

The great penetrating qualities of Et. O makes it useful in sterilization of medical and surgical appliances such as : Catherters Needles Plastic disposable syringes Ø In their final plastic packaging just prior to shipment 33

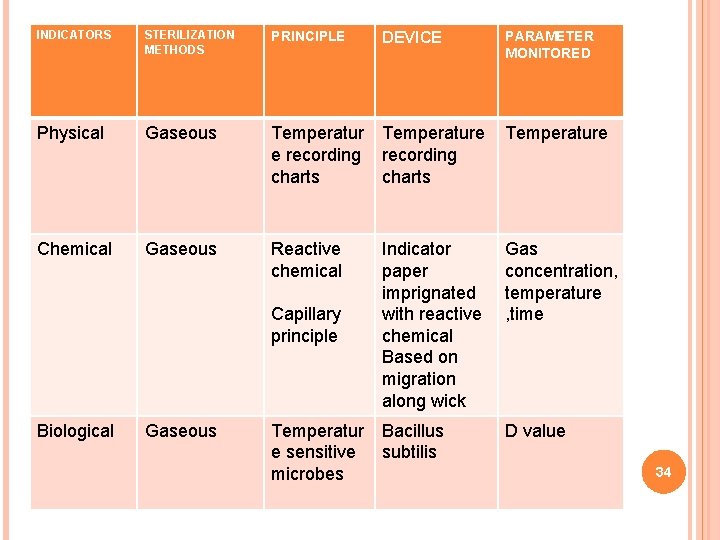
INDICATORS STERILIZATION METHODS PRINCIPLE Physical Gaseous Temperature e recording charts Chemical Gaseous Reactive chemical Capillary principle Biological Gaseous Temperatur e sensitive microbes DEVICE PARAMETER MONITORED Indicator paper imprignated with reactive chemical Based on migration along wick Gas concentration, temperature , time Bacillus subtilis D value 34

35

STERILIZATION BY RADIATION Both, X rays and Gamma rays have wavelength shorter than the wavelength of ultraviolet light. X rays, which have wavelength of 0. 1 to 40 nm, and gamma rays, which have even shorter wavelength, are forms of radiation, so named because it can dislodge electrons from atoms, creating ions These forms of radiation kill microorganisms by damaging DNA and produces peroxides, which act as powerful oxidizing agents in cells. 36

High-energy gamma irradiation is used mainly in the healthcare industries to sterilize disposable medical devices. Pharmaceutical companies now radiation sterilize drugs such as ophthalmic preparations, topical ointments, veterinary products, and parenterals. Regulatory pressure to adopt terminal-sterilization processes has promoted radiation sterilization. 37

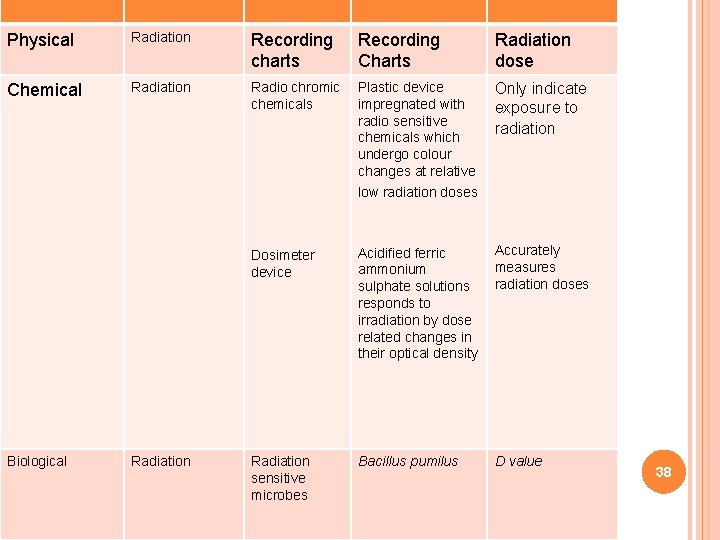
RADIATIONRadiation STERILIZATION Recording Physical charts Chemical Biological Radiation Charts Radiation dose Radio chromic Plastic device Only indicate chemicals impregnated with exposure to radio sensitive radiation chemicals which undergo colour changes at relative low radiation doses Dosimeter device Accurately Acidified ferric measures ammonium sulphate solutions radiation doses responds to irradiation by dose related changes in their optical density Radiation sensitive microbes Bacillus pumilus D value 38

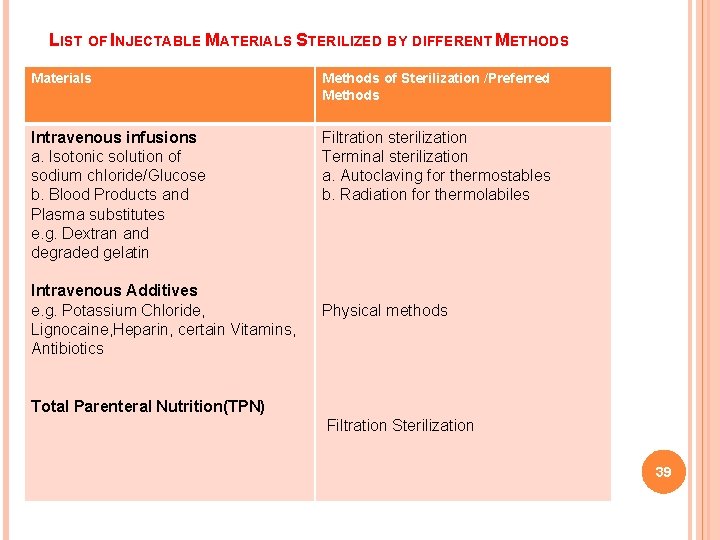
LIST OF INJECTABLE MATERIALS STERILIZED BY DIFFERENT METHODS Materials Methods of Sterilization /Preferred Methods Intravenous infusions a. Isotonic solution of sodium chloride/Glucose b. Blood Products and Plasma substitutes e. g. Dextran and degraded gelatin Filtration sterilization Terminal sterilization a. Autoclaving for thermostables b. Radiation for thermolabiles Intravenous Additives e. g. Potassium Chloride, Lignocaine, Heparin, certain Vitamins, Antibiotics Physical methods Total Parenteral Nutrition(TPN) Filtration Sterilization 39

List of Non injectable sterile fluids Sterilized Materials Methods of Sterilization /Preferred Methods Non injectable waters Urological irrigation solution Peritoneal dialysis and heamodialysis solution Inhaler solution Filtration sterilization / Terminal sterilization by Autoclaving 40

EVALUATION AND IN PROCESS MONITORING OF STERILIZATION PROCEDURES 41

STERILIZATION MONITORING Sterilization is a process designed to kill all microbes. Because we can't actually test whether all microbes are killed during the sterilization process. the next best thing is to determine whether the process kills the most resistant microbe. If so, we may assume all others have been killed as well. 42

Demonstrating the death of bacterial spores provides the main guarantee of sterilization, because it assess the process directly using live resistant microorganisms. Using bacterial spores to monitor the sterilization process is referred to as biologic monitoring (or spore-testing) and the bacterial spores used for monitoring the sterilization process are referred to as biologic indicators (BIs). 43

TYPES OF BIOLOGICAL INDICATORS BIs contain the bacterial spores used for monitoring. The spores used are Geobacillus stearothermophilus (for testing steam or chemical vapor sterilization) or Bacillus subtilis (for testing dry-heat or ethylene oxide gas sterilization). Currently, no BIs are available for routine testing of liquid chemical sterilants or disinfectants. 44

SPORE STRIPS The medium that in turn is incubated for two to seven days at Ø 55°C/131°F (for G. stearothermophilus) or at 37°C/98. 6°F (for B. subtilis ). If live bacterial spores still are present, they will grow and produce cloudiness and/or change the color of the growth medium, indicating sterilization failure. Spore strip BIs can be used to monitor all forms of heat sterilization. 45

SELF-CONTAINED VIAL This comprises both a spore strip or disk and an ampule filled with growth medium, contained in a plastic vial with a vented cap to permit entrance of the sterilizing agent into the vial After processing through the sterilizer, either the vial is squeezed or the cap is pushed down to break the internal ampule, which mixes the growth medium with the spores. The vial is then incubated at 55°C/131°F, and if live bacterial spores still are present, they will grow and change the color of the growth medium, indicating sterilization failure. 46

SPORE STRIP SELF CONTAINED VIAL 47

CHEMICAL INDICATORS Chemical indicators change color or form when exposed to specific high temperatures or to the sterilizing conditions within a sterilizer. This is referred to as chemical monitoring (or process monitoring). 48

MECHANICAL MONITORING Mechanical monitoring involves observing and recording the physical aspects e. g. , Ø temperature, Ø pressure, Ø time of the cycle when the sterilizer is being operated. 49

STERILITY ASSURANCE LEVEL Sterility assurance level (SAL) is a term used to describe the probability of a single unit being non-sterile after it has been subjected to the sterilization process SAL is also used to describe the killing efficacy of a sterilization process, where a very effective sterilization process has a very low SAL. 50

Each log reduction (10− 1) represents a 90% reduction in microbial population. So a process shown to achieve a "6 -log reduction" (10− 6) will reduce a population from a million organisms (106) to very close to zero, theoretically. It is common to employ overkill cycles to provide greatest assurance of sterility. 51

KILLING KINETICS Thermal death point: The lowest temperature at which all the bacteria in a population will be killed. Thermal death time: The length of time before all microorganisms in a population will be killed at a certain temperature • D-value and Z-value 52

FACTORS THAT AFFECT CELL DEATH Time number of cells type of microorganism method of killing concentration or intensity temperature p. H presence of organic material development of resistance 53

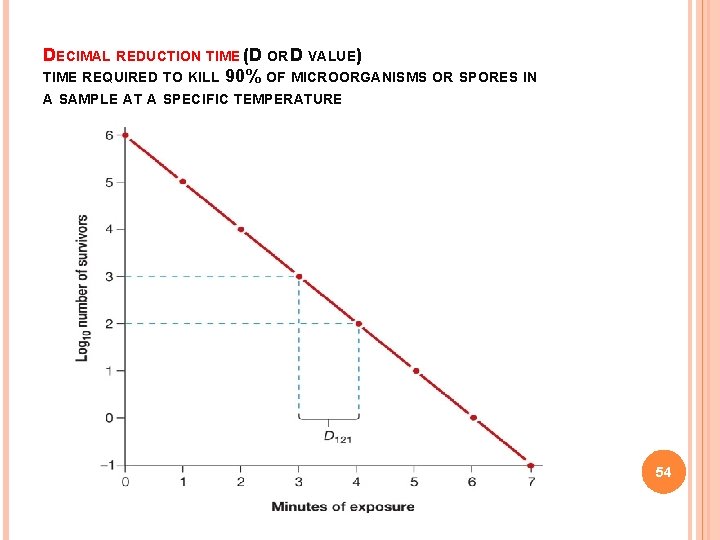
DECIMAL REDUCTION TIME (D OR D VALUE) TIME REQUIRED TO KILL 90% OF MICROORGANISMS OR SPORES IN A SAMPLE AT A SPECIFIC TEMPERATURE 54

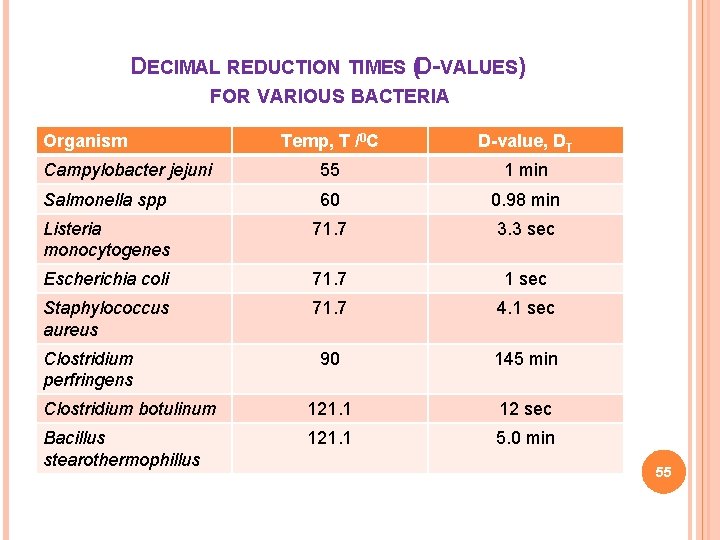
DECIMAL REDUCTION TIMES (D-VALUES) FOR VARIOUS BACTERIA Organism Temp, T /0 C D-value, DT Campylobacter jejuni 55 1 min Salmonella spp 60 0. 98 min Listeria monocytogenes 71. 7 3. 3 sec Escherichia coli 71. 7 1 sec Staphylococcus aureus 71. 7 4. 1 sec 90 145 min Clostridium botulinum 121. 1 12 sec Bacillus stearothermophillus 121. 1 5. 0 min Clostridium perfringens 55

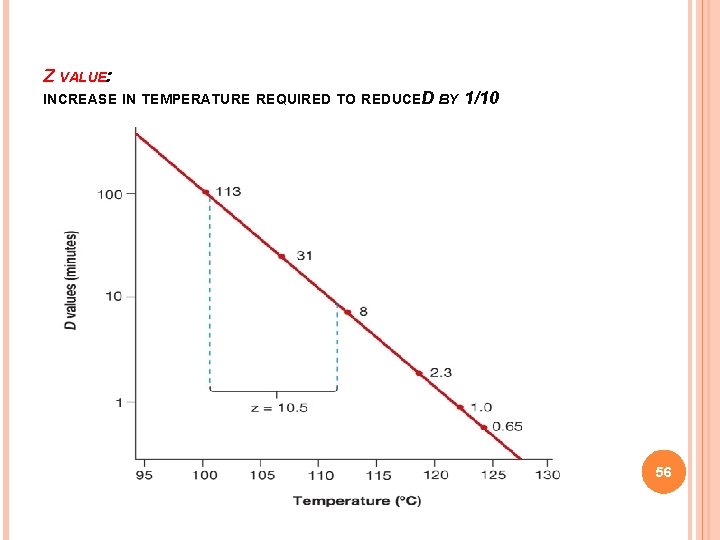
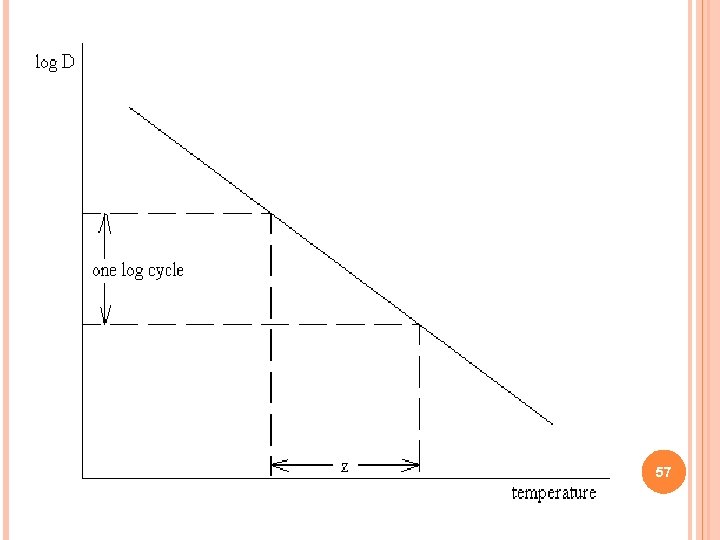
Z VALUE: INCREASE IN TEMPERATURE REQUIRED TO REDUCED BY 1/10 56

57

WHY MOST MICRO ORGANISMS DO NOT DIE IN A LINEAR FASHION? The mechanisms of death are not mono molecular (different proteins are degraded or coagulated at different speeds) The proportion of life essential proteineous “targets” varies with the age of the micro organisms, their life cycle, their food supply etc. Susceptibility to heat varies thereof non linearly with limited predictability 58

CONCLUSION Ø A detailed investigation of sterilization methods and the production of parenterals may uncover circumstantial evidence to support a final decision, follow-up of validation before making final decisions for the product release. 59

REFERENCES I. Pharmaceutical dosage forms, parenteral medications Edited by HERBERT. A. LIEBERMAN and LEON LACHMAN second edition pg 406 - 439 II. Pharmaceutical Dosage Forms and Drug Delivery Systems, H C Ansel, L V Allen pg 445 -503 III. U. S. P. 30 – NF 25, APPENDIX: 71 IV. Sterilization in theory and practice of industrial pharmacy 3 rd edition pg 234 -313 V. Pharmaceutical Dosage Forms: Parenteral Medications, Third Edition: Volume 2: Facility Design, Sterilization and Processing By Sandeep Nema and John D Ludwig VI. Development of Biopharmaceutical Parenteral Dosage Forms (Drugs and the Pharmaceutical Sciences) pg 171 -221 60

WEB SOURCES http: //www. gmp-compliance. org v http: //www. pda. org/ v http: //www. pharmtech. com v http: //www. etcsterilization. com/ v http: //www. consteril. com/ v Association for European Safety & Infection Control v http: //www. aesic. eu v 61

62
 Santhi priya hot
Santhi priya hot Santhi soundararajan
Santhi soundararajan Panchayat raj institutions accounting software
Panchayat raj institutions accounting software Nathan ong
Nathan ong Tanu malik
Tanu malik Priya narayanan naac
Priya narayanan naac Panchayati raj institutions accounting software (priasoft)
Panchayati raj institutions accounting software (priasoft) Priya raghubir
Priya raghubir Nee priya prabhuni sevakai
Nee priya prabhuni sevakai Yesu ni wangu wa uzima wamilele
Yesu ni wangu wa uzima wamilele Relative ppp
Relative ppp Priya dehedkar
Priya dehedkar Kriyakala
Kriyakala Priya yesu nirminchitivi
Priya yesu nirminchitivi Priya ramaswami
Priya ramaswami Sindhu priya ks
Sindhu priya ks Priya yesu nirminchithivi song
Priya yesu nirminchithivi song Cmu distributed systems
Cmu distributed systems Quality control test for parenterals
Quality control test for parenterals Leaker test for parenterals
Leaker test for parenterals Leaker test for parenterals
Leaker test for parenterals Clarity test for parenterals
Clarity test for parenterals Nurses responsibility in sterilization
Nurses responsibility in sterilization Sterilization and disinfection
Sterilization and disinfection Differentiate between disinfection and sterilization
Differentiate between disinfection and sterilization Classification of sterilization
Classification of sterilization Chemster medical clinic
Chemster medical clinic Parasafe sterilization
Parasafe sterilization Define disinfection
Define disinfection Forced sterilization
Forced sterilization Methods of instrument processing
Methods of instrument processing Physical and chemical methods of sterilization slideshare
Physical and chemical methods of sterilization slideshare Oven labelled
Oven labelled Advantage of autoclave
Advantage of autoclave Radiant streak method
Radiant streak method Sterilization and disinfection
Sterilization and disinfection Beta propiolactone
Beta propiolactone What is sterilization
What is sterilization Process of sterilization
Process of sterilization Spaulding classification system
Spaulding classification system Tubal sterilization
Tubal sterilization Package sterilization
Package sterilization Forced sterilization
Forced sterilization What is batch sterilization
What is batch sterilization Advantages of batch sterilization
Advantages of batch sterilization Sterilization by mechanical methods
Sterilization by mechanical methods Sterilization and disinfection
Sterilization and disinfection What is batch sterilization
What is batch sterilization Principles of heat preservation
Principles of heat preservation Mechanical method of sterilization slideshare
Mechanical method of sterilization slideshare What is sterilization
What is sterilization Pacific bio labs
Pacific bio labs Low temperature sterilization
Low temperature sterilization Tass eye syndrome
Tass eye syndrome Mechanical method of sterilization
Mechanical method of sterilization Sterilization quality control
Sterilization quality control Al noor ajax
Al noor ajax Sterilization and disinfection in microbiology lab
Sterilization and disinfection in microbiology lab Cold sterilization
Cold sterilization Performance qualification protocol
Performance qualification protocol Gamma irradiation sterilization process
Gamma irradiation sterilization process Physical methods of food preservation
Physical methods of food preservation Principle of autoclave ppt
Principle of autoclave ppt