14 4 Gases Mixtures and Movements Chapter 14
































- Slides: 52

14. 4 Gases: Mixtures and Movements > Chapter 14 The Behavior of Gases 14. 1 Properties of Gases 14. 2 The Gas Laws 14. 3 Ideal Gases 14. 4 Gases: Mixtures and Movements 1 Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

14. 4 Gases: Mixtures and Movements > CHEMISTRY & YOU Why do balloons filled with helium deflate faster than balloons filled with air? The surface of a latex balloon has tiny pores through which gas particles can pass. The rate at which the balloon deflates depends on the gas it contains. 2 Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

14. 4 Gases: Mixtures and Movements > Dalton’s Law How is the total pressure of a mixture of gases related to the partial pressures of the component gases? 3 Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

14. 4 Gases: Mixtures and Movements > Dalton’s Law Gas pressure results from collisions of particles in a gas with an object. 4 Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

14. 4 Gases: Mixtures and Movements > Dalton’s Law Gas pressure results from collisions of particles in a gas with an object. • If the number of particles increases in a given volume, more collisions occur. 5 Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

14. 4 Gases: Mixtures and Movements > Dalton’s Law Gas pressure results from collisions of particles in a gas with an object. • If the number of particles increases in a given volume, more collisions occur. • If the average kinetic energy of the particles increases, more collisions occur. 6 Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

14. 4 Gases: Mixtures and Movements > Dalton’s Law Gas pressure results from collisions of particles in a gas with an object. • If the number of particles increases in a given volume, more collisions occur. • If the average kinetic energy of the particles increases, more collisions occur. • In both cases, the pressure increases. 7 Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

14. 4 Gases: Mixtures and Movements > Dalton’s Law Particles in a mixture of gases at the same temperature have the same kinetic energy. 8 Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

14. 4 Gases: Mixtures and Movements > Dalton’s Law Particles in a mixture of gases at the same temperature have the same kinetic energy. • The kind of particle is not important. 9 Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

14. 4 Gases: Mixtures and Movements > Dalton’s Law Particles in a mixture of gases at the same temperature have the same kinetic energy. • The kind of particle is not important. • The contribution each gas in a mixture makes to the total pressure is called the partial pressure exerted by that gas. 10 Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

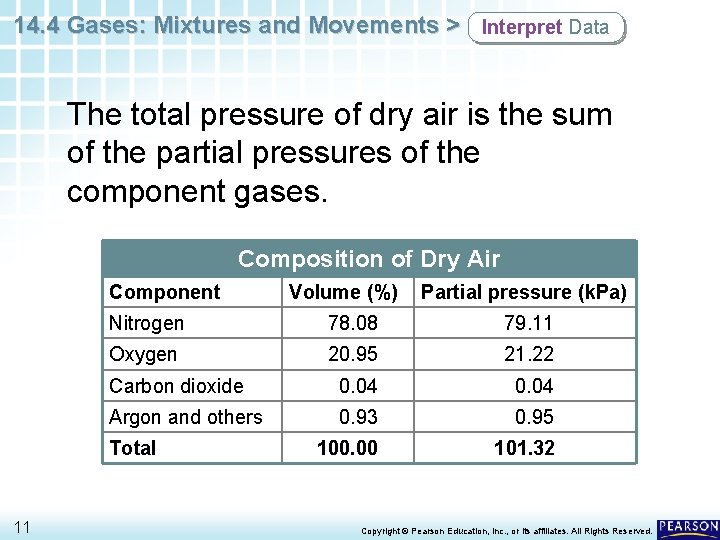
14. 4 Gases: Mixtures and Movements > Interpret Data The total pressure of dry air is the sum of the partial pressures of the component gases. Composition of Dry Air Component Partial pressure (k. Pa) Nitrogen 78. 08 79. 11 Oxygen 20. 95 21. 22 Carbon dioxide 0. 04 Argon and others 0. 93 0. 95 100. 00 101. 32 Total 11 Volume (%) Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

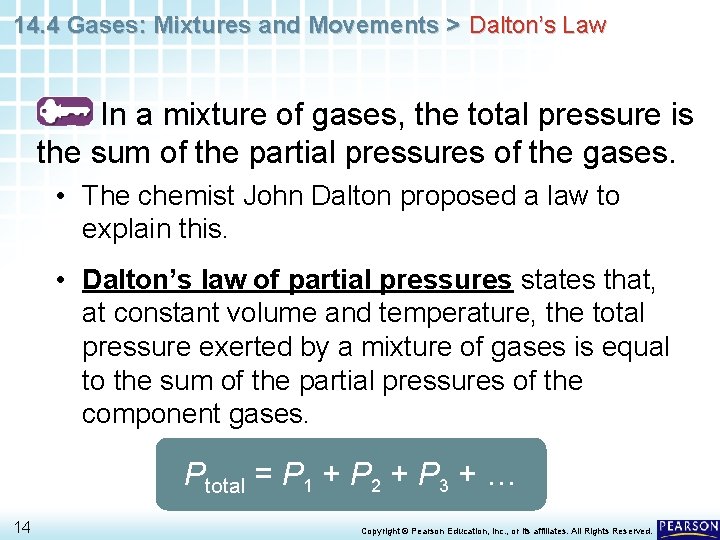
14. 4 Gases: Mixtures and Movements > Dalton’s Law In a mixture of gases, the total pressure is the sum of the partial pressures of the gases. 12 Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

14. 4 Gases: Mixtures and Movements > Dalton’s Law In a mixture of gases, the total pressure is the sum of the partial pressures of the gases. • The chemist John Dalton proposed a law to explain this. • Dalton’s law of partial pressures states that, at constant volume and temperature, the total pressure exerted by a mixture of gases is equal to the sum of the partial pressures of the component gases. 13 Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

14. 4 Gases: Mixtures and Movements > Dalton’s Law In a mixture of gases, the total pressure is the sum of the partial pressures of the gases. • The chemist John Dalton proposed a law to explain this. • Dalton’s law of partial pressures states that, at constant volume and temperature, the total pressure exerted by a mixture of gases is equal to the sum of the partial pressures of the component gases. Ptotal = P 1 + P 2 + P 3 + … 14 Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

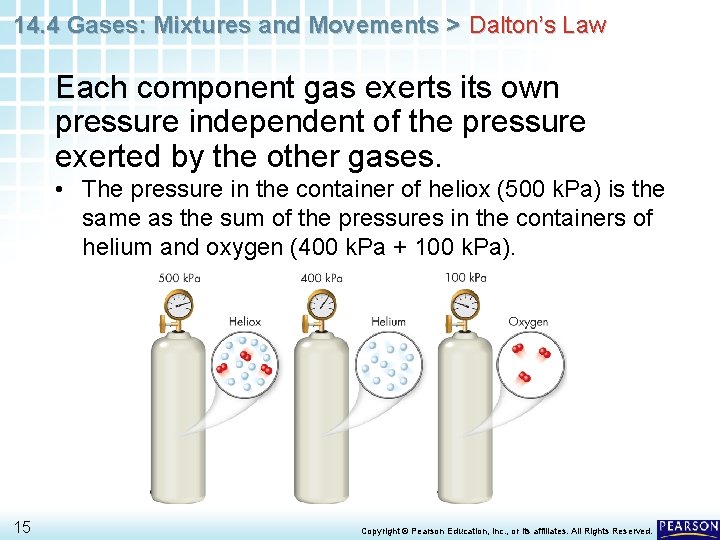
14. 4 Gases: Mixtures and Movements > Dalton’s Law Each component gas exerts its own pressure independent of the pressure exerted by the other gases. • The pressure in the container of heliox (500 k. Pa) is the same as the sum of the pressures in the containers of helium and oxygen (400 k. Pa + 100 k. Pa). 15 Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

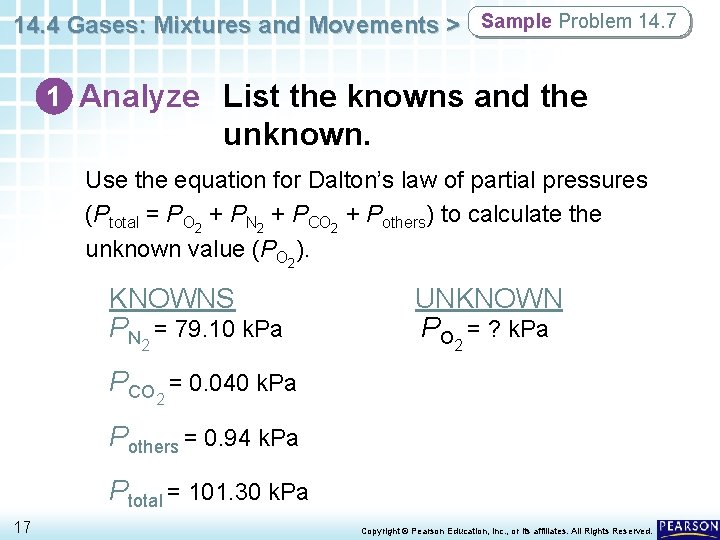
14. 4 Gases: Mixtures and Movements > Sample Problem 14. 7 Using Dalton’s Law of Partial Pressures Air contains oxygen, nitrogen, carbon dioxide, and trace amounts of other gases. What is the partial pressure of oxygen (PO 2) at 101. 30 k. Pa of total pressure if the partial pressures of nitrogen, carbon dioxide, and other gases are 79. 10 k. Pa, 0. 040 k. Pa, and 0. 94 k. Pa, respectively? 16 Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

14. 4 Gases: Mixtures and Movements > Sample Problem 14. 7 1 Analyze List the knowns and the unknown. Use the equation for Dalton’s law of partial pressures (Ptotal = PO 2 + PN 2 + PCO 2 + Pothers) to calculate the unknown value (PO 2). KNOWNS PN 2 = 79. 10 k. Pa UNKNOWN PO 2 = ? k. Pa PCO 2 = 0. 040 k. Pa Pothers = 0. 94 k. Pa Ptotal = 101. 30 k. Pa 17 Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

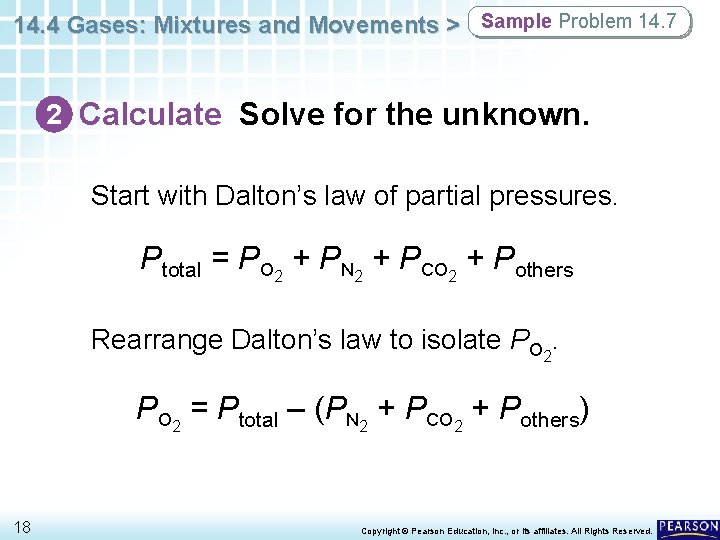
14. 4 Gases: Mixtures and Movements > Sample Problem 14. 7 2 Calculate Solve for the unknown. Start with Dalton’s law of partial pressures. Ptotal = PO 2 + PN 2 + PCO 2 + Pothers Rearrange Dalton’s law to isolate PO 2 = Ptotal – (PN 2 + PCO 2 + Pothers) 18 Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

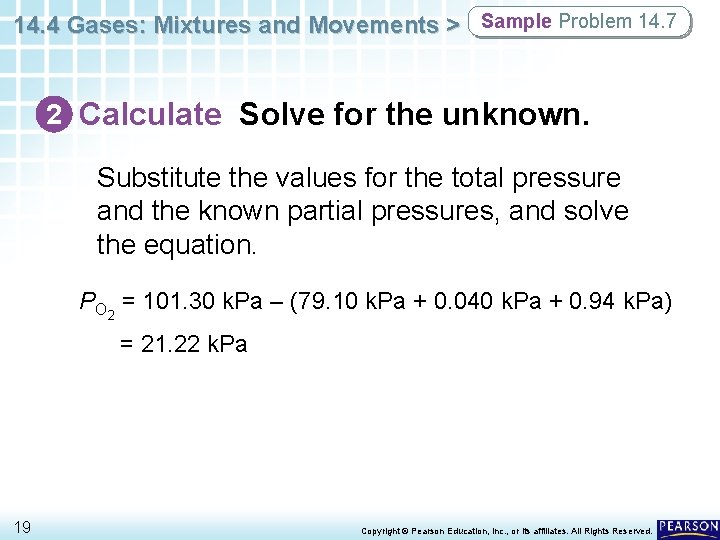
14. 4 Gases: Mixtures and Movements > Sample Problem 14. 7 2 Calculate Solve for the unknown. Substitute the values for the total pressure and the known partial pressures, and solve the equation. PO 2 = 101. 30 k. Pa – (79. 10 k. Pa + 0. 040 k. Pa + 0. 94 k. Pa) = 21. 22 k. Pa 19 Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

14. 4 Gases: Mixtures and Movements > Sample Problem 14. 7 3 Evaluate Does this result make sense? • The partial pressure of oxygen must be smaller than that of nitrogen because Ptotal is only 101. 30 k. Pa. • The other partial pressures are small, so the calculated answer of 21. 22 k. Pa seems reasonable. 20 Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

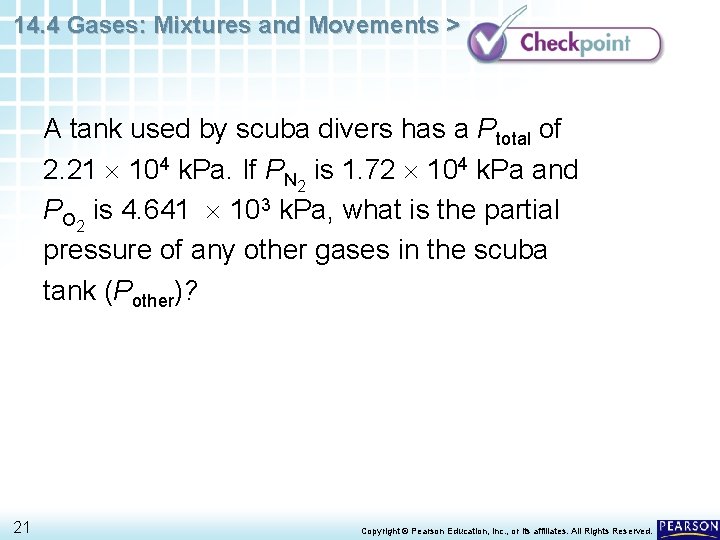
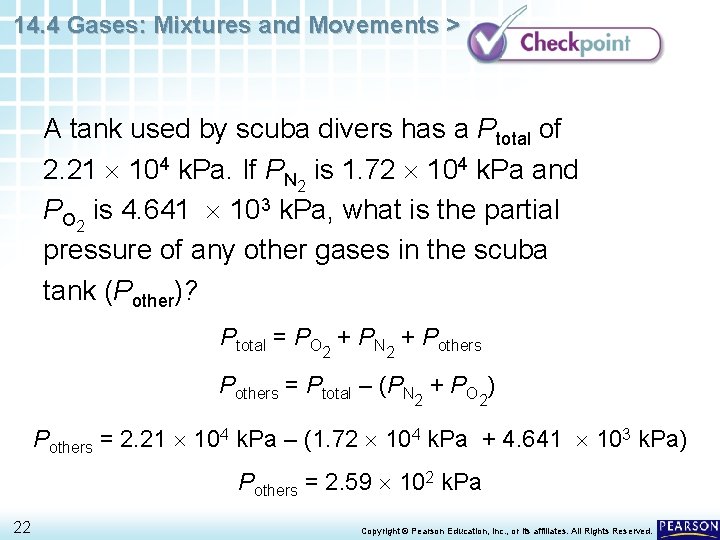
14. 4 Gases: Mixtures and Movements > A tank used by scuba divers has a Ptotal of 2. 21 104 k. Pa. If PN 2 is 1. 72 104 k. Pa and PO 2 is 4. 641 103 k. Pa, what is the partial pressure of any other gases in the scuba tank (Pother)? 21 Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

14. 4 Gases: Mixtures and Movements > A tank used by scuba divers has a Ptotal of 2. 21 104 k. Pa. If PN 2 is 1. 72 104 k. Pa and PO 2 is 4. 641 103 k. Pa, what is the partial pressure of any other gases in the scuba tank (Pother)? Ptotal = PO + PN + Pothers 2 2 Pothers = Ptotal – (PN + PO ) 2 2 Pothers = 2. 21 104 k. Pa – (1. 72 104 k. Pa + 4. 641 103 k. Pa) Pothers = 2. 59 102 k. Pa 22 Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

14. 4 Gases: Mixtures and Movements > Graham’s Law How does the molar mass of a gas affect the rate at which the gas diffuses or effuses? 23 Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

14. 4 Gases: Mixtures and Movements > Graham’s Law • If you open a perfume bottle in one corner of a room, at some point, a person standing in the opposite corner will be able to smell the perfume. 24 Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

14. 4 Gases: Mixtures and Movements > Graham’s Law • If you open a perfume bottle in one corner of a room, at some point, a person standing in the opposite corner will be able to smell the perfume. • Molecules in the perfume evaporate and diffuse, or spread out, through the air in the room. 25 Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

14. 4 Gases: Mixtures and Movements > Graham’s Law Diffusion is the tendency of molecules to move toward areas of lower concentration until the concentration is uniform throughout. 26 Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

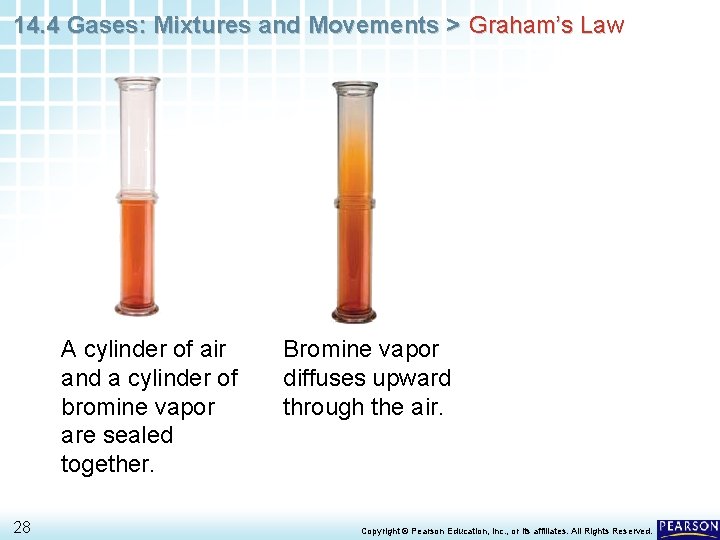
14. 4 Gases: Mixtures and Movements > Graham’s Law A cylinder of air and a cylinder of bromine vapor are sealed together. 27 Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

14. 4 Gases: Mixtures and Movements > Graham’s Law A cylinder of air and a cylinder of bromine vapor are sealed together. 28 Bromine vapor diffuses upward through the air. Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

14. 4 Gases: Mixtures and Movements > Graham’s Law A cylinder of air and a cylinder of bromine vapor are sealed together. 29 Bromine vapor diffuses upward through the air. After several hours, bromine vapors reach the top of the column. Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

14. 4 Gases: Mixtures and Movements > Graham’s Law During effusion, a gas escapes through a tiny hole in its container. 30 Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

14. 4 Gases: Mixtures and Movements > Graham’s Law During effusion, a gas escapes through a tiny hole in its container. • With effusion and diffusion, the type of particle is important. 31 Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

14. 4 Gases: Mixtures and Movements > Graham’s Law Gases of lower molar mass diffuse and effuse faster than gases of higher molar mass. 32 Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

14. 4 Gases: Mixtures and Movements > Graham’s Law Thomas Graham’s Contribution Graham’s law of effusion states that the rate of effusion of a gas is inversely proportional to the square root of the gas’s molar mass. 33 Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

14. 4 Gases: Mixtures and Movements > Graham’s Law Thomas Graham’s Contribution Graham’s law of effusion states that the rate of effusion of a gas is inversely proportional to the square root of the gas’s molar mass. • This law can also be applied to the diffusion of gases. 34 Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

14. 4 Gases: Mixtures and Movements > Graham’s Law Thomas Graham’s Contribution Graham’s law of effusion states that the rate of effusion of a gas is inversely proportional to the square root of the gas’s molar mass. • This law can also be applied to the diffusion of gases. • If two objects with different masses have the same kinetic energy, the lighter object must move faster. 35 Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

14. 4 Gases: Mixtures and Movements > CHEMISTRY & YOU Why do balloons filled with helium deflate faster than balloons filled with air? Use Graham’s law of effusion to explain your answer. 36 Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

14. 4 Gases: Mixtures and Movements > CHEMISTRY & YOU Why do balloons filled with helium deflate faster than balloons filled with air? Use Graham’s law of effusion to explain your answer. Molecules of helium have a lower mass than the average mass of air molecules, so helium molecules effuse through the tiny pores in a balloon faster than air molecules do. 37 Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

14. 4 Gases: Mixtures and Movements > Graham’s Law Comparing Effusion Rates Suppose you have two balloons, one filled with helium and the other filled with air. • If the balloons are the same temperature, the particles in each balloon have the same average kinetic energy. • But helium atoms are less massive than oxygen or nitrogen molecules. 38 Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

14. 4 Gases: Mixtures and Movements > Graham’s Law Comparing Effusion Rates Suppose you have two balloons, one filled with helium and the other filled with air. • If the balloons are the same temperature, the particles in each balloon have the same average kinetic energy. • But helium atoms are less massive than oxygen or nitrogen molecules. • So the molecules in air move more slowly than helium atoms with the same kinetic energy. 39 Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

14. 4 Gases: Mixtures and Movements > Graham’s Law Because the rate of effusion is related only to a particle’s speed, Graham’s law can be written as follows for two gases, A and B. Rate. A Rate. B 40 = molar mass. B molar mass. A Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

14. 4 Gases: Mixtures and Movements > Sample Problem 14. 8 Comparing Effusion Rates How much faster does helium (He) effuse than nitrogen (N 2) at the same temperature? 41 Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

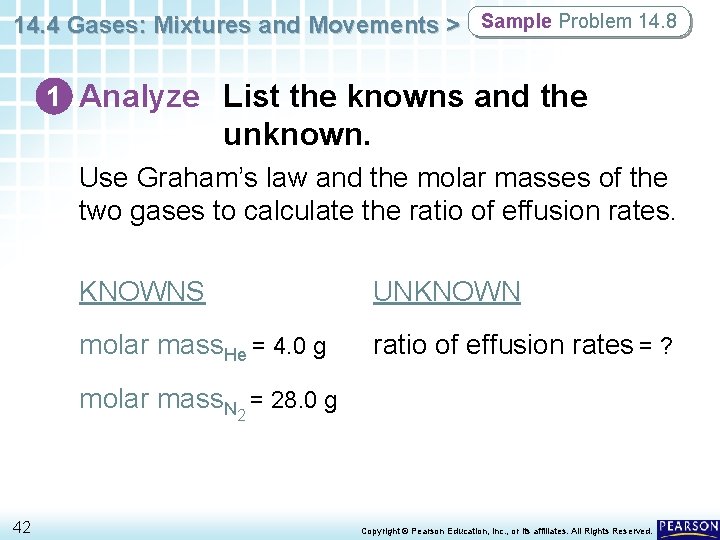
14. 4 Gases: Mixtures and Movements > Sample Problem 14. 8 1 Analyze List the knowns and the unknown. Use Graham’s law and the molar masses of the two gases to calculate the ratio of effusion rates. KNOWNS UNKNOWN molar mass. He = 4. 0 g ratio of effusion rates = ? molar mass. N 2 = 28. 0 g 42 Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

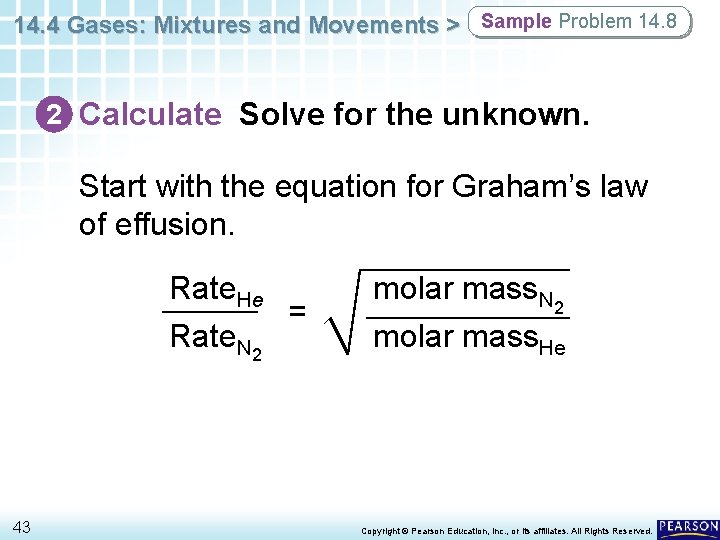
14. 4 Gases: Mixtures and Movements > Sample Problem 14. 8 2 Calculate Solve for the unknown. Start with the equation for Graham’s law of effusion. Rate. He = Rate. N 2 43 molar mass. N 2 molar mass. He Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

14. 4 Gases: Mixtures and Movements > Sample Problem 14. 8 2 Calculate Solve for the unknown. Substitute the molar masses of nitrogen and helium into the equation. Rate. He Rate. N 2 44 = 28. 0 g 4. 0 g = 7. 0 = 2. 7 Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

14. 4 Gases: Mixtures and Movements > Sample Problem 14. 8 3 Evaluate Does this result make sense? Helium atoms are less massive than nitrogen molecules, so it makes sense that helium effuses faster than nitrogen. 45 Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

14. 4 Gases: Mixtures and Movements > Which of the following gas particles will diffuse fastest if all of these gases are at the same temperature and pressure? 46 A. SO 2 C. N 2 O B. Cl 2 D. Hg Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

14. 4 Gases: Mixtures and Movements > Which of the following gas particles will diffuse fastest if all of these gases are at the same temperature and pressure? 47 A. SO 2 C. N 2 O B. Cl 2 D. Hg Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

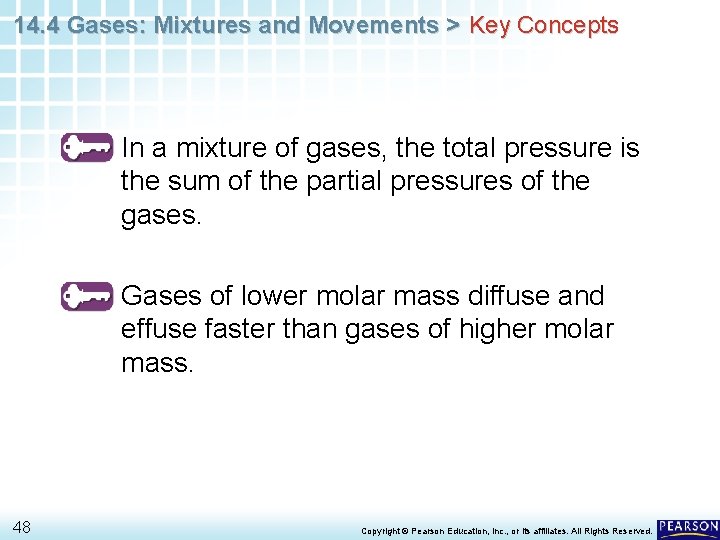
14. 4 Gases: Mixtures and Movements > Key Concepts In a mixture of gases, the total pressure is the sum of the partial pressures of the gases. Gases of lower molar mass diffuse and effuse faster than gases of higher molar mass. 48 Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

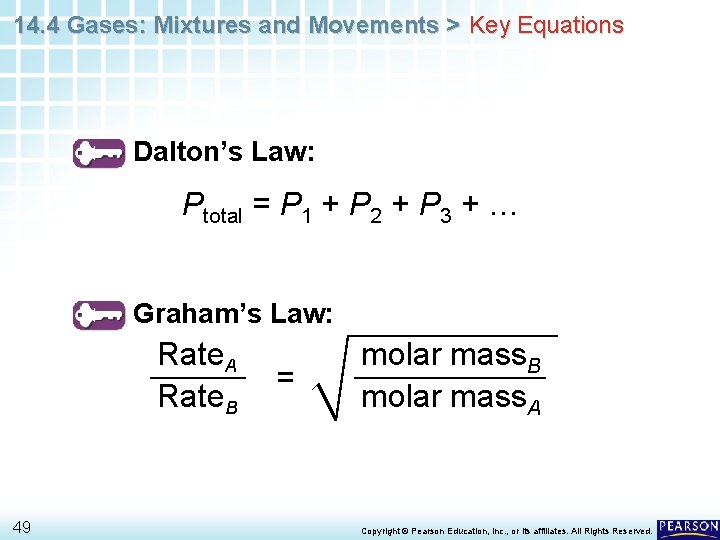
14. 4 Gases: Mixtures and Movements > Key Equations Dalton’s Law: Ptotal = P 1 + P 2 + P 3 + … Graham’s Law: Rate. A Rate. B 49 = molar mass. B molar mass. A Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

14. 4 Gases: Mixtures and Movements > Glossary Terms • partial pressure: the contribution each gas in a mixture of gases makes to the total pressure • Dalton’s law of partial pressures: at constant volume and temperature, the total pressure exerted by a mixture of gases is equal to the sum of the partial pressures of the component gases • diffusion: the tendency of molecules to move toward areas of lower concentration until the concentration is uniform throughout 50 Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

14. 4 Gases: Mixtures and Movements > Glossary Terms • effusion: the process that occurs when a gas escapes through a tiny hole in its container • Graham’s law of effusion: the rate of effusion of a gas is inversely proportional to the square root of its molar mass; this relationship is also true for the diffusion of gases 51 Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

14. 4 Gases: Mixtures and Movements > END OF 14. 4 52 Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.
 14.4 gases mixtures and movements answer key
14.4 gases mixtures and movements answer key Different axial and locomotor movements
Different axial and locomotor movements Heterogeneous mixture def
Heterogeneous mixture def Chapter 13 solutions chemistry
Chapter 13 solutions chemistry Chapter 14 mixtures and solutions
Chapter 14 mixtures and solutions Chapter 14 solids liquids and gases worksheet answers
Chapter 14 solids liquids and gases worksheet answers Chapter 14 solids liquids and gases
Chapter 14 solids liquids and gases Examples of mass movement
Examples of mass movement Examples of mixtures and solutions
Examples of mixtures and solutions Chapter 11 review gases section 1
Chapter 11 review gases section 1 Chapter 11 review gases section 1
Chapter 11 review gases section 1 Gas laws formula
Gas laws formula Chapter 14 the behavior of gases
Chapter 14 the behavior of gases Gas laws
Gas laws Elements, compounds and mixtures worksheet
Elements, compounds and mixtures worksheet Which are pure substances
Which are pure substances How do you classify uniform and non-uniform mixtures?
How do you classify uniform and non-uniform mixtures? Oblong pan with round depressions
Oblong pan with round depressions Gse grade 7 unit 3 answer key
Gse grade 7 unit 3 answer key Mixtures of organic substances and a medicinal agent are:
Mixtures of organic substances and a medicinal agent are: Mixtures solubility and acid/base solutions answer key
Mixtures solubility and acid/base solutions answer key Which of the following dinner items is a pure substance?
Which of the following dinner items is a pure substance? Flowchart matter
Flowchart matter Periodic table mixtures
Periodic table mixtures Classifying elements compounds and mixtures
Classifying elements compounds and mixtures Elements compounds and mixtures ks3
Elements compounds and mixtures ks3 Substances and mixtures
Substances and mixtures Fossweb mixtures and solutions
Fossweb mixtures and solutions Mixtures images
Mixtures images Is a granola bar homogeneous or heterogeneous
Is a granola bar homogeneous or heterogeneous Pure substances and mixtures graphic organizer
Pure substances and mixtures graphic organizer Elements compounds and mixtures oh my
Elements compounds and mixtures oh my Plasma h2o brain teaser
Plasma h2o brain teaser Elements compounds and mixtures quiz
Elements compounds and mixtures quiz A compund
A compund Mixtures and solutions quiz
Mixtures and solutions quiz What's the difference between pure substances and mixtures
What's the difference between pure substances and mixtures Design and control of concrete mixtures
Design and control of concrete mixtures How can matter be classified
How can matter be classified Lightweight concrete mix ratio
Lightweight concrete mix ratio Boron
Boron Expansion of solids liquids and gases examples
Expansion of solids liquids and gases examples Buoyancyability
Buoyancyability Venn diagram for solids liquids and gases
Venn diagram for solids liquids and gases 20 examples of liquids
20 examples of liquids Molecular theory of gases and liquids
Molecular theory of gases and liquids Example of solid liquid and gas
Example of solid liquid and gas Lesson outline lesson 1 solids liquids and gases answer key
Lesson outline lesson 1 solids liquids and gases answer key Particle movement in solids liquids and gases
Particle movement in solids liquids and gases Unit 24 cutting with oxyfuels and other gases
Unit 24 cutting with oxyfuels and other gases Solid liquid and gases
Solid liquid and gases How does sound travel through solids liquids and gases
How does sound travel through solids liquids and gases Liquid information
Liquid information