Lewis Dot Chapter 8 Another model The localized

- Slides: 19

Lewis Dot Chapter 8

Another model § The localized electron model assumes compounds are held together by sharing electron pairs. § Pairs of electrons may be • localized pairs or • bonding pairs. § There are 3 parts to this.

The LE Model § Description of the electrons in a molecule using Lewis model. § Prediction of the geometry of the molecule using valence shell electronpair repulsion (VSEPR). § Description of atomic orbitals used by atoms to share electrons or hold lone pairs (Molecular Orbital model in chapter 9).

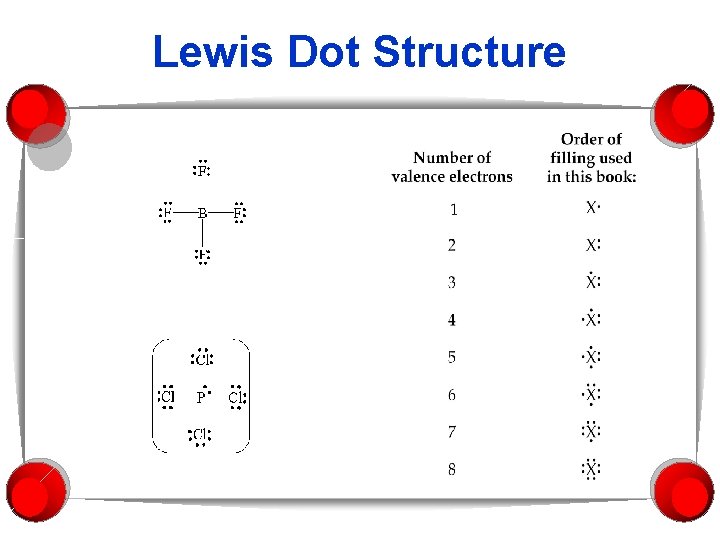
Lewis Dot Structure


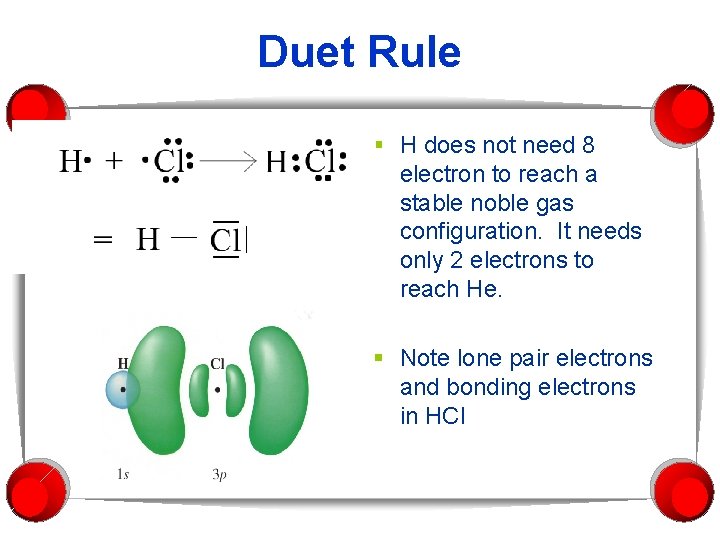
Duet Rule § H does not need 8 electron to reach a stable noble gas configuration. It needs only 2 electrons to reach He. § Note lone pair electrons and bonding electrons in HCl

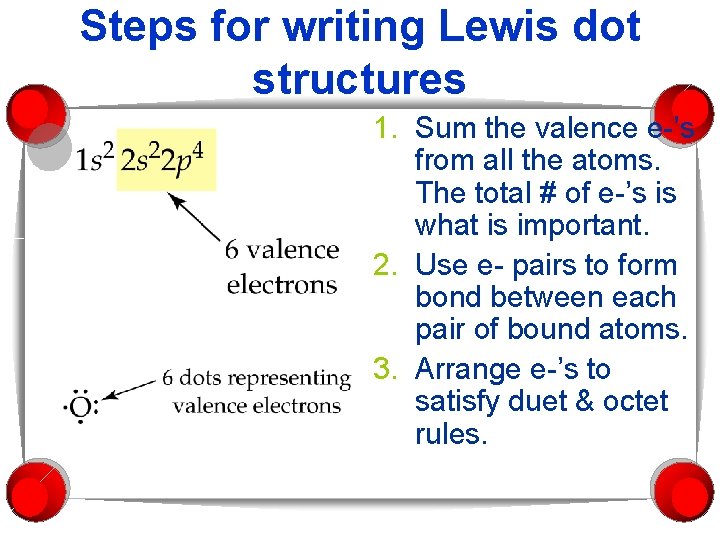
Steps for writing Lewis dot structures 1. Sum the valence e-’s from all the atoms. The total # of e-’s is what is important. 2. Use e- pairs to form bond between each pair of bound atoms. 3. Arrange e-’s to satisfy duet & octet rules.

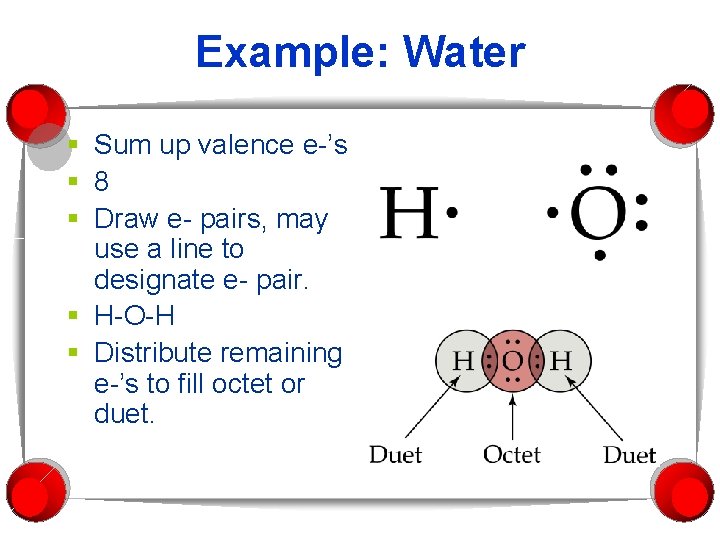
Example: Water § Sum up valence e-’s § 8 § Draw e- pairs, may use a line to designate e- pair. § H-O-H § Distribute remaining e-’s to fill octet or duet.

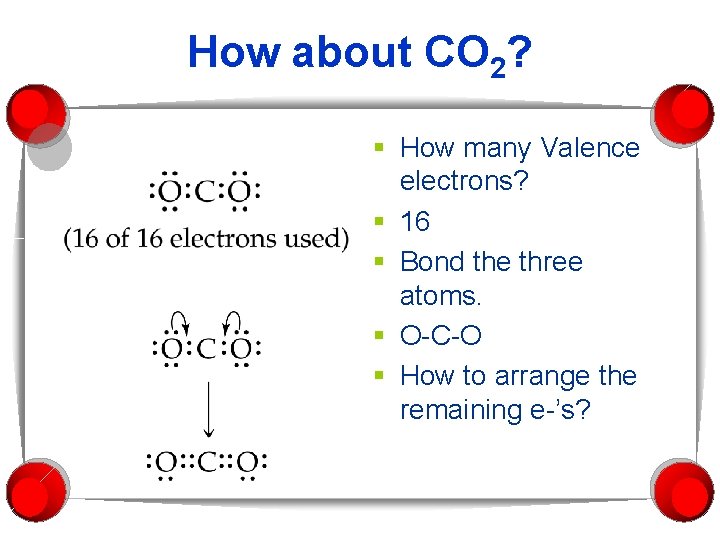
How about CO 2? § How many Valence electrons? § 16 § Bond the three atoms. § O-C-O § How to arrange the remaining e-’s?

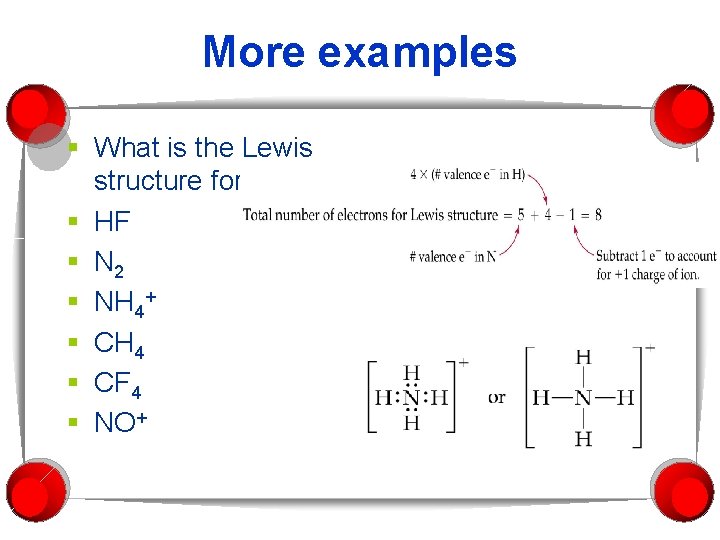
More examples § What is the Lewis structure for: § HF § N 2 § NH 4+ § CH 4 § CF 4 § NO+

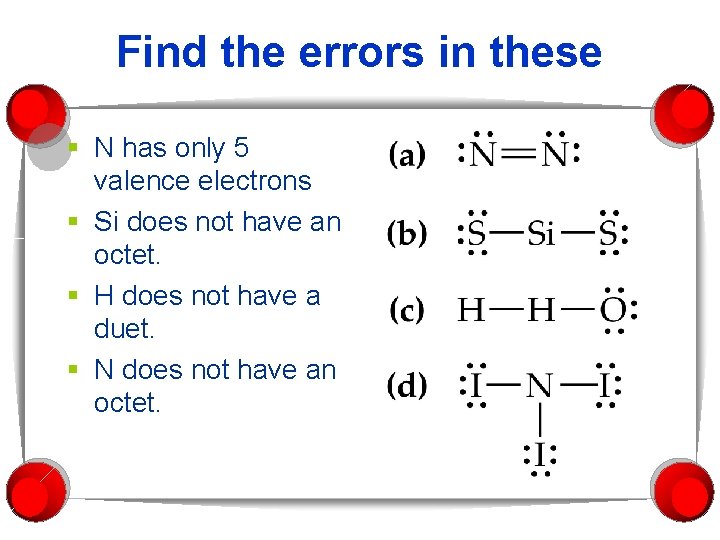
Find the errors in these § N has only 5 valence electrons § Si does not have an octet. § H does not have a duet. § N does not have an octet.

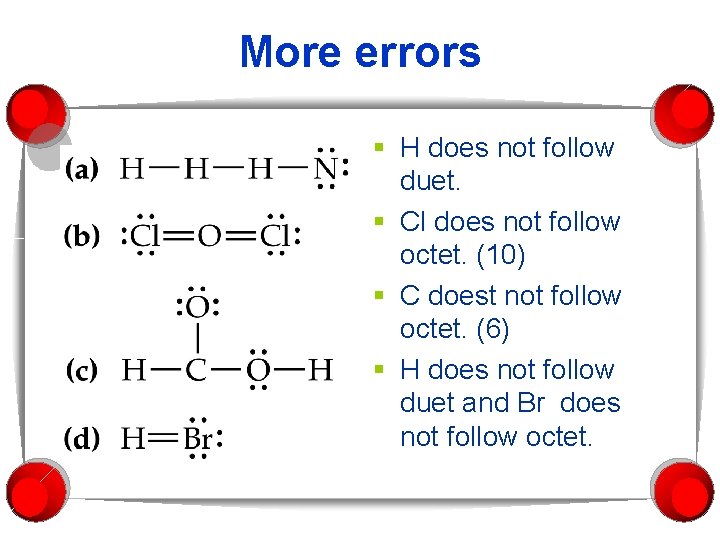
More errors § H does not follow duet. § Cl does not follow octet. (10) § C doest not follow octet. (6) § H does not follow duet and Br does not follow octet.


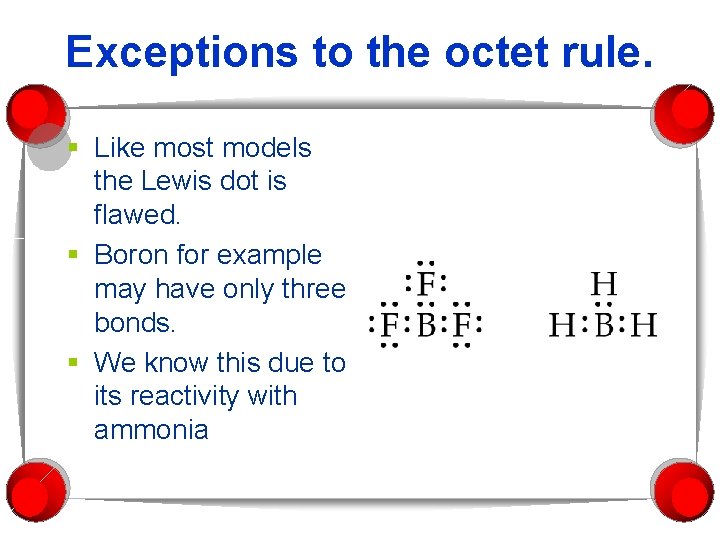
Exceptions to the octet rule. § Like most models the Lewis dot is flawed. § Boron for example may have only three bonds. § We know this due to its reactivity with ammonia

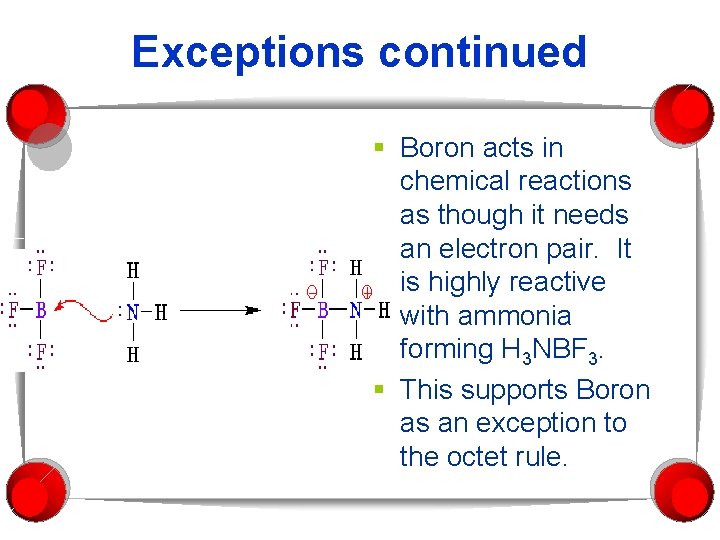
Exceptions continued § Boron acts in chemical reactions as though it needs an electron pair. It is highly reactive with ammonia forming H 3 NBF 3. § This supports Boron as an exception to the octet rule.

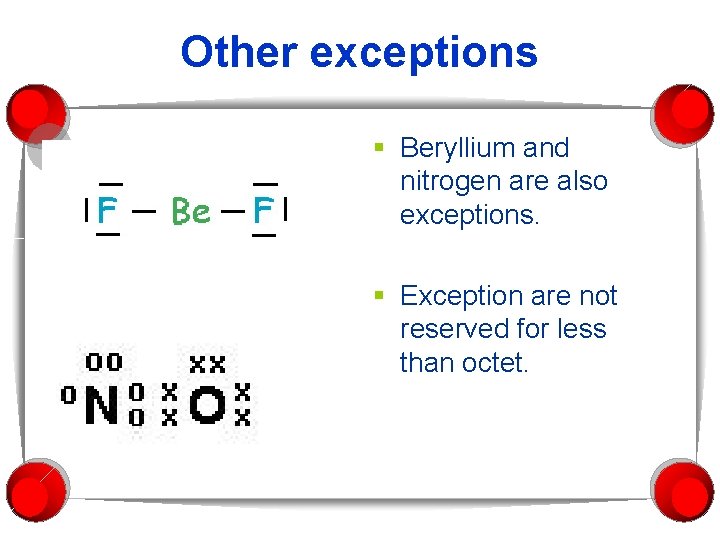
Other exceptions § Beryllium and nitrogen are also exceptions. § Exception are not reserved for less than octet.

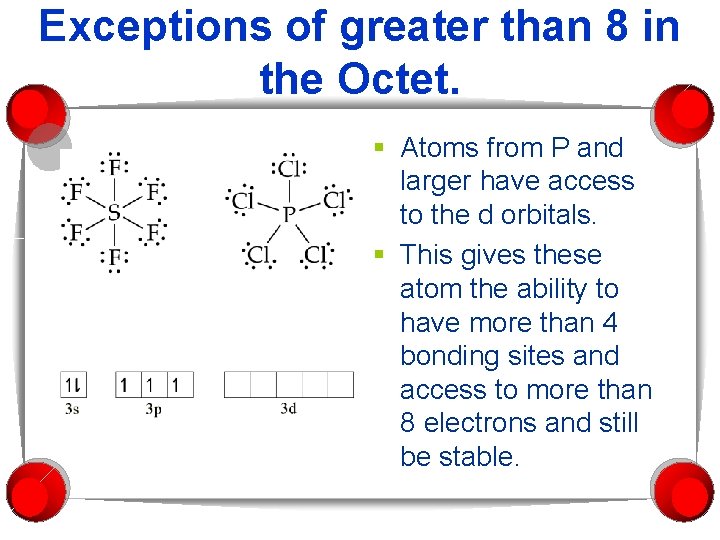
Exceptions of greater than 8 in the Octet. § Atoms from P and larger have access to the d orbitals. § This gives these atom the ability to have more than 4 bonding sites and access to more than 8 electrons and still be stable.

Summary § Second row elements C, N, O & F always follow the octet rule. § Second row B, & Be may have less than 8. These electron deficient molecules are highly reactive. § 2 nd row elements may never exceed the octet since they do not have access to d orbitals in the second energy level.

Summary continued § Third row and higher elements often satisfy the octet rule but may exceed the rule as they have access to the d orbital. § When writing the Lewis structure, follow the octet rule. If electrons remain, only then may you add extra electron pairs to the element having available d orbitals.