Sterile Products and Aseptic Techniques for the Pharmacy



















- Slides: 33

Sterile Products and Aseptic Techniques for the Pharmacy Technician CHAPTER 9 Quality Control and Assurance Sterile Products and Aseptic Techniques for the Pharmacy Technician, Second Edition Mike Johnston Copyright © 2011 by Pearson Education, Inc. All rights reserved.

The Seven Rights • • Right patient Right medication Right dose Right route of administration Right time Right technique Right documentation Sterile Products and Aseptic Techniques for the Pharmacy Technician, Second Edition Mike Johnston Copyright © 2011 by Pearson Education, Inc. All rights reserved.

Quality Assurance Functions • • • Written policies & procedures Documentation Personnel training System checks & process validation Daily assessment of all operations Sterile Products and Aseptic Techniques for the Pharmacy Technician, Second Edition Mike Johnston Copyright © 2011 by Pearson Education, Inc. All rights reserved.

Quality Improvement • Ensures that integrity is not lost due to – Lack of training – Equipment failure – Outdated facilities – Other noncompliance issues Sterile Products and Aseptic Techniques for the Pharmacy Technician, Second Edition Mike Johnston Copyright © 2011 by Pearson Education, Inc. All rights reserved.

Quality Improvement (cont’d) • Process includes – Monitoring trends – Conducting audits – Identifying & analyzing problems – Taking corrective measures – Conducting testing – Suggesting change Sterile Products and Aseptic Techniques for the Pharmacy Technician, Second Edition Mike Johnston Copyright © 2011 by Pearson Education, Inc. All rights reserved.

Guidelines FDA • Publishes Good Manufacturing Practices (GMP) • GMP covers compounding sterile products • Other guidelines: manufacture of sterile products • Guidelines include quality system regulations Sterile Products and Aseptic Techniques for the Pharmacy Technician, Second Edition Mike Johnston Copyright © 2011 by Pearson Education, Inc. All rights reserved.

Guidelines (cont’d) FDA (cont’d) • Regulations make standards consistent with requirements Sterile Products and Aseptic Techniques for the Pharmacy Technician, Second Edition Mike Johnston Copyright © 2011 by Pearson Education, Inc. All rights reserved.

Guidelines (cont’d) The Joint Commission • Publishes standards on – Pharmaceuticals – Facilities that manufacture, store, & deliver them • Constantly revises standards to – Improve compliance – Keep up with drugs & their manufacturing processes Sterile Products and Aseptic Techniques for the Pharmacy Technician, Second Edition Mike Johnston Copyright © 2011 by Pearson Education, Inc. All rights reserved.

Guidelines (cont’d) • Performs stringent inspections at timed intervals • Offers accreditation for health care organizations • Offers support to help comply with accreditation Sterile Products and Aseptic Techniques for the Pharmacy Technician, Second Edition Mike Johnston Copyright © 2011 by Pearson Education, Inc. All rights reserved.

Guidelines (cont’d) CDC • Focus: preventing spread of disease/infection • Same focus as goal of aseptic compounding • Provides info for hospital environment controls Sterile Products and Aseptic Techniques for the Pharmacy Technician, Second Edition Mike Johnston Copyright © 2011 by Pearson Education, Inc. All rights reserved.

Guidelines (cont’d) ASHP • Publishes Guidelines on Quality Assurance for Pharmacy-Prepared Sterile Products • This document – Outlines considerations that are subjected to quality testing & assurance – Addresses all areas impacted by quality assurance Sterile Products and Aseptic Techniques for the Pharmacy Technician, Second Edition Mike Johnston Copyright © 2011 by Pearson Education, Inc. All rights reserved.

Guidelines (cont’d) USP • Published USP Chapter 797 – First enforceable national standards for sterile compounding – Adopted by state boards, JCAHO, FDA Miscellaneous • Other local, state, & national agencies • Find out what rules apply in your state Sterile Products and Aseptic Techniques for the Pharmacy Technician, Second Edition Mike Johnston Copyright © 2011 by Pearson Education, Inc. All rights reserved.

ASHP Guidelines on QA for CSPs: Risk Level 1 • Products that are – Stored at room temperature – Completely administered within 28 hrs of prep – Unpreserved – Sterile – Prepared for administration to >1 patient – Prepared by closed-system aseptic transfer Sterile Products and Aseptic Techniques for the Pharmacy Technician, Second Edition Mike Johnston Copyright © 2011 by Pearson Education, Inc. All rights reserved.

ASHP Guidelines on QA for CSPs: Risk Level 2 • Products that are – Administered beyond 28 hrs after prep – Stored at room temperature – Batch-prepared without preservatives – Intended for use by >1 patient – Compounded by complex, numerous manipulations using closed-system aseptic transfer Sterile Products and Aseptic Techniques for the Pharmacy Technician, Second Edition Mike Johnston Copyright © 2011 by Pearson Education, Inc. All rights reserved.

ASHP Guidelines on QA for CSPs: Risk Level 3 • Products that are – Compounded from nonsterile § Ingredients/components § Containers § Equipment – Prepared by combining multiple ingredients § Sterile or nonsterile § Using open-system transfer before terminal sterilization Sterile Products and Aseptic Techniques for the Pharmacy Technician, Second Edition Mike Johnston Copyright © 2011 by Pearson Education, Inc. All rights reserved.

USP 797: Low-Risk Level CSPs • Any CSP that is prepared – Using proper aseptic technique – In an ISO Class 5 or better environment – Using only sterile ingredients & supplies – With 3 or less commercially available products/packages – With 2 or less entries into any one sterile IV bag or vial Sterile Products and Aseptic Techniques for the Pharmacy Technician, Second Edition Mike Johnston Copyright © 2011 by Pearson Education, Inc. All rights reserved.

USP 797: Low-Risk Level CSPs (cont’d) • Any CSP that is prepared (cont’d) – Limited to § Opening ampules § Transferring liquids in syringes to sterile containers § Penetrating disinfected vial stoppers – At least 48 hrs storage @ room temp – 14 days or less refrigerated – 45 days or less frozen Sterile Products and Aseptic Techniques for the Pharmacy Technician, Second Edition Mike Johnston Copyright © 2011 by Pearson Education, Inc. All rights reserved.

USP 797: Medium-Risk Level CSPs • Any CSP that is prepared (cont’d) – As more than 1 individual or small doses administered to: § Multiple patients § One patient on multiple occasions – By complex manipulations § Of long durations § That involve processes other than single-volume transfer Sterile Products and Aseptic Techniques for the Pharmacy Technician, Second Edition Mike Johnston Copyright © 2011 by Pearson Education, Inc. All rights reserved.

USP 797: Medium-Risk Level CSPs (cont’d) • Any CSP that is prepared (cont’d) – 30 hrs or less storage @ room temp – 9 days or less refrigerated – 45 days or less frozen Sterile Products and Aseptic Techniques for the Pharmacy Technician, Second Edition Mike Johnston Copyright © 2011 by Pearson Education, Inc. All rights reserved.

USP 797: High-Risk Level CSPs • Any CSP that is prepared (cont’d) – By compounding using nonsterile ingredients – Using sterile contents of commercially manufactured products – Without antimicrobial preservatives – Exposed to less than ISO Class 5 environment for more than 1 hr – By improperly gowned personnel Sterile Products and Aseptic Techniques for the Pharmacy Technician, Second Edition Mike Johnston Copyright © 2011 by Pearson Education, Inc. All rights reserved.

USP 797: High-Risk Level CSPs (cont'd) • Any CSP that is prepared (cont’d) – With nonsterile water-containing products that are stored for more than 6 hrs – 24 hrs storage or less @ room temperature – 3 days or less refrigerated – 45 days or less frozen Sterile Products and Aseptic Techniques for the Pharmacy Technician, Second Edition Mike Johnston Copyright © 2011 by Pearson Education, Inc. All rights reserved.

USP 797: Environmental Sampling Testing • Should occur – Upon commissioning of new facilities & equipment – After any servicing of facilities & equipment – As part of periodic recertification of facilities/equipment – In response to § Issues with compounded sterile products § Observed improper technique of personnel Sterile Products and Aseptic Techniques for the Pharmacy Technician, Second Edition Mike Johnston Copyright © 2011 by Pearson Education, Inc. All rights reserved.

USP 797: Environmental Sampling Testing (cont’d) • Should occur (cont’d) – In response to § Issues with compounded sterile products § Observed improper technique of personnel § If a CSP is a potential source of patient-related infection Sterile Products and Aseptic Techniques for the Pharmacy Technician, Second Edition Mike Johnston Copyright © 2011 by Pearson Education, Inc. All rights reserved.

USP 797: Cleaning & Disinfecting of Compounding Area • Primary engineering control must be cleaned – At beginning of each shift – Before each batch – At least every 30 min after previous disinfection § During continuous compounding activities § After spills § When suspected contamination occurs Sterile Products and Aseptic Techniques for the Pharmacy Technician, Second Edition Mike Johnston Copyright © 2011 by Pearson Education, Inc. All rights reserved.

USP 797: Cleaning & Disinfecting of Compounding Area (cont’d) • Counters, work surfaces, floors must be cleaned – Daily when no aseptic compounding is occurring • Walls, ceilings, storage shelving must be cleaned monthly Sterile Products and Aseptic Techniques for the Pharmacy Technician, Second Edition Mike Johnston Copyright © 2011 by Pearson Education, Inc. All rights reserved.

USP 797: Other Guidelines • Policies & procedures – Needed in every pharmacy – Address updating, accessibility to personnel, accuracy, SOP, monitoring – All personnel must know policies & procedures Sterile Products and Aseptic Techniques for the Pharmacy Technician, Second Edition Mike Johnston Copyright © 2011 by Pearson Education, Inc. All rights reserved.

USP 797: Other Guidelines (cont’d) • Personnel, education, & evaluation – Personnel must constantly § Update skills § Continue education – Must have process of evaluating personnel’s § Skills (assessment) § Knowledge Sterile Products and Aseptic Techniques for the Pharmacy Technician, Second Edition Mike Johnston Copyright © 2011 by Pearson Education, Inc. All rights reserved.

USP 797: Other Guidelines (cont’d) • Storage & handling inside & outside pharmacy – How drugs are packaged, prepared, delivered – Need for refrigeration – Risk of contamination if stored/handled improperly Sterile Products and Aseptic Techniques for the Pharmacy Technician, Second Edition Mike Johnston Copyright © 2011 by Pearson Education, Inc. All rights reserved.

USP 797: Other Guidelines (cont’d) • Facilities & equipment – All compounding areas are held to high standards – All equipment & supplies must be § Installed properly § Used properly § Maintained § Monitored Sterile Products and Aseptic Techniques for the Pharmacy Technician, Second Edition Mike Johnston Copyright © 2011 by Pearson Education, Inc. All rights reserved.

USP 797: Other Guidelines (cont’d) • Aseptic technique, product preparation, & garb – Manufacturing processes § Require constant evaluation & improvement § Have many checkpoints – Focus on prevention of microbial contamination Sterile Products and Aseptic Techniques for the Pharmacy Technician, Second Edition Mike Johnston Copyright © 2011 by Pearson Education, Inc. All rights reserved.

USP 797: Other Guidelines (cont’d) • Process validation – Documenting/proving that process will consistently produce product meeting specifications • End-product evaluation – Pharmacist’s “final check” – Inspections with periodic sampling/testing of batches manufactured under same conditions Sterile Products and Aseptic Techniques for the Pharmacy Technician, Second Edition Mike Johnston Copyright © 2011 by Pearson Education, Inc. All rights reserved.

Documentation and Labeling • All products dispensed must be labeled • Every piece of equipment must have records • SOPs illustrate how a product is made/processed • Required patient info for each product – Patient diagnosis, IV regimen, dosages, BSA, lab values Sterile Products and Aseptic Techniques for the Pharmacy Technician, Second Edition Mike Johnston Copyright © 2011 by Pearson Education, Inc. All rights reserved.

Documentation and Labeling (cont’d) • Required product info for each product (cont’d) – Ingredients, prep/process, labeling, warnings, storage/handling, expiration date, batch/lot ID numbers Sterile Products and Aseptic Techniques for the Pharmacy Technician, Second Edition Mike Johnston Copyright © 2011 by Pearson Education, Inc. All rights reserved.
 Sterile products and aseptic techniques
Sterile products and aseptic techniques Sterile products and aseptic techniques
Sterile products and aseptic techniques Sterile products and aseptic techniques
Sterile products and aseptic techniques Sterile vs non sterile compounding
Sterile vs non sterile compounding Aseptic hand washing technique pharmacy
Aseptic hand washing technique pharmacy Gmp sterile pharmaceutical products
Gmp sterile pharmaceutical products Gmp for sterile pharmaceutical products
Gmp for sterile pharmaceutical products 15:4 observing standard precautions
15:4 observing standard precautions Chapter 15 infection control
Chapter 15 infection control 15:8 using sterile techniques
15:8 using sterile techniques Chapter 14:8 using sterile techniques
Chapter 14:8 using sterile techniques Chapter 15:7 cleaning with an ultrasonic unit
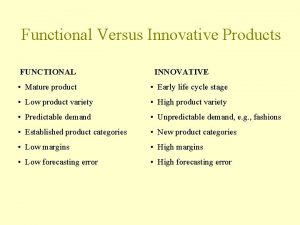
Chapter 15:7 cleaning with an ultrasonic unit List 5 innovative and 5 functional products
List 5 innovative and 5 functional products Antt safeguards
Antt safeguards Use of aseptic hood
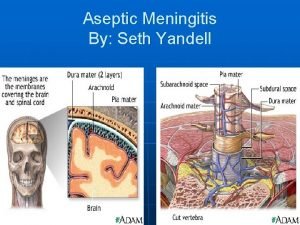
Use of aseptic hood Kernig and brudzinski signs
Kernig and brudzinski signs Introduction of gowning
Introduction of gowning Medical vs surgical aseptic hand washing
Medical vs surgical aseptic hand washing Ekisha shoes
Ekisha shoes Aseptic technique clipart
Aseptic technique clipart Micro critical aseptic field
Micro critical aseptic field Lafws
Lafws Aseptic packaging
Aseptic packaging Nsw health
Nsw health Marketing mix of pepsi and coca cola
Marketing mix of pepsi and coca cola Usp 797 laminar flow hood cleaning
Usp 797 laminar flow hood cleaning Sterile workflow optimization
Sterile workflow optimization Sterile pyuria
Sterile pyuria Sterile field border
Sterile field border Sterile lupiner
Sterile lupiner Compounding sterile preparations quiz
Compounding sterile preparations quiz Sterile compounding calculations
Sterile compounding calculations Sterile flower
Sterile flower What process is this
What process is this