Lecture 3 30 09 2008 Lecture simple mixtures





- Slides: 52

Lecture 3 30 -09 -2008 • Lecture: – simple mixtures (cont) – colligative properties – membrane potential – Debye-Hückel limiting law – two-component phase diagrams – new problems • Last lecture problems

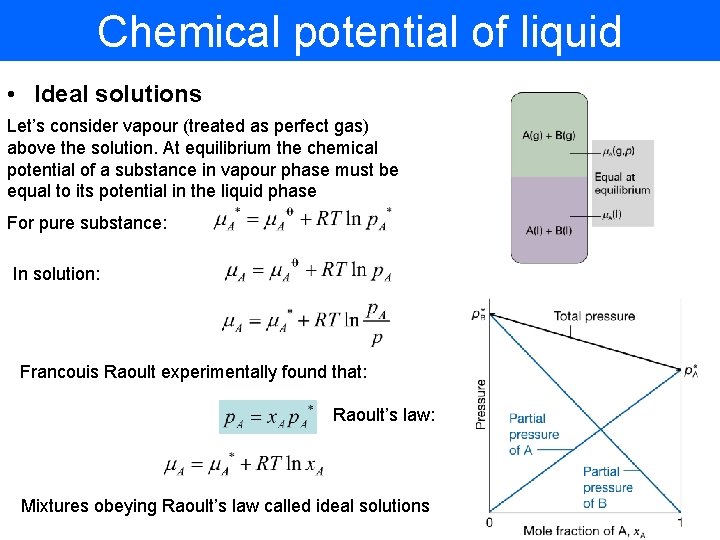
Chemical potential of liquid • Ideal solutions Let’s consider vapour (treated as perfect gas) above the solution. At equilibrium the chemical potential of a substance in vapour phase must be equal to its potential in the liquid phase For pure substance: In solution: Francouis Raoult experimentally found that: Raoult’s law: Mixtures obeying Raoult’s law called ideal solutions

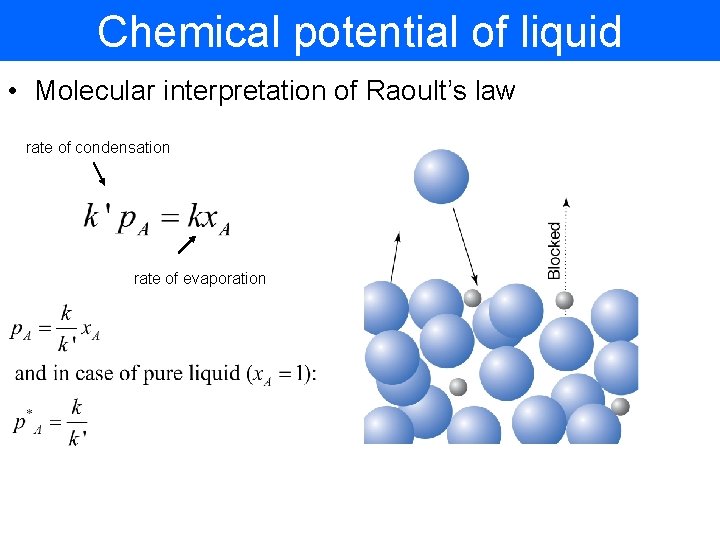
Chemical potential of liquid • Molecular interpretation of Raoult’s law rate of condensation rate of evaporation

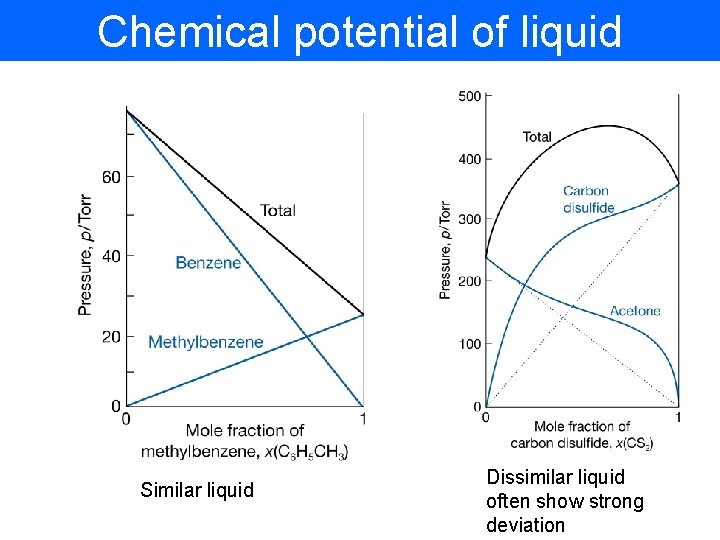
Chemical potential of liquid Similar liquid Dissimilar liquid often show strong deviation

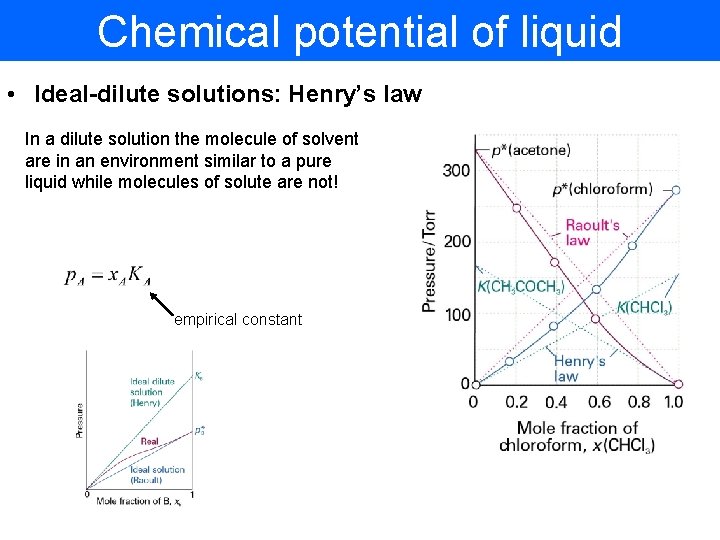
Chemical potential of liquid • Ideal-dilute solutions: Henry’s law In a dilute solution the molecule of solvent are in an environment similar to a pure liquid while molecules of solute are not! empirical constant

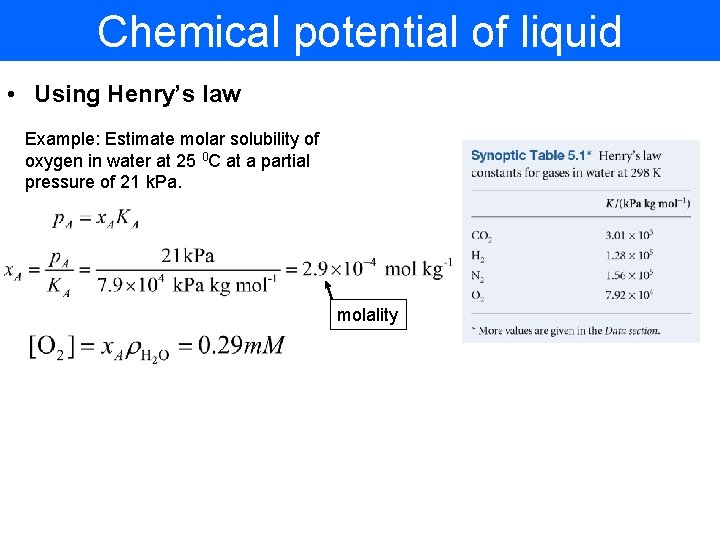
Chemical potential of liquid • Using Henry’s law Example: Estimate molar solubility of oxygen in water at 25 0 C at a partial pressure of 21 k. Pa. molality

Liquid mixtures • Ideal solutions If Raoult’s law applied to we have: From molecular prospective it means that interactions of A-A, A-B, and B-B are the same.

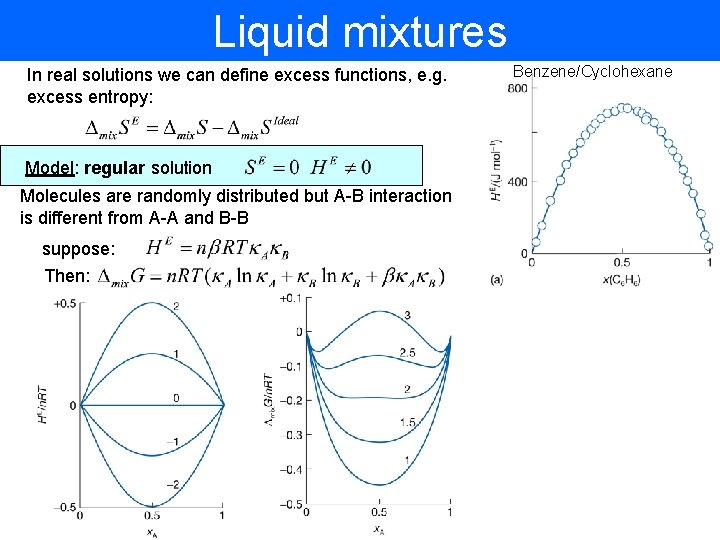
Liquid mixtures In real solutions we can define excess functions, e. g. excess entropy: Model: regular solution Molecules are randomly distributed but A-B interaction is different from A-A and B-B suppose: Then: Benzene/Cyclohexane

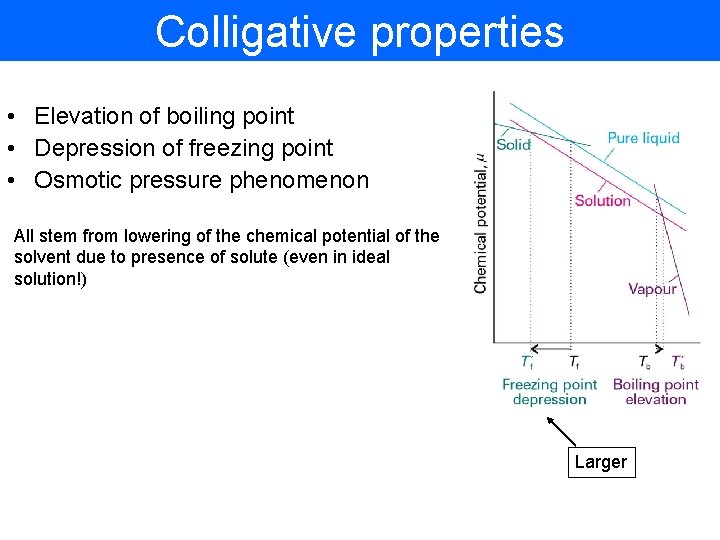
Colligative properties • Elevation of boiling point • Depression of freezing point • Osmotic pressure phenomenon All stem from lowering of the chemical potential of the solvent due to presence of solute (even in ideal solution!) Larger

Colligative properties • Elevation of boiling point (Here we neglect temperature dependence) For pure liquid:

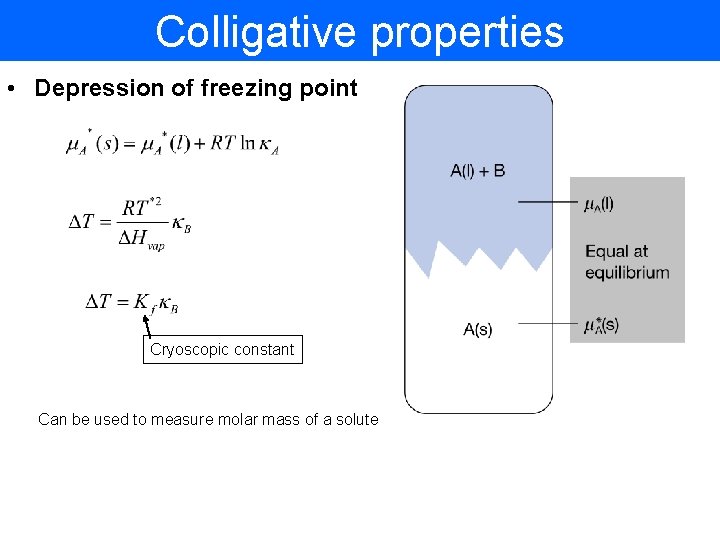
Colligative properties • Depression of freezing point Cryoscopic constant Can be used to measure molar mass of a solute

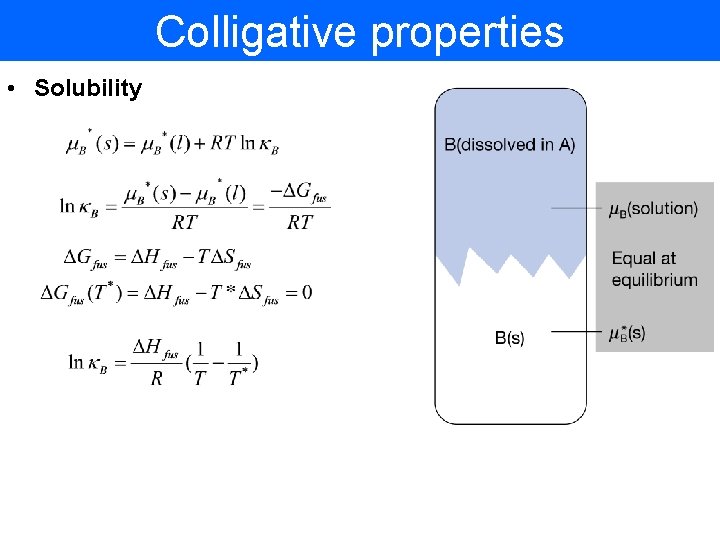
Colligative properties • Solubility

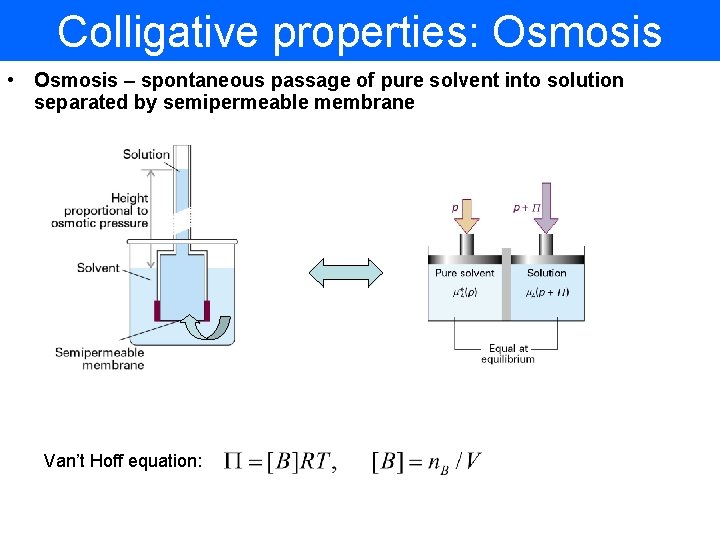
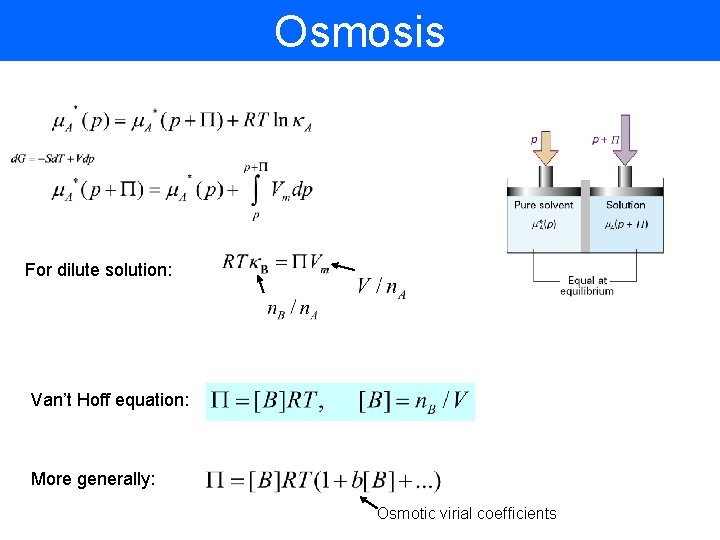
Colligative properties: Osmosis • Osmosis – spontaneous passage of pure solvent into solution separated by semipermeable membrane Van’t Hoff equation:

Osmosis For dilute solution: Van’t Hoff equation: More generally: Osmotic virial coefficients

Osmosis: Examples • Calculate osmotic pressure exhibited by 0. 1 M solutions of mannitol and Na. Cl. Mannitol (C 6 H 8(OH)6)

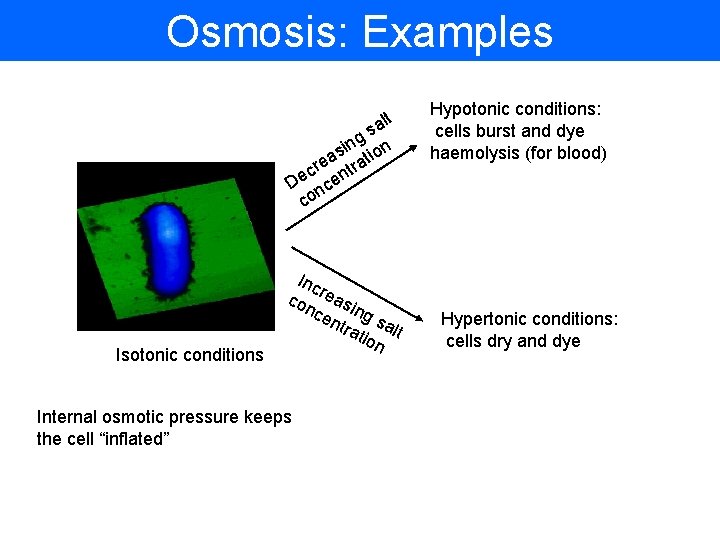
Osmosis: Examples lt a s g n si tion a cre ntra e D nce co Isotonic conditions Inc con reasi cen ng s tra alt tion Internal osmotic pressure keeps the cell “inflated” Hypotonic conditions: cells burst and dye haemolysis (for blood) Hypertonic conditions: cells dry and dye

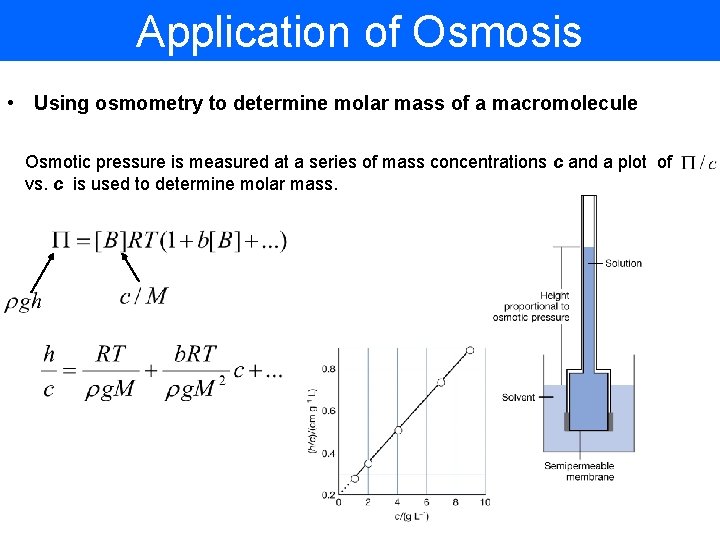
Application of Osmosis • Using osmometry to determine molar mass of a macromolecule Osmotic pressure is measured at a series of mass concentrations c and a plot of vs. c is used to determine molar mass.

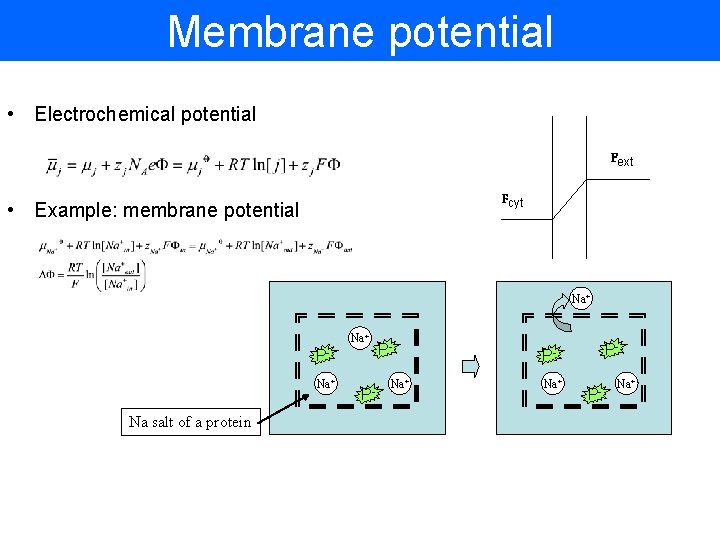
Membrane potential • Electrochemical potential Fext Fcyt • Example: membrane potential Na+ PNa+ Na salt of a protein P- PNa+ P- Na+

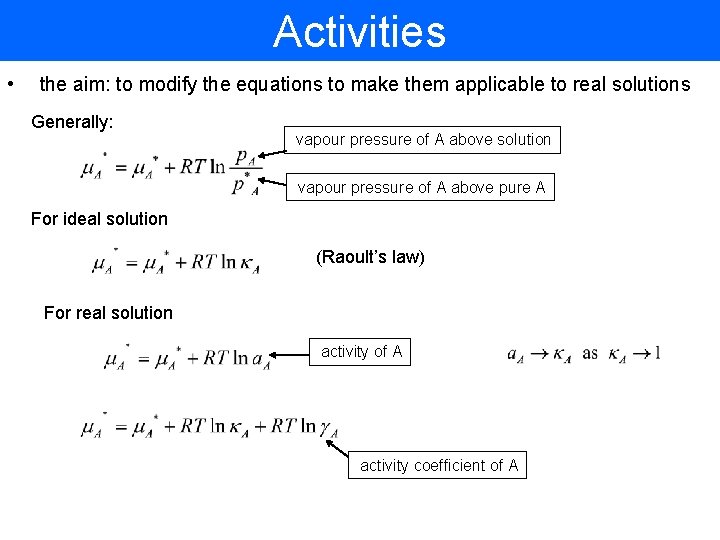
Activities • the aim: to modify the equations to make them applicable to real solutions Generally: vapour pressure of A above solution vapour pressure of A above pure A For ideal solution (Raoult’s law) For real solution activity of A activity coefficient of A

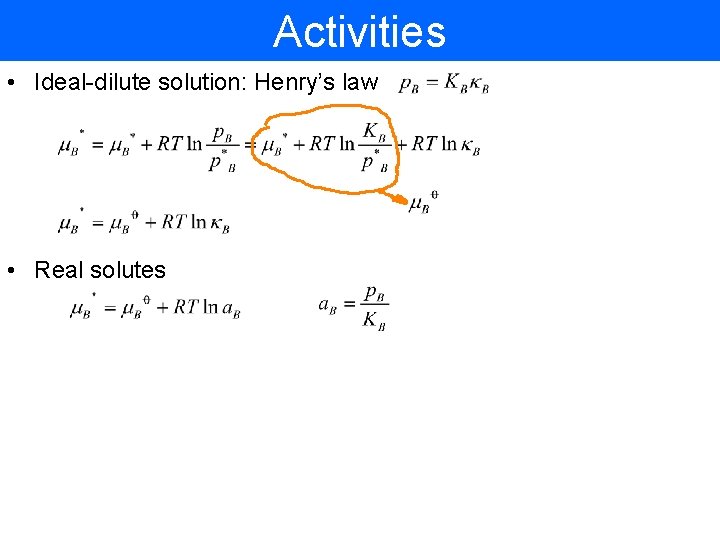
Activities • Ideal-dilute solution: Henry’s law • Real solutes

Example: Biological standard state • Biological standard state: let’s define chemical potential of hydrogen at p. H=7

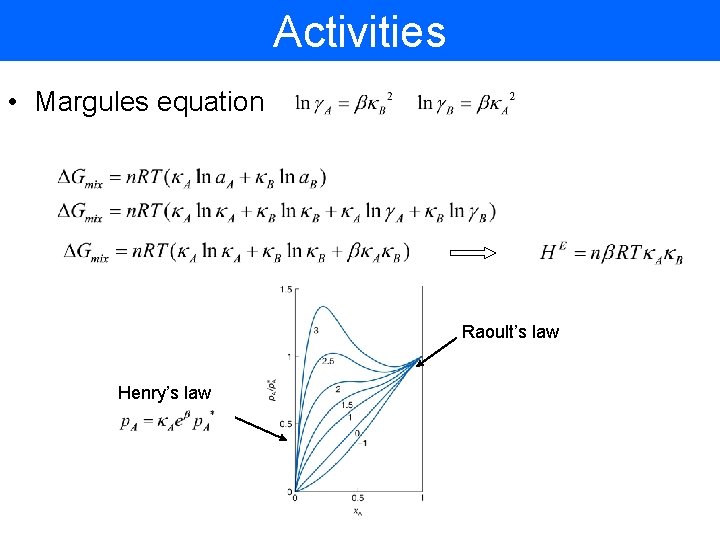
Activities • Margules equation Raoult’s law Henry’s law

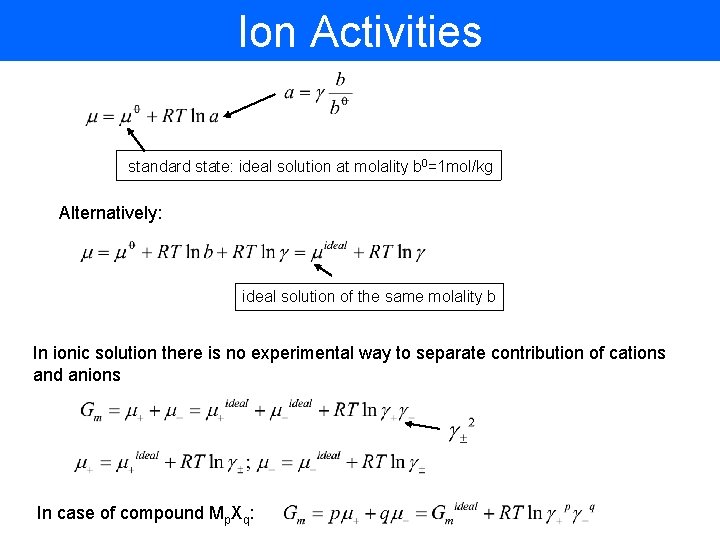
Ion Activities standard state: ideal solution at molality b 0=1 mol/kg Alternatively: ideal solution of the same molality b In ionic solution there is no experimental way to separate contribution of cations and anions In case of compound Mp. Xq:

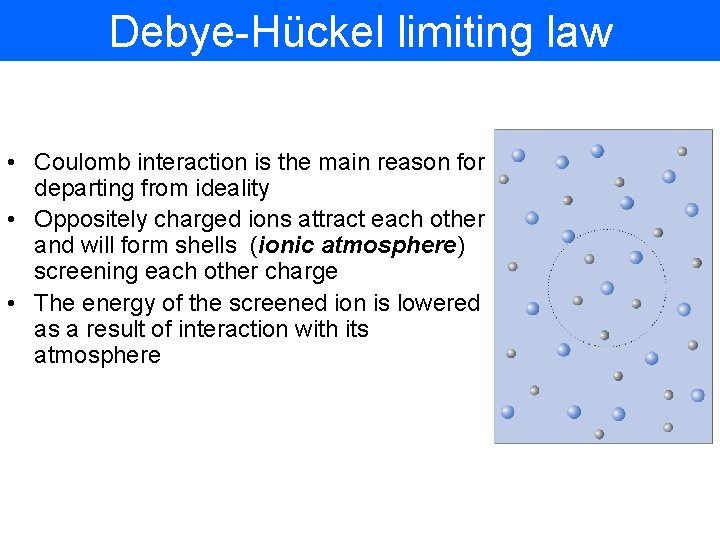
Debye-Hückel limiting law • Coulomb interaction is the main reason for departing from ideality • Oppositely charged ions attract each other and will form shells (ionic atmosphere) screening each other charge • The energy of the screened ion is lowered as a result of interaction with its atmosphere

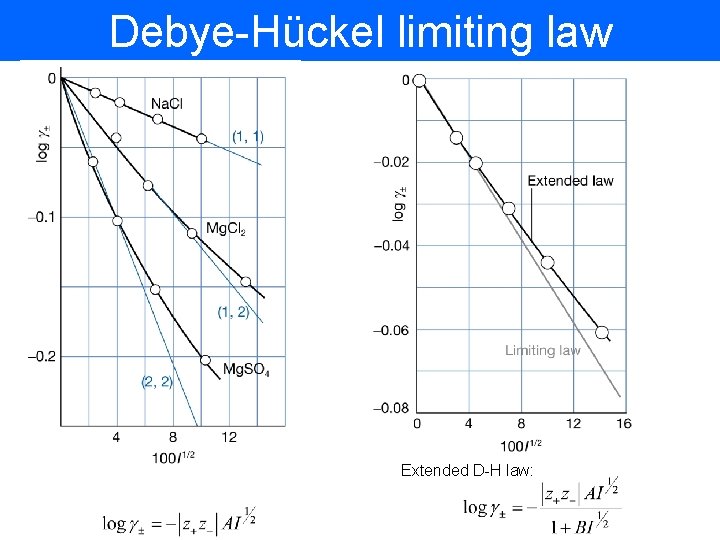
Debye-Hückel limiting law In a limit of low concentration the activity coefficient can be calculated as: Ionic strength of the solution Example: calculate mean activity coefficient of 5 m. M solution of KCL at 25 C.

Debye-Hückel limiting law Extended D-H law:

Phase Diagrams

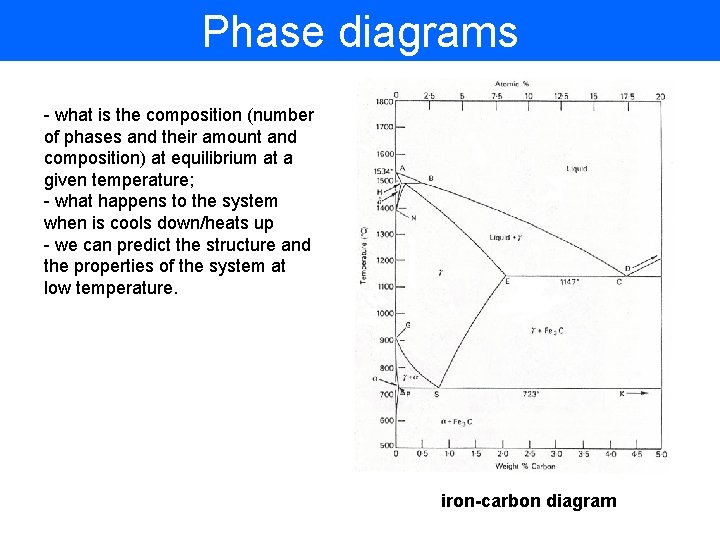
Phase diagrams - what is the composition (number of phases and their amount and composition) at equilibrium at a given temperature; - what happens to the system when is cools down/heats up - we can predict the structure and the properties of the system at low temperature. iron-carbon diagram

Phase diagrams water-surfactant-oil That’s the base of all modern engineering from swiss knife to food and cosmetics! iron-carbon diagram

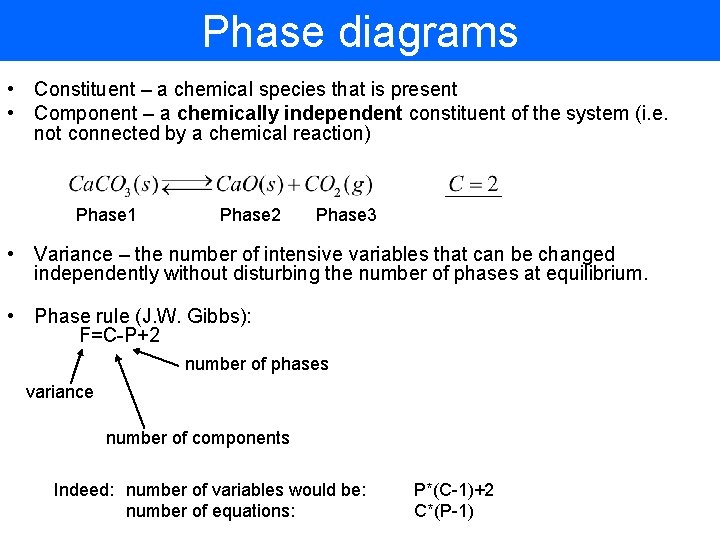
Phase diagrams • Constituent – a chemical species that is present • Component – a chemically independent constituent of the system (i. e. not connected by a chemical reaction) Phase 1 Phase 2 Phase 3 • Variance – the number of intensive variables that can be changed independently without disturbing the number of phases at equilibrium. • Phase rule (J. W. Gibbs): F=C-P+2 number of phases variance number of components Indeed: number of variables would be: number of equations: P*(C-1)+2 C*(P-1)

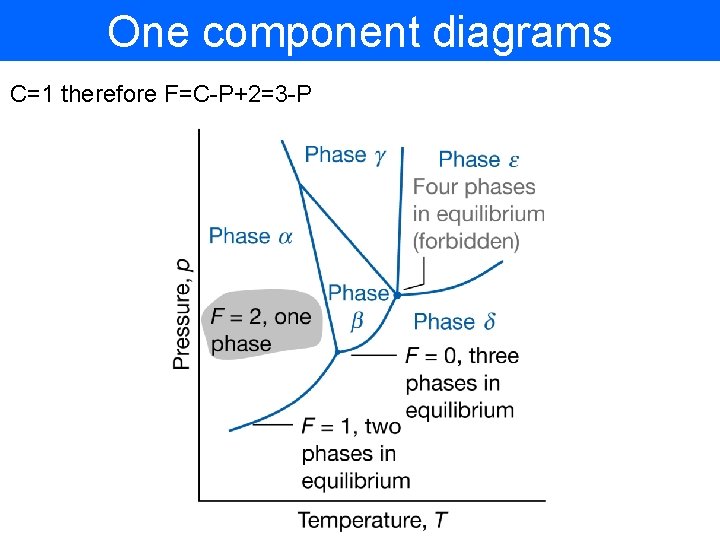
One component diagrams C=1 therefore F=C-P+2=3 -P

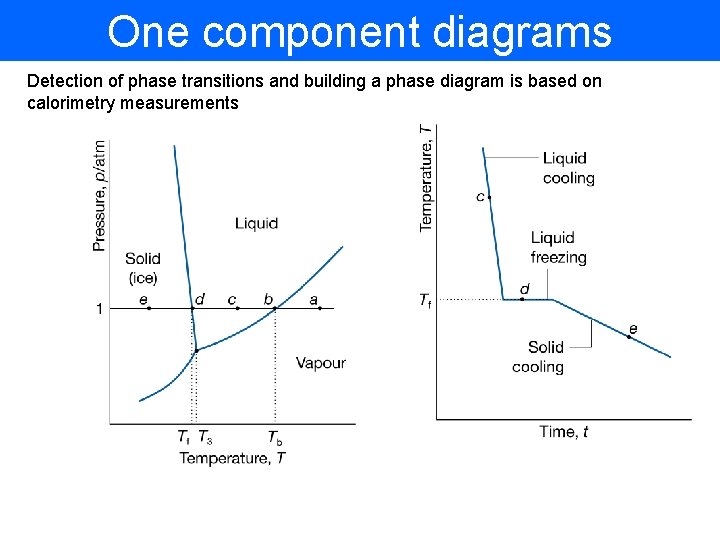
One component diagrams Detection of phase transitions and building a phase diagram is based on calorimetry measurements

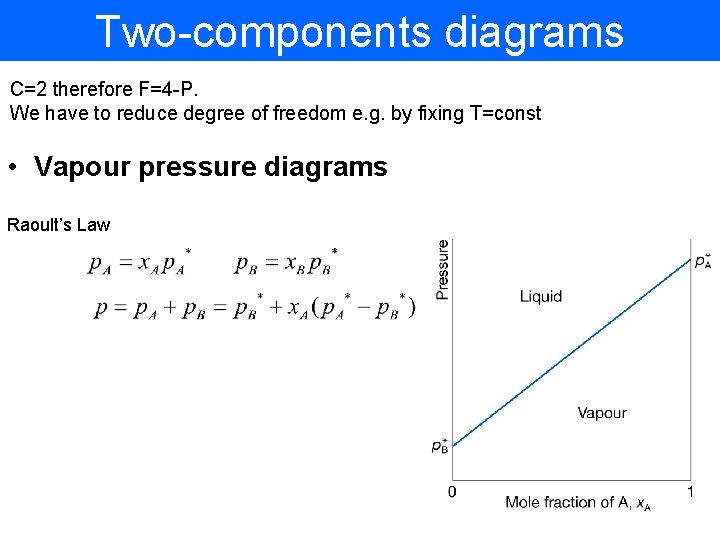
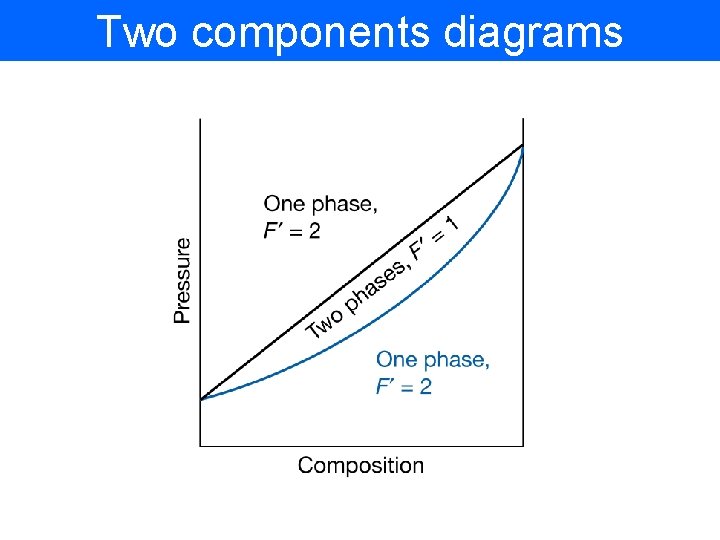
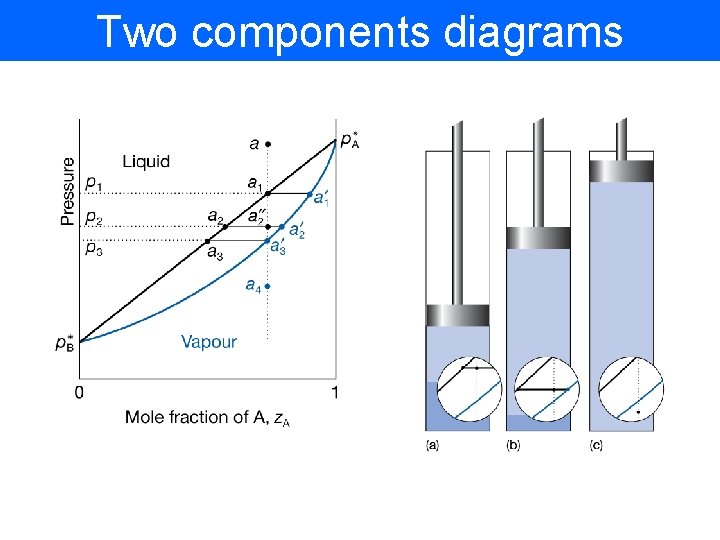
Two-components diagrams C=2 therefore F=4 -P. We have to reduce degree of freedom e. g. by fixing T=const • Vapour pressure diagrams Raoult’s Law

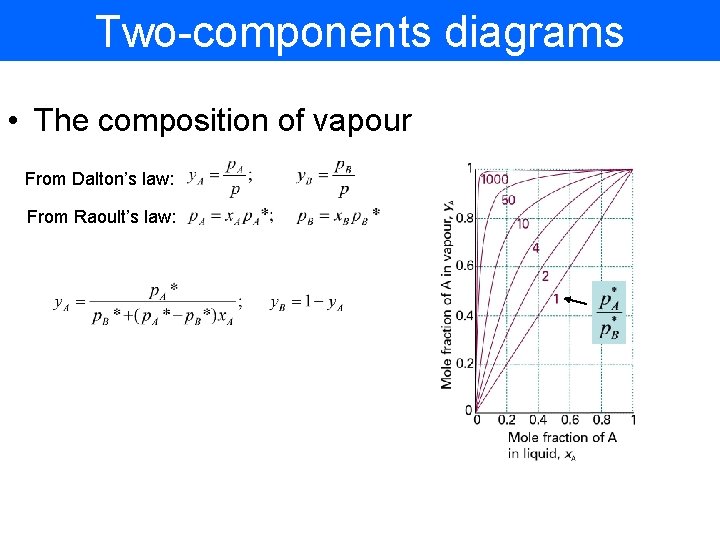
Two-components diagrams • The composition of vapour From Dalton’s law: From Raoult’s law:

Two components diagrams

Two components diagrams

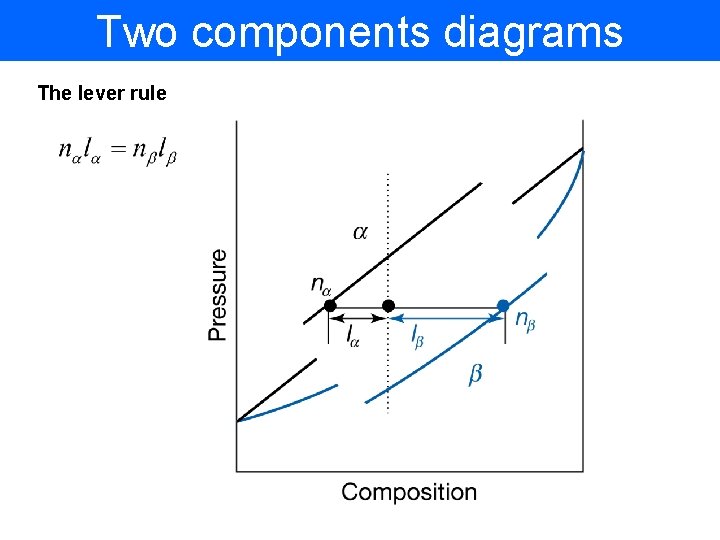
Two components diagrams The lever rule

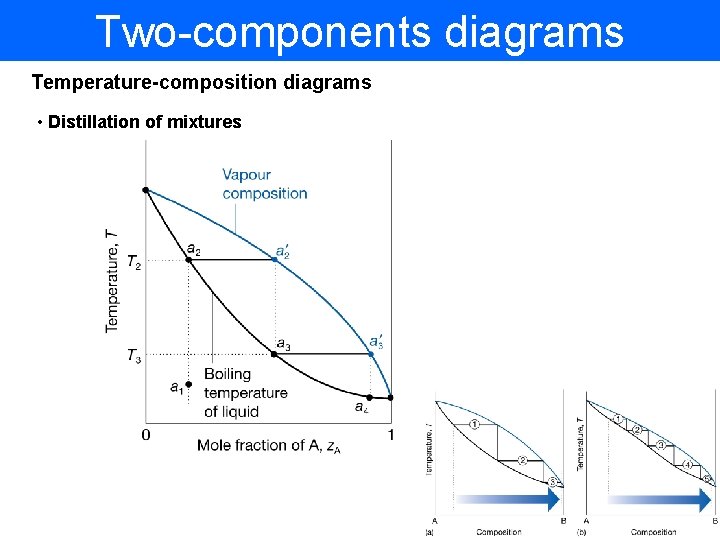
Two-components diagrams Temperature-composition diagrams • Distillation of mixtures

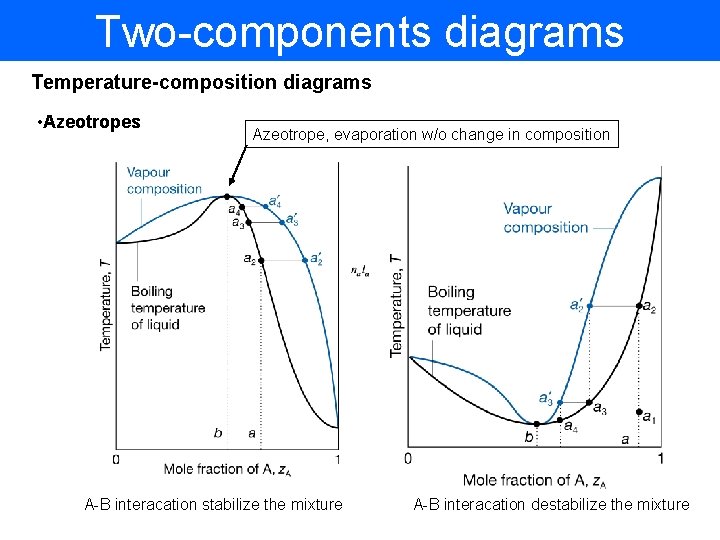
Two-components diagrams Temperature-composition diagrams • Azeotropes Azeotrope, evaporation w/o change in composition A-B interacation stabilize the mixture A-B interacation destabilize the mixture

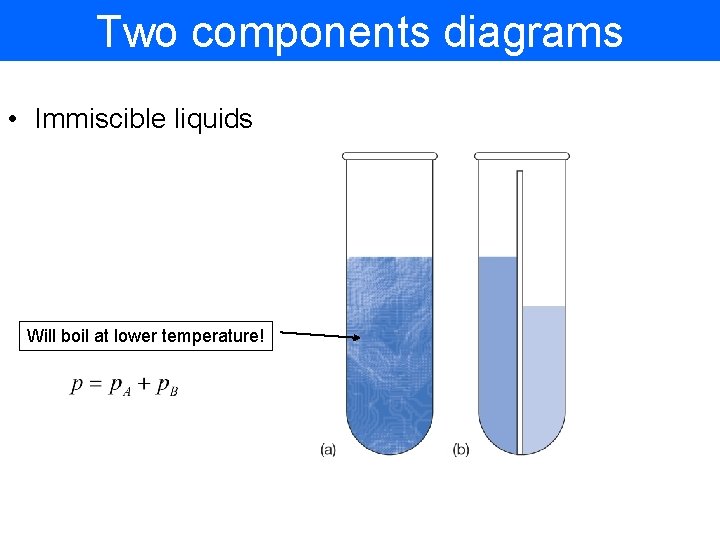
Two components diagrams • Immiscible liquids Will boil at lower temperature!

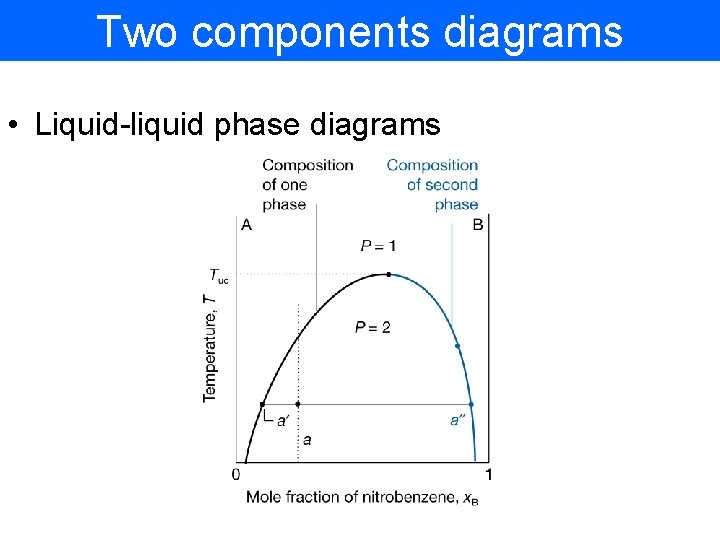
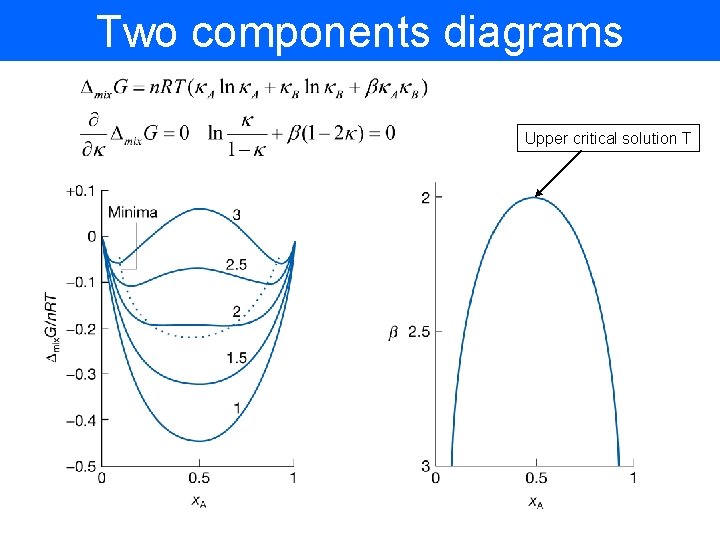
Two components diagrams • Liquid-liquid phase diagrams

Two components diagrams Upper critical solution T

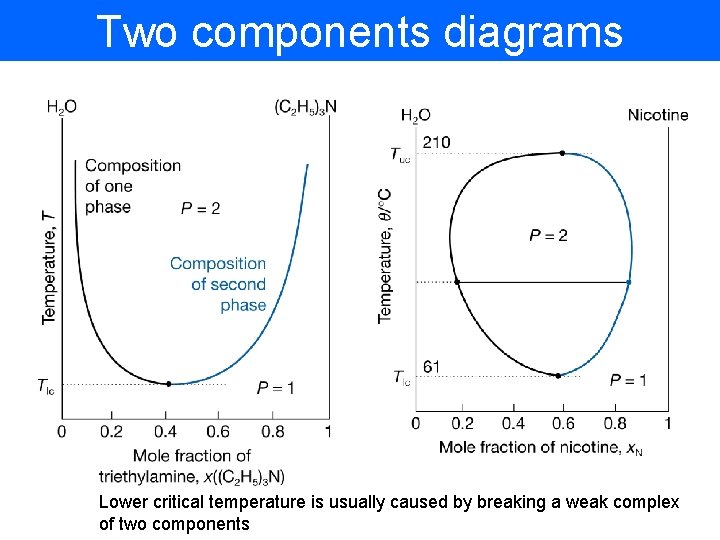
Two components diagrams Lower critical temperature is usually caused by breaking a weak complex of two components

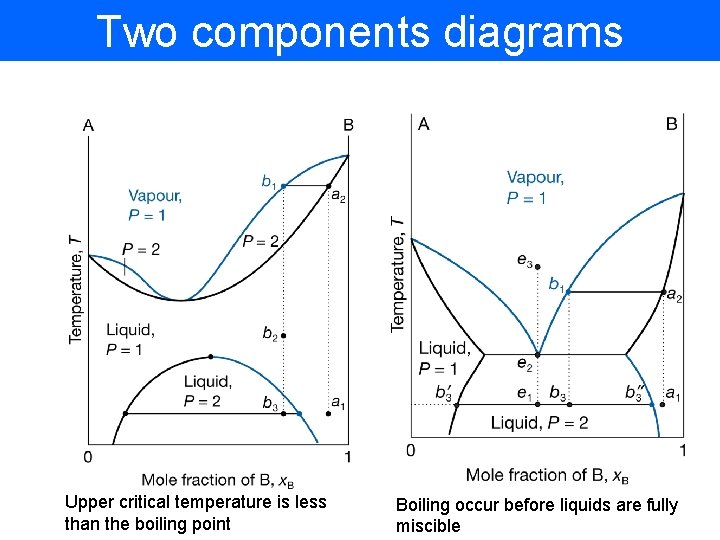
Two components diagrams Upper critical temperature is less than the boiling point Boiling occur before liquids are fully miscible

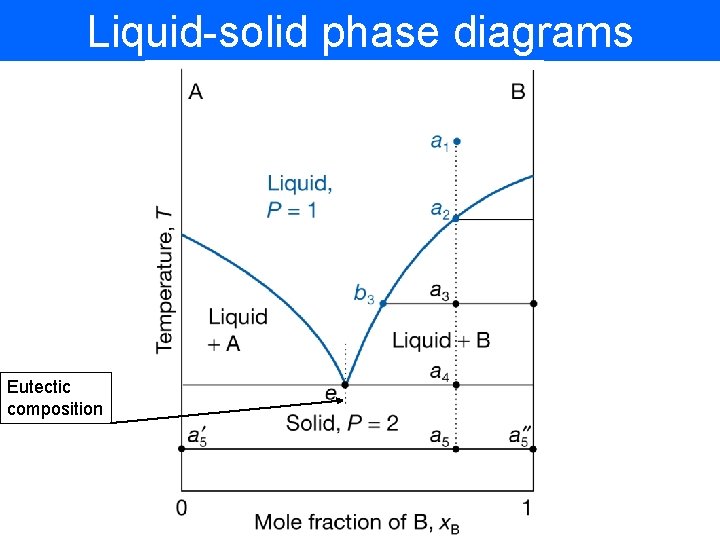
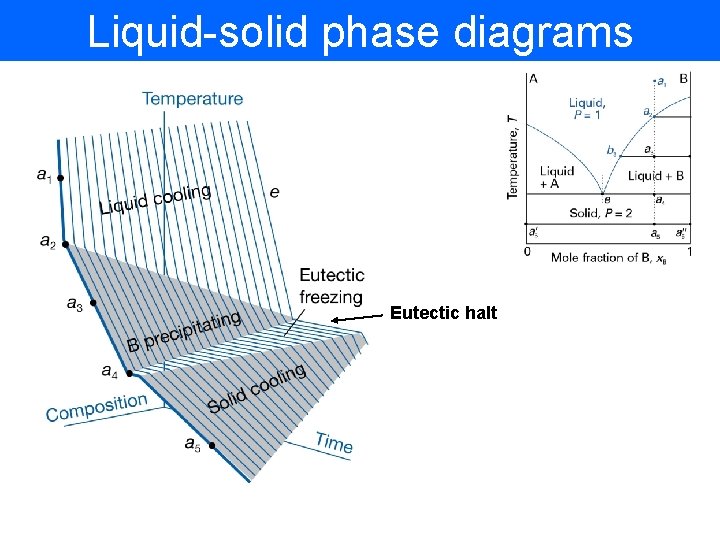
Liquid-solid phase diagrams Eutectic composition

Liquid-solid phase diagrams Eutectic halt

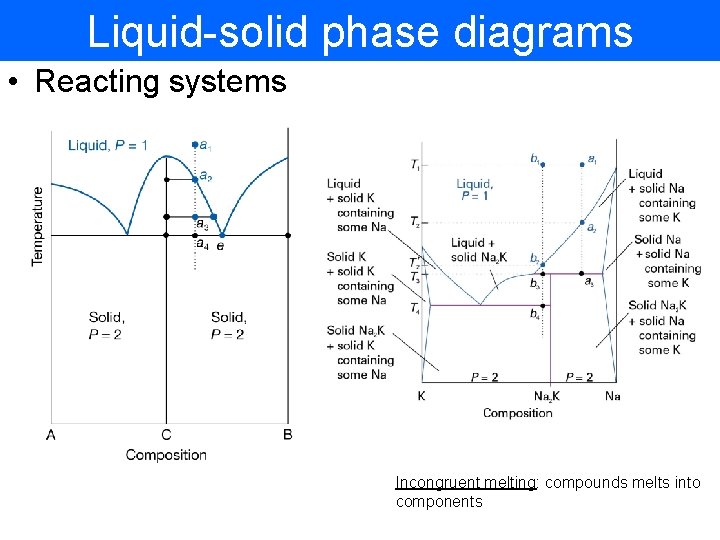
Liquid-solid phase diagrams • Reacting systems Incongruent melting: compounds melts into components

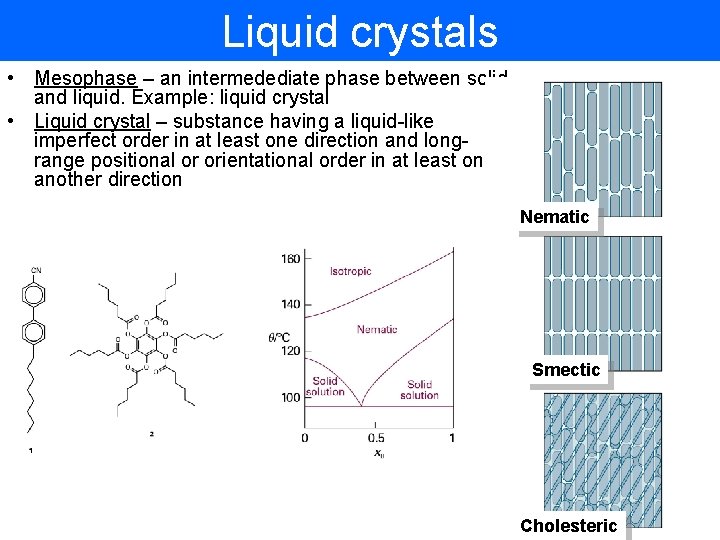
Liquid crystals • Mesophase – an intermedediate phase between solid and liquid. Example: liquid crystal • Liquid crystal – substance having a liquid-like imperfect order in at least one direction and longrange positional or orientational order in at least one another direction Nematic Smectic Cholesteric

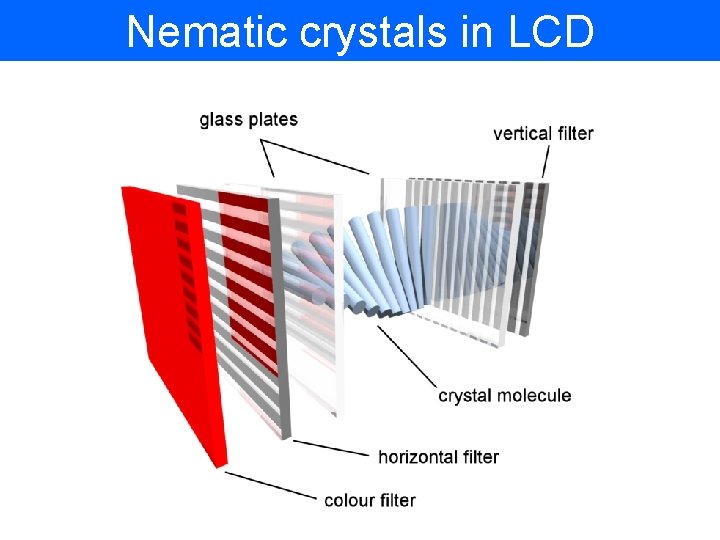
Nematic crystals in LCD

Problems I • 5. 6 a The addition of 100 g of a compound to 750 g of CCl 4 lowered the freezing point of the solvent by 10. 5 K. Calculate the molar mass of the compound. • 5. 14 a The osmotic pressure of solution of polystyrene in toluene were measured at 25 C and the pressure was expressed in terms of the height of the solvent of density 1. 004 g/cm 3. Calculate the molar mass of polystyrene: c [g/dm 3] 2. 042 6. 613 9. 521 12. 602 h [cm] 0. 592 1. 910 2. 750 3. 600 • 5. 20(a) Estimate the mean ionic activity coefficient and activity of a solution that is 0. 010 mol kg– 1 Ca. Cl 2(aq) and 0. 030 mol kg – 1 Na. F(aq).

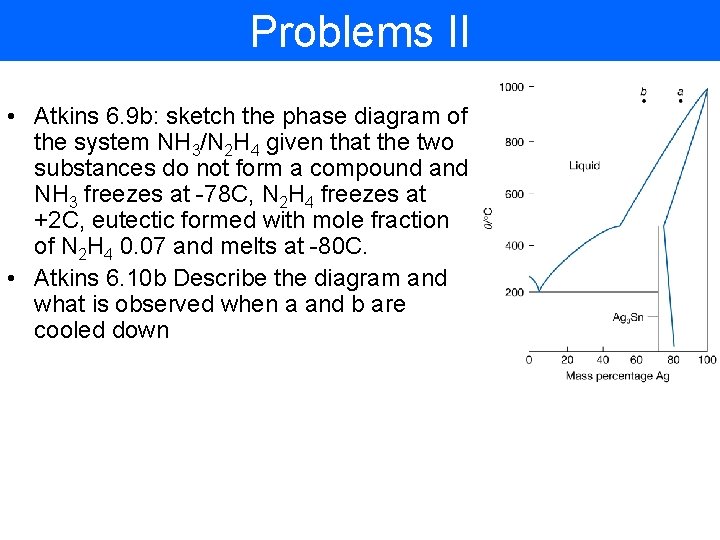
Problems II • Atkins 6. 9 b: sketch the phase diagram of the system NH 3/N 2 H 4 given that the two substances do not form a compound and NH 3 freezes at -78 C, N 2 H 4 freezes at +2 C, eutectic formed with mole fraction of N 2 H 4 0. 07 and melts at -80 C. • Atkins 6. 10 b Describe the diagram and what is observed when a and b are cooled down

Home problem analysis • 4. 7 a: An open vessel containing (a) water, (b) benzene, (c) mercury stands in a laboratory measuring 5. 0 m 3. 0 m at 25 C. What mass of each substance will be found in the air if there is no ventilation? (The vapour pressures are (a) 3. 2 k. Pa, (b) 13. 1 k. Pa, (c) 0. 23 Pa. ) • 4. 9 a Calculate the melting point of ice under a pressure of 50 bar. Assume that the density of ice under these conditions is approximately 0. 92 g cm– 3 and that of liquid water is 1. 00 g cm – 3. • 5. 2 a At 25 C, the density of a 50 per cent by mass ethanol– water solution is 0. 914 g cm– 3. Given that the partial molar volume of water in the solution is 17. 4 cm 3 mol– 1, calculate the partial molar volume of the ethanol.