IV REUNIN GIDOGGCP Inmunoterapia en Cncer de Pulmn











- Slides: 61

IV REUNIÓN GIDO-GGCP Inmunoterapia en Cáncer de Pulmón Dr. José Muñoz Langa Servicio Oncología Médica Hospital Universitario y Politécnico La Fe Baiona, 25 Abril 2015)

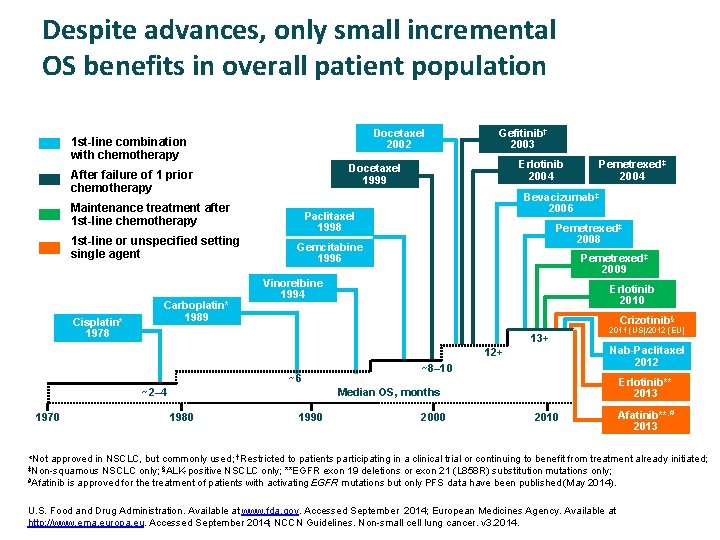
Despite advances, only small incremental OS benefits in overall patient population Docetaxel 2002 1 st-line combination with chemotherapy Maintenance treatment after 1 st-line chemotherapy Cisplatin* 1978 Carboplatin* 1989 Erlotinib 2004 Docetaxel 1999 After failure of 1 prior chemotherapy 1 st-line or unspecified setting single agent Gefitinib† 2003 Bevacizumab‡ 2006 Paclitaxel 1998 Pemetrexed‡ 2008 Gemcitabine 1996 Pemetrexed‡ 2009 Vinorelbine 1994 Erlotinib 2010 Crizotinib§ 13+ ~6 ~2– 4 2011 (US)/2012 (EU) Nab-Paclitaxel 2012 12+ 1970 Pemetrexed‡ 2004 ~8– 10 Erlotinib** 2013 Median OS, months 1980 1990 2000 2010 Afatinib**, # 2013 *Not approved in NSCLC, but commonly used; †Restricted to patients participating in a clinical trial or continuing to benefit from treatment already initiated; ‡Non-squamous #Afatinib NSCLC only; §ALK-positive NSCLC only; **EGFR exon 19 deletions or exon 21 (L 858 R) substitution mutations only; is approved for the treatment of patients with activating EGFR mutations but only PFS data have been published (May 2014). U. S. Food and Drug Administration. Available at www. fda. gov. Accessed September 2014; European Medicines Agency. Available at http: //www. ema. europa. eu. Accessed September 2014; NCCN Guidelines. Non-small cell lung cancer. v 3. 2014.

Immuno-Oncology (I-O): can it address unmet needs in lung cancer? “Immunotherapy represents a new hope for NSCLC patients”, Cancer patient “In any trial you get the odd patient who does very well, but this is an order of magnitude above that. ”, Mick Peake, Glenfield Hospital “The PD-1 antibodies stop lung cancer cells from blocking the body's natural immune response to cancer. A drug that can inhibit PD-1 may be able to treat a variety of cancers, which is very exciting”, Myron Bednar, Hunterdon Regional Cancer Center “The high level of excitement around this space is that immune checkpoint inhibitors may apply to a very broad range of cancer types, as both monotherapies and combination therapies. The data we are seeing so far, including recently released at ASCO, also indicates the mechanism works in patients who have failed previous cancer therapies. ”, Stephen Dunn, Life. Tech Capital “We look forward to the potential for the combination of checkpoint inhibitors, like nivolumab, with small molecule inhibitors in patients with EGFR mutated lung cancer”, Naiyer Rizvi, Memorial Sloan Kettering Cancer Center “The field of immunotherapy has exploded in the last decade, and more patients are benefiting”, Steven O'Day, USC

Objetivos de la Charla: Ø Revisar los fundamentos científicos de la inmuno-oncología en el cáncer de pulmón y en qué se diferencia de otras modalidades de tratamiento. Ø Discutir los últimos avances en inmunoterapia para el tratamiento del cáncer de pulmón. Ø Analizar el problema de la identificación de biomarcadores para las terapias inumono‐oncológicas y su potencial valor predictivo. Ø Valorar el potencial de la inmunoterapia sola o en combinación con otras modalidades de tratamiento en la práctica clínica.

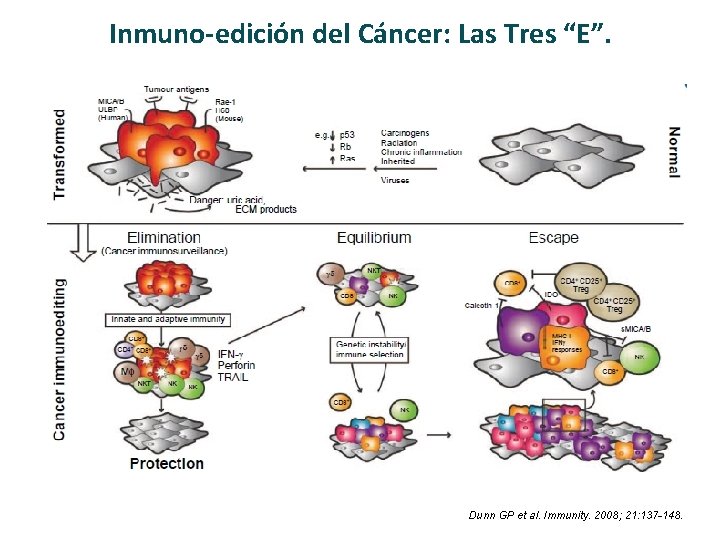
Inmuno-edición del Cáncer: Las Tres “E”. Dunn GP et al. Immunity. 2008; 21: 137 -148.

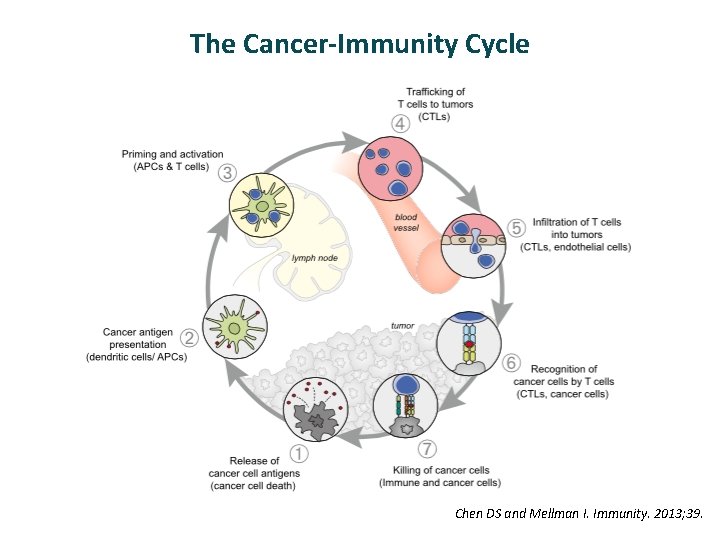
The Cancer-Immunity Cycle Chen DS and Mellman I. Immunity. 2013; 39.

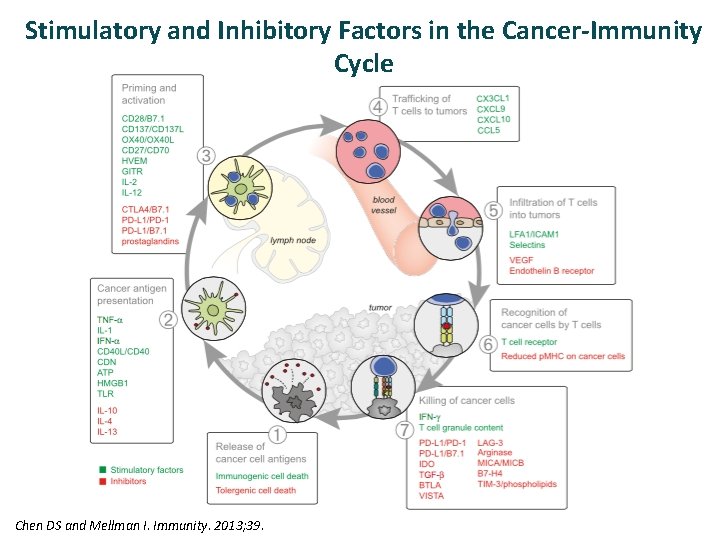
Stimulatory and Inhibitory Factors in the Cancer-Immunity Cycle Chen DS and Mellman I. Immunity. 2013; 39.

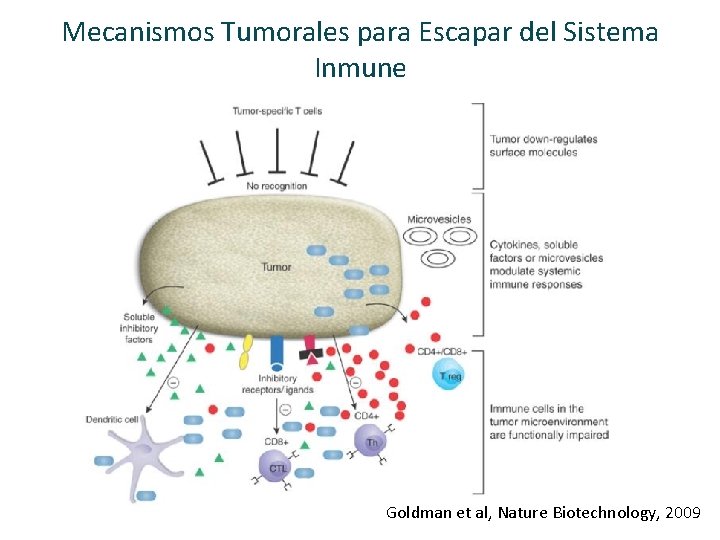
Mecanismos Tumorales para Escapar del Sistema Inmune Goldman et al, Nature Biotechnology, 2009

Mecanismos Tumorales para Escapar del Sistema Inmune

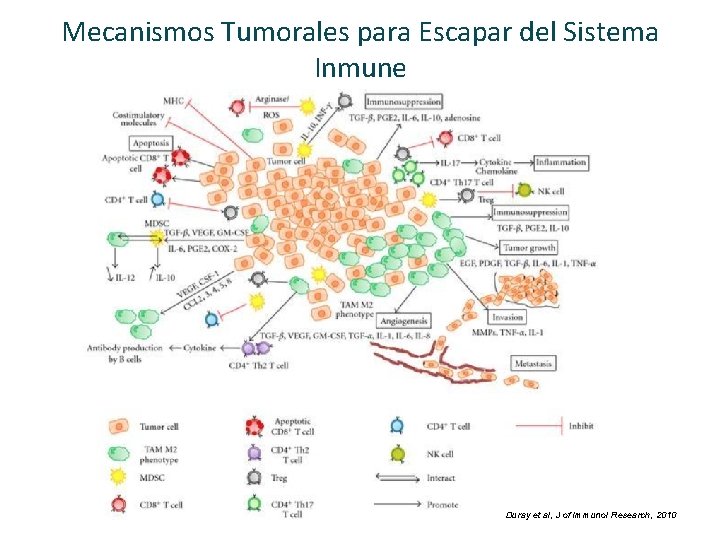
Mecanismos Tumorales para Escapar del Sistema Inmune Duray et al, J of Immunol Research, 2010

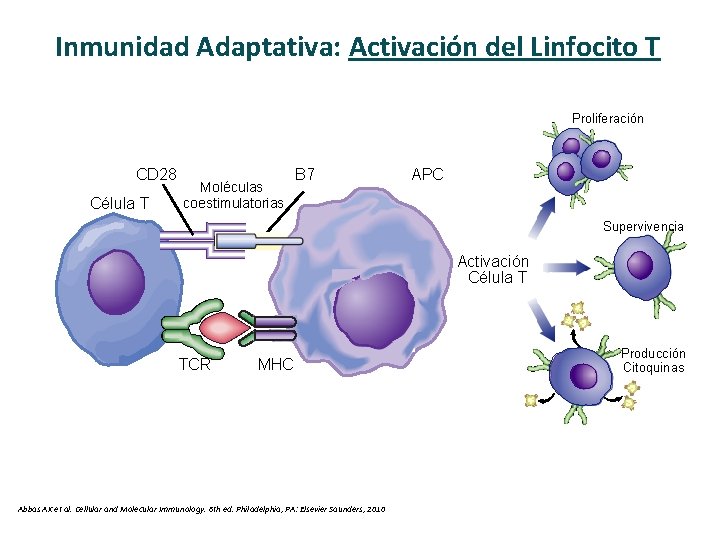
Inmunidad Adaptativa: Activación del Linfocito T Proliferación CD 28 Célula T Moléculas coestimulatorias B 7 APC Supervivencia Activación Célula T TCR MHC Abbas AK et al. Cellular and Molecular Immunology. 6 th ed. Philadelphia, PA: Elsevier Saunders, 2010 Producción Citoquinas

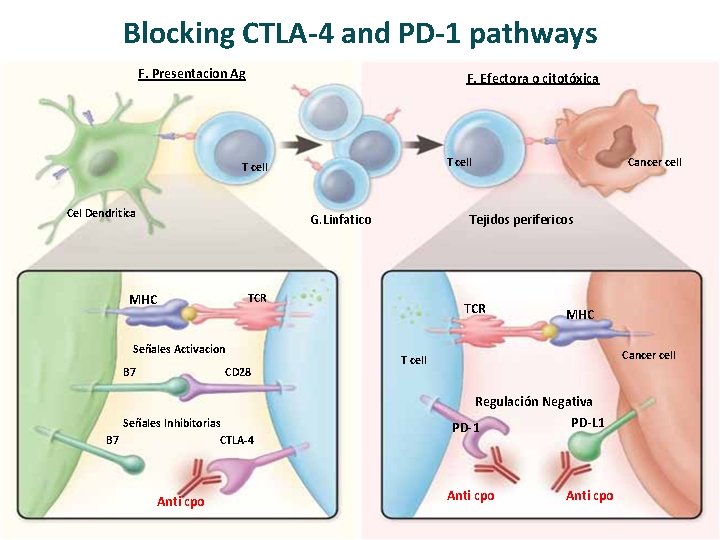
Blocking CTLA-4 and PD-1 pathways F. Presentacion Ag F. Efectora o citotóxica T cell Cel Dendritica G. Linfatico MHC B 7 CD 28 Señales Inhibitorias B 7 CTLA-4 Anti cpo Tejidos perifericos TCR Señales Activacion Cancer cell TCR MHC Cancer cell T cell Regulación Negativa PD-L 1 PD-1 Anti cpo

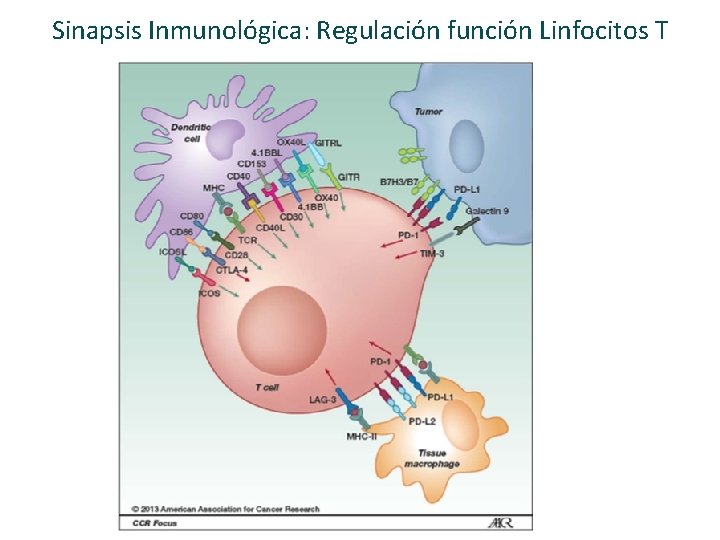
Sinapsis Inmunológica: Regulación función Linfocitos T

Sinapsis Inmunológica: Regulación función Linfocitos T Nirschl CJ Clin Cancer Res 2013

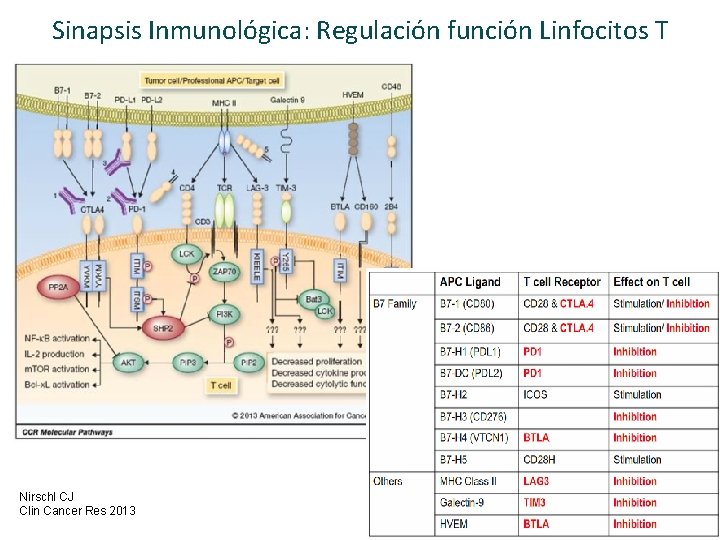
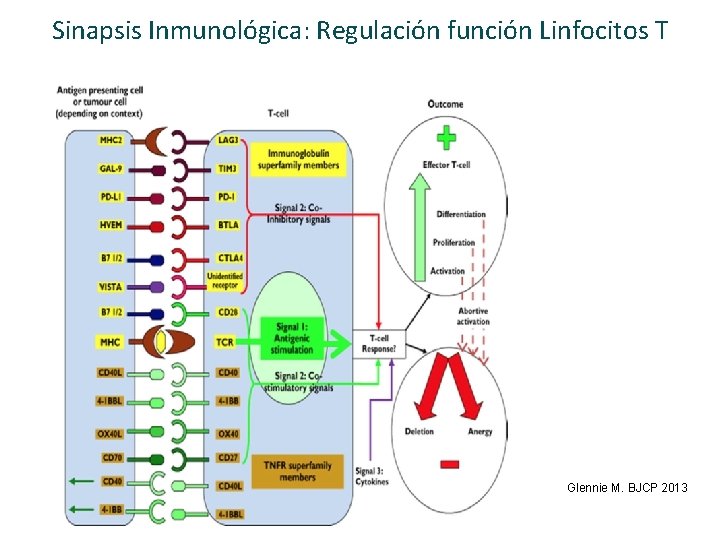
Sinapsis Inmunológica: Regulación función Linfocitos T Glennie M. BJCP 2013

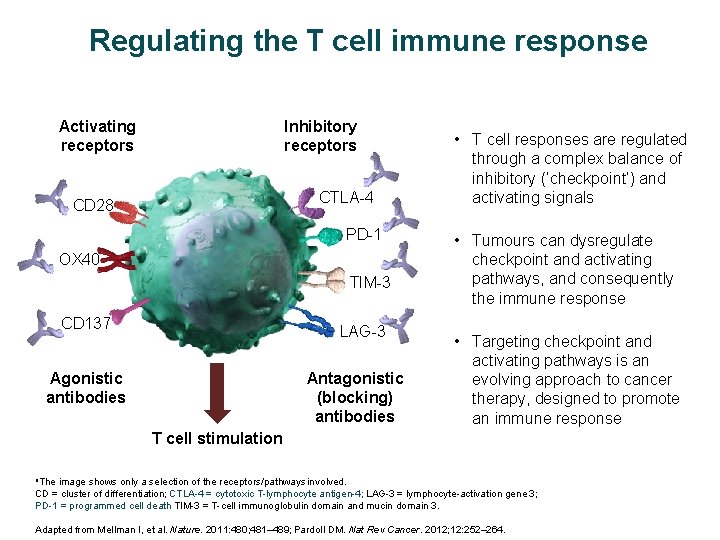
Regulating the T cell immune response Activating receptors Inhibitory receptors CTLA-4 CD 28 PD-1 OX 40 TIM-3 CD 137 LAG-3 Agonistic antibodies Antagonistic (blocking) antibodies • T cell responses are regulated through a complex balance of inhibitory (‘checkpoint’) and activating signals • Tumours can dysregulate checkpoint and activating pathways, and consequently the immune response • Targeting checkpoint and activating pathways is an evolving approach to cancer therapy, designed to promote an immune response T cell stimulation a. The image shows only a selection of the receptors/pathways involved. CD = cluster of differentiation; CTLA-4 = cytotoxic T-lymphocyte antigen-4; LAG-3 = lymphocyte-activation gene 3; PD-1 = programmed cell death TIM-3 = T-cell immunoglobulin domain and mucin domain 3. Adapted from Mellman I, et al. Nature. 2011: 480; 481– 489; Pardoll DM. Nat Rev Cancer. 2012; 12: 252– 264.

Objetivos de la Charla: Ø Revisar los fundamentos científicos de la inmuno‐oncología en el cáncer de pulmón y en qué se diferencia de otras modalidades de tratamiento. Ø Discutir los últimos avances en inmunoterapia para el tratamiento del cáncer de pulmón. Ø Analizar el problema de la identificación de biomarcadores para las terapias inumono‐oncológicas y su potencial valor predictivo. Ø Valorar el potencial de la inmunoterapia sola o en combinación con otras modalidades de tratamiento en la práctica clínica.

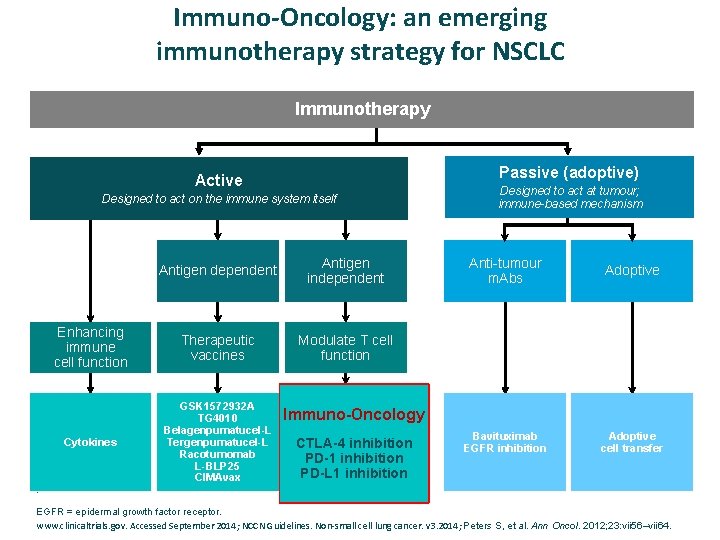
Immuno-Oncology: an emerging immunotherapy strategy for NSCLC Immunotherapy Passive (adoptive) Active Designed to act on the immune system itself Antigen dependent Antigen independent Enhancing immune cell function Therapeutic vaccines Modulate T cell function Cytokines GSK 1572932 A TG 4010 Belagenpumatucel-L Tergenpumatucel-L Racotumomab L-BLP 25 CIMAvax Designed to act at tumour; immune-based mechanism Anti-tumour m. Abs Adoptive Bavituximab EGFR inhibition Adoptive cell transfer Immuno-Oncology CTLA-4 inhibition PD-1 inhibition PD-L 1 inhibition . EGFR = epidermal growth factor receptor. www. clinicaltrials. gov. Accessed September 2014; NCCN Guidelines. Non‐small cell lung cancer. v 3. 2014; Peters S, et al. Ann Oncol. 2012; 23: vii 56–vii 64.

Blocking CTL 4 and PD-1/PD-L 1 Patway Un agente único es capaz de proporcionar beneficio clínico en múltiples líneas de tratamiento y con largos supervivientes.

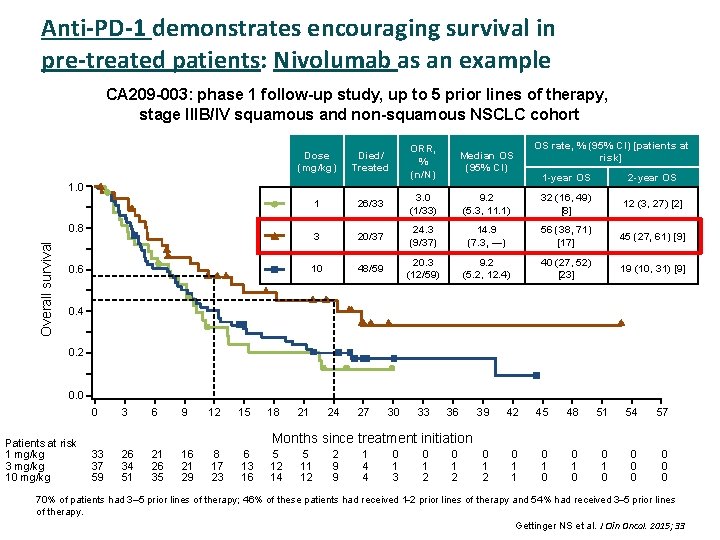
Anti-PD-1 demonstrates encouraging survival in pre-treated patients: Nivolumab as an example CA 209 -003: phase 1 follow-up study, up to 5 prior lines of therapy, stage IIIB/IV squamous and non-squamous NSCLC cohort Dose (mg/kg) Died/ Treated ORR, % (n/N) Median OS (95% CI) 1 26/33 3. 0 (1/33) 3 20/37 10 48/59 1. 0 Overall survival 0. 8 0. 6 OS rate, % (95% CI) [patients at risk] 1 -year OS 2 -year OS 9. 2 (5. 3, 11. 1) 32 (16, 49) [8] 12 (3, 27) [2] 24. 3 (9/37) 14. 9 (7. 3, —) 56 (38, 71) [17] 45 (27, 61) [9] 20. 3 (12/59) 9. 2 (5. 2, 12. 4) 40 (27, 52) [23] 19 (10, 31) [9] 0. 4 0. 2 0. 0 0 Patients at risk 1 mg/kg 3 mg/kg 10 mg/kg 3 6 9 12 15 18 21 24 27 30 33 36 39 42 45 48 51 54 57 0 1 2 0 1 1 0 0 0 0 Months since treatment initiation 33 37 59 26 34 51 21 26 35 16 21 29 8 17 23 6 13 16 5 12 14 5 11 12 2 9 9 1 4 4 0 1 3 0 1 2 70% of patients had 3– 5 prior lines of therapy; 46% of these patients had received 1 2 prior lines of therapy and 54% had received 3 5 prior lines of therapy. Gettinger NS et al. J Clin Oncol. 2015; 33

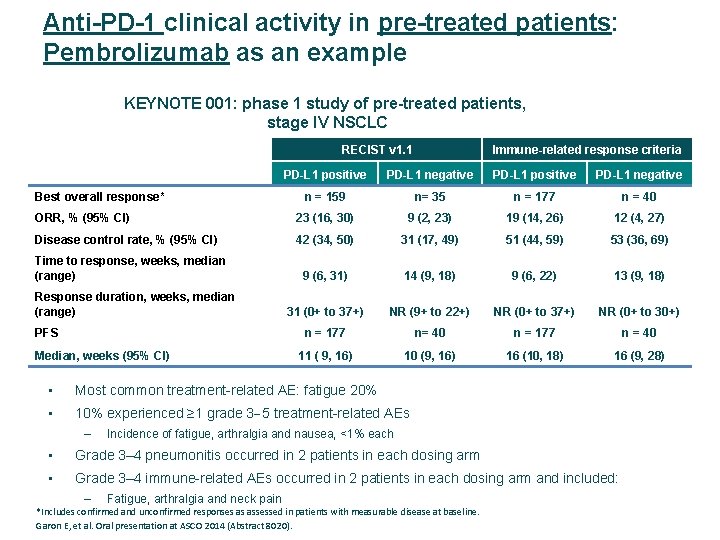
Anti-PD-1 clinical activity in pre-treated patients: Pembrolizumab as an example KEYNOTE 001: phase 1 study of pre-treated patients, stage IV NSCLC RECIST v 1. 1 Immune-related response criteria PD-L 1 positive PD-L 1 negative n = 159 n= 35 n = 177 n = 40 ORR, % (95% CI) 23 (16, 30) 9 (2, 23) 19 (14, 26) 12 (4, 27) Disease control rate, % (95% CI) 42 (34, 50) 31 (17, 49) 51 (44, 59) 53 (36, 69) 9 (6, 31) 14 (9, 18) 9 (6, 22) 13 (9, 18) 31 (0+ to 37+) NR (9+ to 22+) NR (0+ to 37+) NR (0+ to 30+) n = 177 n= 40 n = 177 n = 40 11 ( 9, 16) 10 (9, 16) 16 (10, 18) 16 (9, 28) Best overall response* Time to response, weeks, median (range) Response duration, weeks, median (range) PFS Median, weeks (95% CI) • Most common treatment-related AE: fatigue 20% • 10% experienced ≥ 1 grade 3 5 treatment-related AEs – Incidence of fatigue, arthralgia and nausea, <1% each • Grade 3– 4 pneumonitis occurred in 2 patients in each dosing arm • Grade 3– 4 immune-related AEs occurred in 2 patients in each dosing arm and included: – Fatigue, arthralgia and neck pain *Includes confirmed and unconfirmed responses as assessed in patients with measurable disease at baseline. Garon E, et al. Oral presentation at ASCO 2014 (Abstract 8020).

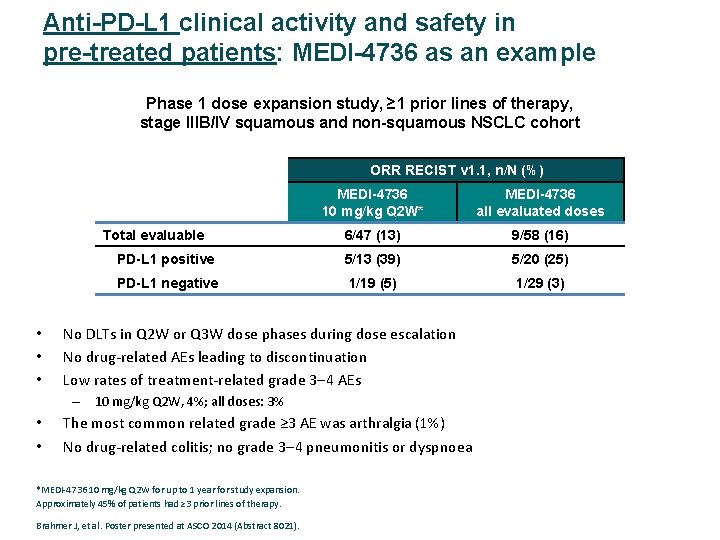
Anti-PD-L 1 clinical activity and safety in pre-treated patients: MEDI-4736 as an example Phase 1 dose expansion study, ≥ 1 prior lines of therapy, stage IIIB/IV squamous and non-squamous NSCLC cohort ORR RECIST v 1. 1, n/N (%) MEDI-4736 10 mg/kg Q 2 W* MEDI-4736 all evaluated doses 6/47 (13) 9/58 (16) PD-L 1 positive 5/13 (39) 5/20 (25) PD-L 1 negative 1/19 (5) 1/29 (3) Total evaluable • • • No DLTs in Q 2 W or Q 3 W dose phases during dose escalation No drug‐related AEs leading to discontinuation Low rates of treatment‐related grade 3 4 AEs – 10 mg/kg Q 2 W, 4%; all doses: 3% • • The most common related grade ≥ 3 AE was arthralgia (1%) No drug‐related colitis; no grade 3 4 pneumonitis or dyspnoea *MEDI‐ 4736 10 mg/kg Q 2 W for up to 1 year for study expansion. Approximately 45% of patients had ≥ 3 prior lines of therapy. Brahmer J, et al. Poster presented at ASCO 2014 (Abstract 8021).

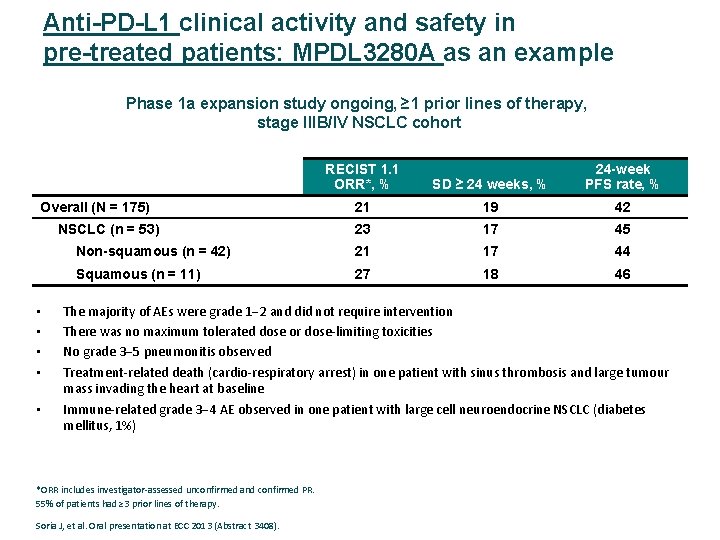
Anti-PD-L 1 clinical activity and safety in pre-treated patients: MPDL 3280 A as an example Phase 1 a expansion study ongoing, ≥ 1 prior lines of therapy, stage IIIB/IV NSCLC cohort RECIST 1. 1 ORR*, % SD ≥ 24 weeks, % 24 -week PFS rate, % 21 19 42 23 17 45 Non-squamous (n = 42) 21 17 44 Squamous (n = 11) 27 18 46 Overall (N = 175) NSCLC (n = 53) • • • The majority of AEs were grade 1 2 and did not require intervention There was no maximum tolerated dose or dose‐limiting toxicities No grade 3 5 pneumonitis observed Treatment‐related death (cardio‐respiratory arrest) in one patient with sinus thrombosis and large tumour mass invading the heart at baseline Immune‐related grade 3 4 AE observed in one patient with large cell neuroendocrine NSCLC (diabetes mellitus, 1%) *ORR includes investigator‐assessed unconfirmed and confirmed PR. 55% of patients had ≥ 3 prior lines of therapy. Soria J, et al. Oral presentation at ECC 2013 (Abstract 3408).

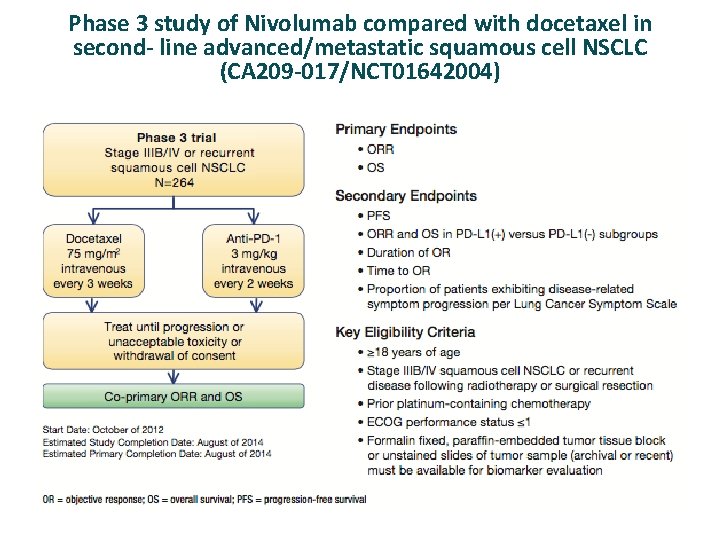
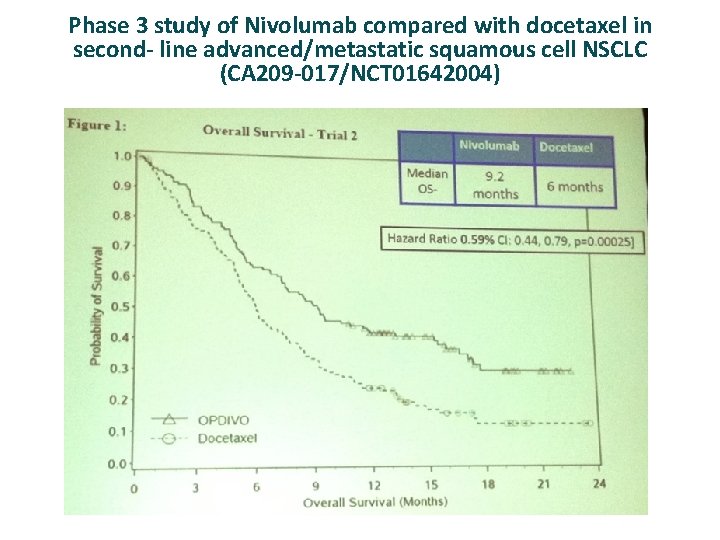
Phase 3 study of Nivolumab compared with docetaxel in second- line advanced/metastatic squamous cell NSCLC (CA 209 -017/NCT 01642004)

Phase 3 study of Nivolumab compared with docetaxel in second- line advanced/metastatic squamous cell NSCLC (CA 209 -017/NCT 01642004)


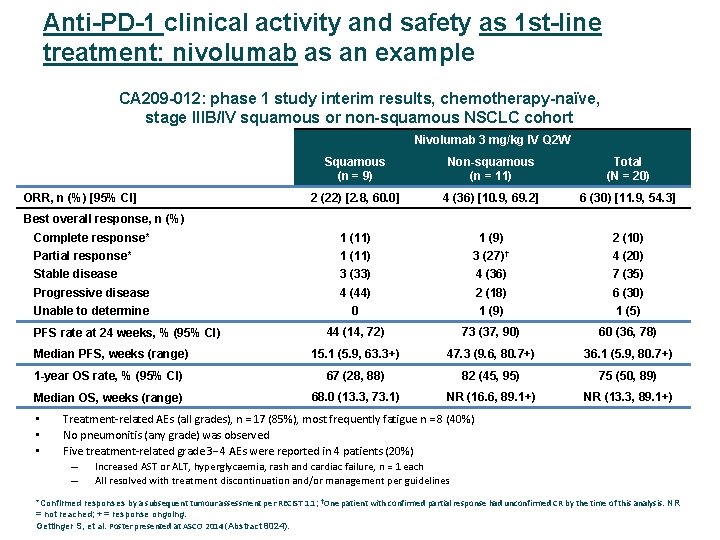
Anti-PD-1 clinical activity and safety as 1 st-line treatment: nivolumab as an example CA 209 -012: phase 1 study interim results, chemotherapy-naïve, stage IIIB/IV squamous or non-squamous NSCLC cohort Nivolumab 3 mg/kg IV Q 2 W Squamous (n = 9) Non-squamous (n = 11) Total (N = 20) 2 (22) [2. 8, 60. 0] 4 (36) [10. 9, 69. 2] 6 (30) [11. 9, 54. 3] Complete response* 1 (11) 1 (9) 2 (10) Partial response* 1 (11) 3 (27)† 4 (20) Stable disease 3 (33) 4 (36) 7 (35) Progressive disease 4 (44) 2 (18) 6 (30) Unable to determine 0 1 (9) 1 (5) 44 (14, 72) 73 (37, 90) 60 (36, 78) Median PFS, weeks (range) 15. 1 (5. 9, 63. 3+) 47. 3 (9. 6, 80. 7+) 36. 1 (5. 9, 80. 7+) 1 -year OS rate, % (95% CI) 67 (28, 88) 82 (45, 95) 75 (50, 89) Median OS, weeks (range) 68. 0 (13. 3, 73. 1) NR (16. 6, 89. 1+) NR (13. 3, 89. 1+) ORR, n (%) [95% CI] Best overall response, n (%) PFS rate at 24 weeks, % (95% CI) • • • Treatment‐related AEs (all grades), n = 17 (85%), most frequently fatigue n = 8 (40%) No pneumonitis (any grade) was observed Five treatment‐related grade 3 4 AEs were reported in 4 patients (20%) – Increased AST or ALT, hyperglycaemia, rash and cardiac failure, n = 1 each – All resolved with treatment discontinuation and/or management per guidelines *Confirmed responses by a subsequent tumour assessment per RECIST 1. 1; †One patient with confirmed partial response had unconfirmed CR by the time of this analysis. NR = not reached; + = response ongoing. Gettinger S, et al. Poster presented at ASCO 2014 (Abstract 8024).

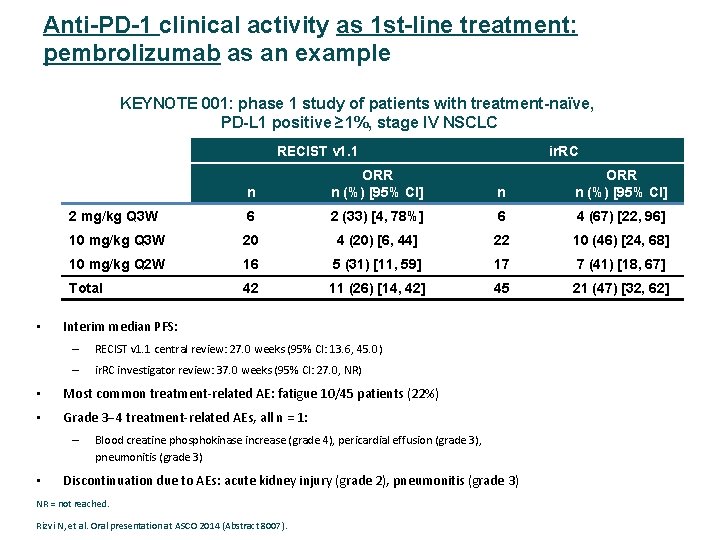
Anti-PD-1 clinical activity as 1 st-line treatment: pembrolizumab as an example KEYNOTE 001: phase 1 study of patients with treatment-naïve, PD-L 1 positive ≥ 1%, stage IV NSCLC RECIST v 1. 1 • n ORR n (%) [95% CI] 2 mg/kg Q 3 W 6 2 (33) [4, 78%] 6 4 (67) [22, 96] 10 mg/kg Q 3 W 20 4 (20) [6, 44] 22 10 (46) [24, 68] 10 mg/kg Q 2 W 16 5 (31) [11, 59] 17 7 (41) [18, 67] Total 42 11 (26) [14, 42] 45 21 (47) [32, 62] Interim median PFS: – RECIST v 1. 1 central review: 27. 0 weeks (95% CI: 13. 6, 45. 0) – ir. RC investigator review: 37. 0 weeks (95% CI: 27. 0, NR) • Most common treatment‐related AE: fatigue 10/45 patients (22%) • Grade 3 4 treatment‐related AEs, all n = 1: – • ir. RC Blood creatine phosphokinase increase (grade 4), pericardial effusion (grade 3), pneumonitis (grade 3) Discontinuation due to AEs: acute kidney injury (grade 2), pneumonitis (grade 3) NR = not reached. Rizvi N, et al. Oral presentation at ASCO 2014 (Abstract 8007).

Blocking CTL 4 and PD-1/PD-L 1 Patway Un agente único es capaz de proporcionar beneficio clínico en múltiples líneas de tratamiento y con largos supervivientes. Toxicidad clase específica con un perfil de seguridad manejable

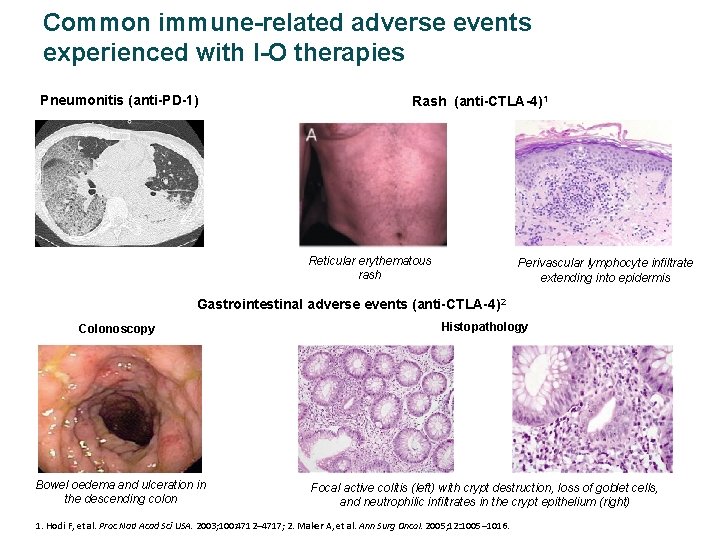
Common immune-related adverse events experienced with I-O therapies Pneumonitis (anti-PD-1) Rash (anti-CTLA-4)1 Reticular erythematous rash Perivascular lymphocyte infiltrate extending into epidermis Gastrointestinal adverse events (anti-CTLA-4)2 Colonoscopy Histopathology Bowel oedema and ulceration in the descending colon Focal active colitis (left) with crypt destruction, loss of goblet cells, and neutrophilic infiltrates in the crypt epithelium (right) 1. Hodi F, et al. Proc Natl Acad Sci USA. 2003; 100: 4712– 4717; 2. Maker A, et al. Ann Surg Oncol. 2005; 12: 1005– 1016.

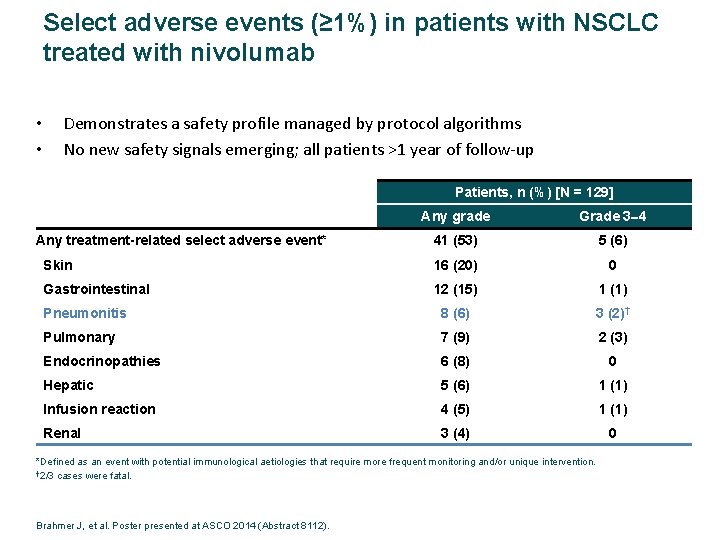
Select adverse events (≥ 1%) in patients with NSCLC treated with nivolumab • • Demonstrates a safety profile managed by protocol algorithms No new safety signals emerging; all patients >1 year of follow‐up Patients, n (%) [N = 129] Any grade Grade 3 4 41 (53) 5 (6) Skin 16 (20) 0 Gastrointestinal 12 (15) 1 (1) Pneumonitis 8 (6) 3 (2)† Pulmonary 7 (9) 2 (3) Endocrinopathies 6 (8) 0 Hepatic 5 (6) 1 (1) Infusion reaction 4 (5) 1 (1) Renal 3 (4) 0 Any treatment-related select adverse event* *Defined as an event with potential immunological aetiologies that require more frequent monitoring and/or unique intervention. † 2/3 cases were fatal. Brahmer J, et al. Poster presented at ASCO 2014 (Abstract 8112).

Inhibition of CTL 4 and PD-1/PD-L 1 Patway Un agente único es capaz de proporcionar beneficio clínico en múltiples líneas de tratamiento y con largos supervivientes. Toxicidad clase específica con un perfil de seguridad manejable Respuestas rápidas y duraderas independientes del tipo histológico y de la presencia mutaciones.

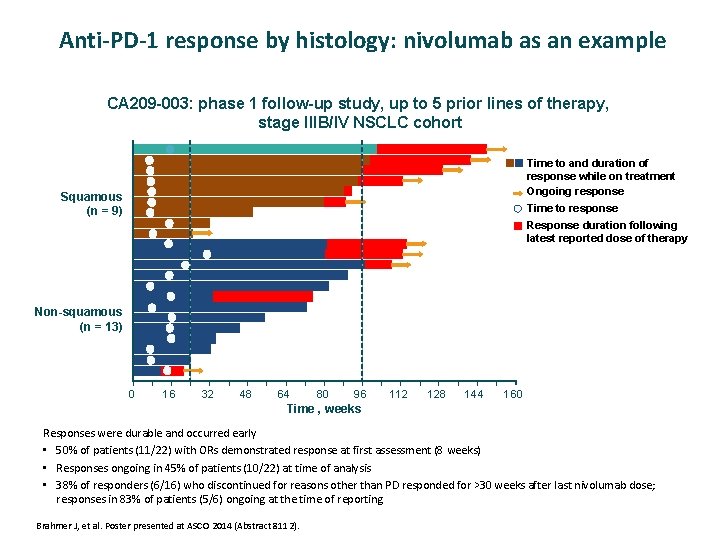
Anti-PD-1 response by histology: nivolumab as an example CA 209 -003: phase 1 follow-up study, up to 5 prior lines of therapy, stage IIIB/IV NSCLC cohort Time to and duration of response while on treatment Ongoing response Squamous (n = 9) Time to response Response duration following latest reported dose of therapy Non-squamous (n = 13) 0 16 32 48 64 80 96 112 128 144 160 Time , weeks Responses were durable and occurred early • 50% of patients (11/22) with ORs demonstrated response at first assessment (8 weeks) • Responses ongoing in 45% of patients (10/22) at time of analysis • 38% of responders (6/16) who discontinued for reasons other than PD responded for >30 weeks after last nivolumab dose; responses in 83% of patients (5/6) ongoing at the time of reporting Brahmer J, et al. Poster presented at ASCO 2014 (Abstract 8112).

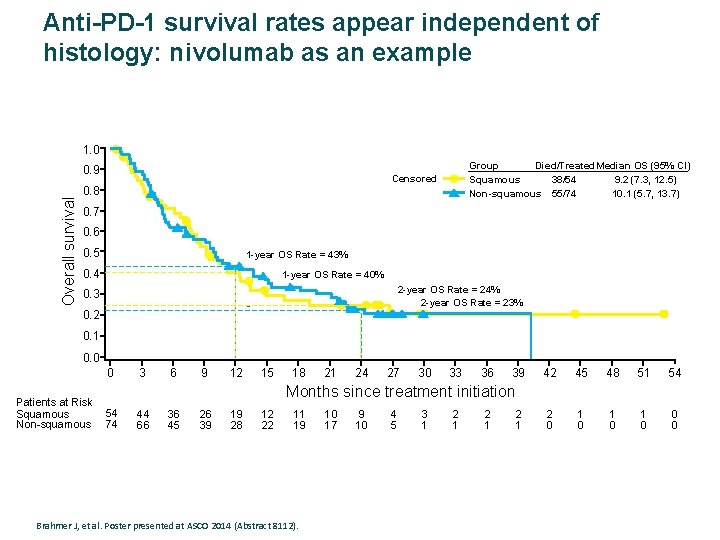
Anti-PD-1 survival rates appear independent of histology: nivolumab as an example 1. 0 Overall survival 0. 9 Group Died/Treated. Median OS (95% CI) Squamous 38/54 9. 2 (7. 3, 12. 5) Non-squamous 55/74 10. 1 (5. 7, 13. 7) Censored 0. 8 0. 7 0. 6 0. 5 1 -year OS Rate = 43% 0. 4 1 -year OS Rate = 40% 2 -year OS Rate = 24% 2 -year OS Rate = 23% 0. 3 0. 2 0. 1 0. 0 0 Patients at Risk Squamous Non-squamous 3 6 9 12 15 18 21 24 27 30 33 36 39 42 45 48 51 54 2 0 1 0 1 0 0 0 Months since treatment initiation 54 74 44 66 36 45 26 39 19 28 12 22 11 19 Brahmer J, et al. Poster presented at ASCO 2014 (Abstract 8112). 10 17 9 10 4 5 3 1 2 1 2 1

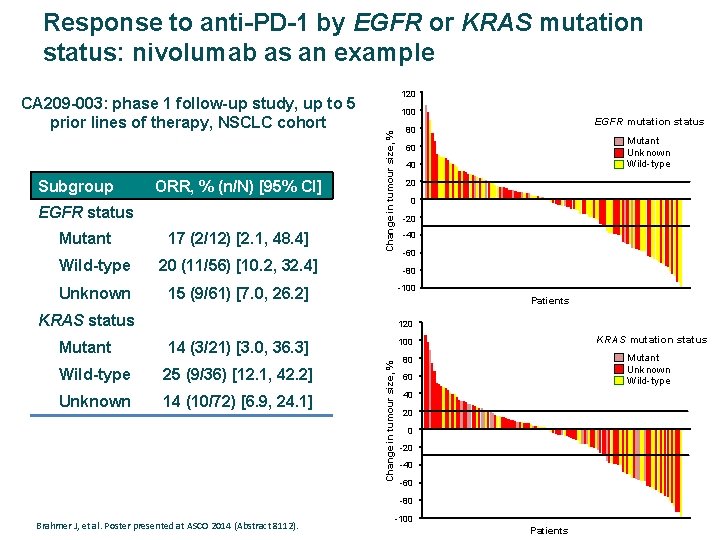
Response to anti-PD-1 by EGFR or KRAS mutation status: nivolumab as an example Subgroup ORR, % (n/N) [95% CI] EGFR status Mutant 17 (2/12) [2. 1, 48. 4] Wild-type 20 (11/56) [10. 2, 32. 4] Unknown 15 (9/61) [7. 0, 26. 2] 120 100 EGFR mutation status 80 Change in tumour size, % CA 209 -003: phase 1 follow-up study, up to 5 prior lines of therapy, NSCLC cohort Mutant Unknown Wild-type 60 40 20 0 -20 -40 -60 -80 -100 Patients KRAS status 120 14 (3/21) [3. 0, 36. 3] Wild-type 25 (9/36) [12. 1, 42. 2] Unknown 14 (10/72) [6. 9, 24. 1] KRAS mutation status 100 Change in tumour size, % Mutant Unknown Wild-type 80 60 40 20 0 -20 -40 -60 -80 Brahmer J, et al. Poster presented at ASCO 2014 (Abstract 8112). -100 Patients

Objetivos de la Charla: Ø Revisar los fundamentos científicos de la inmuno‐oncología en el cáncer de pulmón y en qué se diferencia de otras modalidades de tratamiento. Ø Discutir los últimos avances en inmunoterapia para el tratamiento del cáncer de pulmón. Ø Analizar el problema de la identificación de biomarcadores para las terapias inumono-oncológicas y su potencial valor predictivo. Ø Valorar el potencial de la inmunoterapia sola o en combinación con otras modalidades de tratamiento en la práctica clínica.

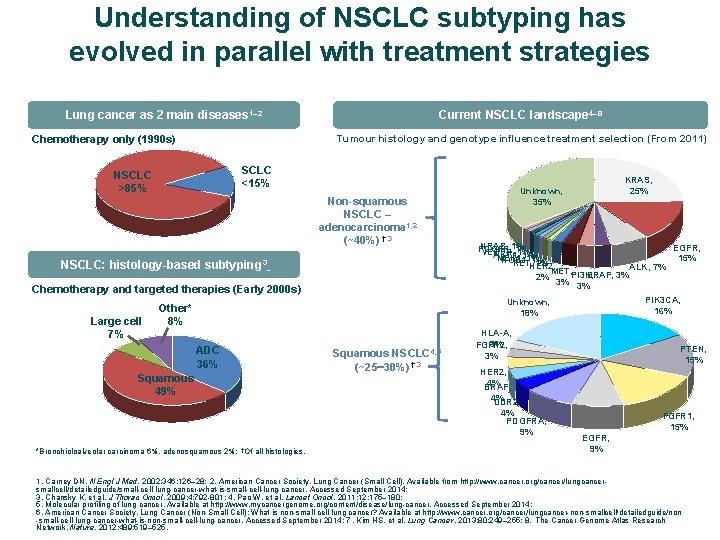
Understanding of NSCLC subtyping has evolved in parallel with treatment strategies Current NSCLC landscape 4– 8 Lung cancer as 2 main diseases 1– 2 Tumour histology and genotype influence treatment selection (From 2011) Chemotherapy only (1990 s) SCLC <15% NSCLC >85% Non-squamous NSCLC – adenocarcinoma 1, 2 (~40%) †, 3 NSCLC: histology-based subtyping 3 Chemotherapy and targeted therapies (Early 2000 s) Large cell 7% KRAS, 25% Unknown, 35% NRAS, 1% EGFR, PDGFR, 1% VEGFR, 1% AKT 1, 1% MEK 1, 1% 15% ROS 1, 1% RET, HER 2, 1% ALK, 7% MET, PI 3 K, BRAF, 3% 2% 3% 3% PIK 3 CA, 16% Unknown, 18% Other* 8% ADC 36% Squamous 49% *Bronchioloalveolar carcinoma 6%, adenosquamous 2%; †Of all histologies. Squamous NSCLC 4, 5 (~25 30%) †, 3 HLA-A, 3% FGFR 2, 3% † HER 2, 4% BRAF, 4% DDR 2, 4% PDGFRA, 9% PTEN, 15% FGFR 1, 15% EGFR, 9% 1. Carney DN. N Engl J Med. 2002; 346: 126– 28; 2. American Cancer Society. Lung Cancer (Small Cell). Available from http: //www. cancer. org/cancer/lungcancersmallcell/detailedguide/small-cell-lung-cancer-what-is-small-cell-lung-cancer. Accessed September 2014; 3. Chansky K, et al. J Thorac Oncol. 2009; 4: 792 -801; 4. Pao W, et al. Lancet Oncol. 2011; 12: 175– 180; 5. Molecular profiling of lung cancer. Available at http: //www. mycancergenome. org/content/disease/lung-cancer. Accessed September 2014; 6. American Cancer Society. Lung Cancer (Non-Small Cell); What is non-small cell lung cancer? Available at http: //www. cancer. org/cancer/lungcancer-non-smallcell/detailedguide/non -small-cell-lung-cancer-what-is-non-small-cell-lung-cancer. Accessed September 2014; 7. Kim HS, et al. Lung Cancer. 2013; 80: 249– 255; 8. The Cancer Genome Atlas Research Network. Nature. 2012; 489: 519– 525.

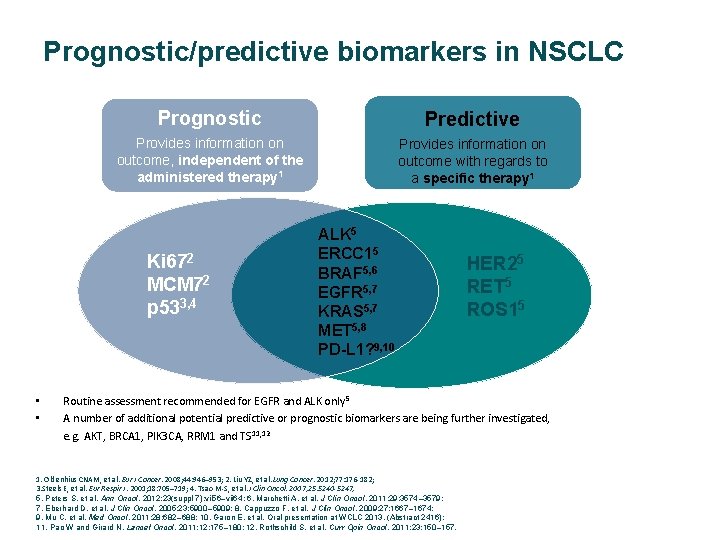
Prognostic/predictive biomarkers in NSCLC Prognostic Predictive Provides information on outcome, independent of the administered therapy 1 Provides information on outcome with regards to a specific therapy 1 Ki 672 MCM 72 p 533, 4 • • ALK 5 ERCC 15 BRAF 5, 6 EGFR 5, 7 KRAS 5, 7 MET 5, 8 PD-L 1? 9, 10 HER 25 RET 5 ROS 15 Routine assessment recommended for EGFR and ALK only 5 A number of additional potential predictive or prognostic biomarkers are being further investigated, e. g. AKT, BRCA 1, PIK 3 CA, RRM 1 and TS 11, 12 1. Oldenhius CNAM, et al. Eur J Cancer. 2008; 44: 946– 953; 2. Liu YZ, et al. Lung Cancer. 2012; 77: 176‐ 182; 3. Steels E, et al. Eur Respir J. 2001; 18: 705– 719; 4. Tsao M‐S, et al. J Clin Oncol. 2007; 25: 5240 -5247; 5. Peters S, et al. Ann Oncol. 2012; 23(suppl 7): vii 56–vii 64; 6. Marchetti A, et al. J Clin Oncol. 2011; 29: 3574– 3579; 7. Eberhard D, et al. J Clin Oncol. 2005; 23: 5900– 5909; 8. Cappuzzo F, et al. J Clin Oncol. 2009; 27: 1667– 1674; 9. Mu C, et al. Med Oncol. 2011; 28: 682– 688; 10. Garon E, et al. Oral presentation at WCLC 2013. (Abstract 2416); 11. Pao W and Girard N. Lancet Oncol. 2011; 12: 175– 180; 12. Rothschild S, et al. Curr Opin Oncol. 2011; 23: 150– 157.

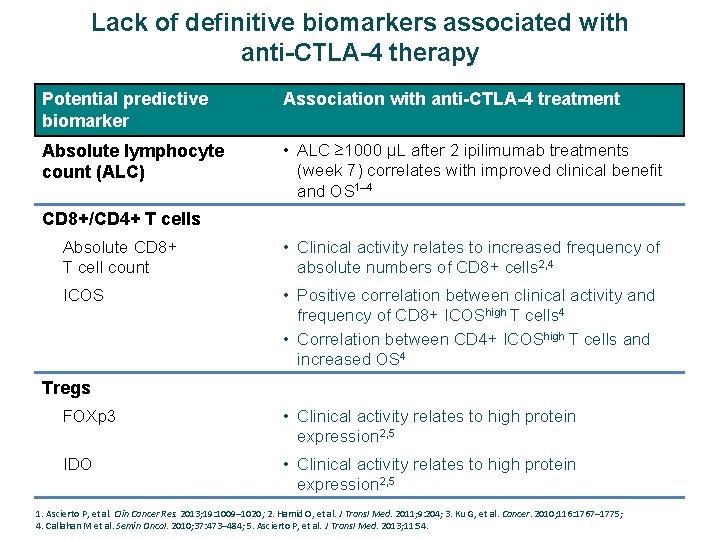
Lack of definitive biomarkers associated with anti-CTLA-4 therapy Potential predictive biomarker Association with anti-CTLA-4 treatment Absolute lymphocyte count (ALC) • ALC ≥ 1000 μL after 2 ipilimumab treatments (week 7) correlates with improved clinical benefit and OS 1– 4 CD 8+/CD 4+ T cells Absolute CD 8+ T cell count • Clinical activity relates to increased frequency of absolute numbers of CD 8+ cells 2, 4 ICOS • Positive correlation between clinical activity and frequency of CD 8+ ICOShigh T cells 4 • Correlation between CD 4+ ICOShigh T cells and increased OS 4 Tregs FOXp 3 • Clinical activity relates to high protein expression 2, 5 IDO • Clinical activity relates to high protein expression 2, 5 1. Ascierto P, et al. Clin Cancer Res. 2013; 19: 1009– 1020; 2. Hamid O, et al. J Transl Med. 2011; 9: 204; 3. Ku G, et al. Cancer. 2010; 116: 1767– 1775; 4. Callahan M et al. Semin Oncol. 2010; 37: 473– 484; 5. Ascierto P, et al. J Transl Med. 2013; 11: 54.

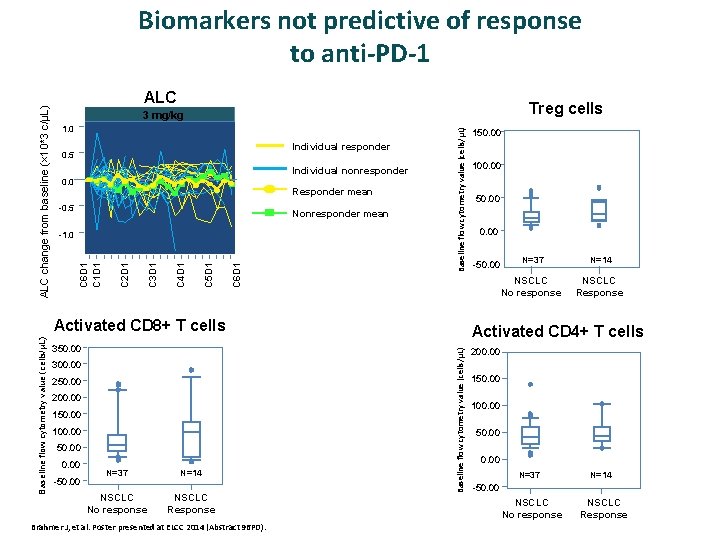
ALC Treg cells 1. 0 Individual responder 0. 5 Individual nonresponder 0. 0 Responder mean -0. 5 Nonresponder mean C 6 D 1 C 5 D 1 C 4 D 1 C 3 D 1 C 2 D 1 -1. 0 Baseline flow cytometry value (cells/μL) 3 mg/kg C 6 D 1 C 1 D 1 ALC change from baseline (× 10*3 c/μL) Biomarkers not predictive of response to anti-PD-1 350. 00 300. 00 250. 00 200. 00 150. 00 100. 00 50. 00 -50. 00 N=37 NSCLC No response N=14 NSCLC Response Brahmer J, et al. Poster presented at ELCC 2014 (Abstract 96 PD). 100. 00 50. 00 -50. 00 N=37 N=14 NSCLC No response NSCLC Response Activated CD 4+ T cells Baseline flow cytometry value (cells/μL) Activated CD 8+ T cells 150. 00 200. 00 150. 00 100. 00 50. 00 N=37 N=14 -50. 00 NSCLC No response NSCLC Response

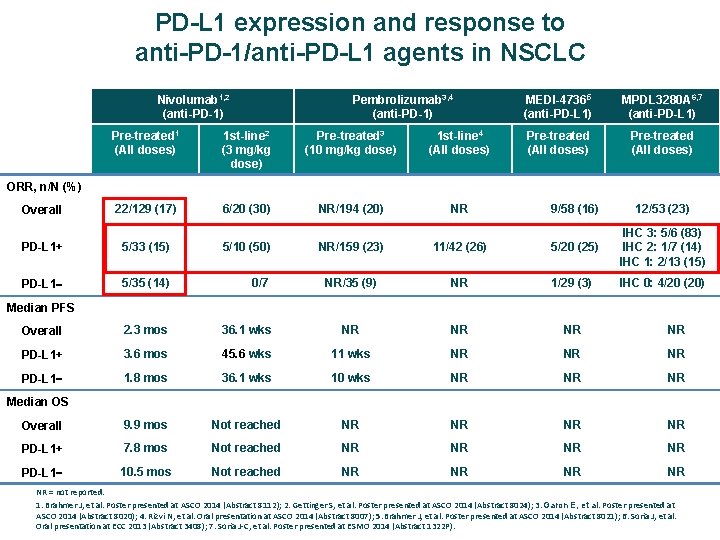
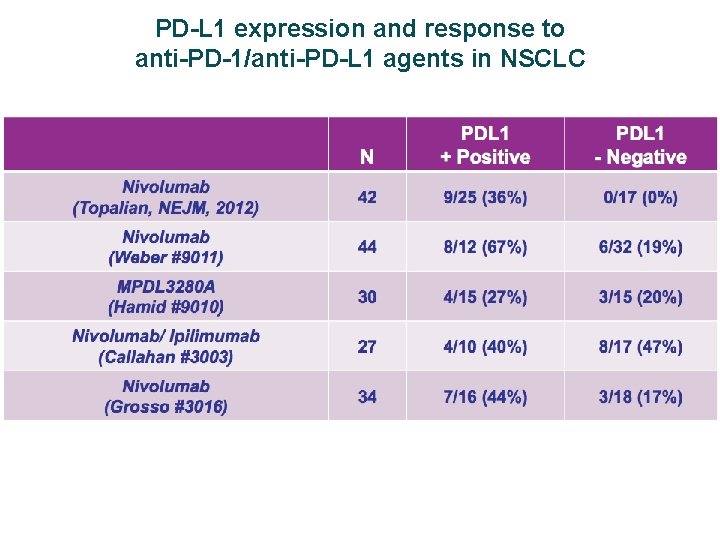
PD-L 1 expression and response to anti-PD-1/anti-PD-L 1 agents in NSCLC Nivolumab 1, 2 (anti-PD-1) Pembrolizumab 3, 4 (anti-PD-1) MEDI-47365 (anti-PD-L 1) MPDL 3280 A 6, 7 (anti-PD-L 1) Pre-treated (All doses) Pre-treated 1 (All doses) 1 st-line 2 (3 mg/kg dose) Pre-treated 3 (10 mg/kg dose) 1 st-line 4 (All doses) Overall 22/129 (17) 6/20 (30) NR/194 (20) NR 9/58 (16) 12/53 (23) PD-L 1+ 5/33 (15) 5/10 (50) NR/159 (23) 11/42 (26) 5/20 (25) IHC 3: 5/6 (83) IHC 2: 1/7 (14) IHC 1: 2/13 (15) PD-L 1 5/35 (14) 0/7 NR/35 (9) NR 1/29 (3) IHC 0: 4/20 (20) Overall 2. 3 mos 36. 1 wks NR NR PD-L 1+ 3. 6 mos 45. 6 wks 11 wks NR NR NR PD-L 1 1. 8 mos 36. 1 wks 10 wks NR NR NR Overall 9. 9 mos Not reached NR NR PD-L 1+ 7. 8 mos Not reached NR NR PD-L 1 10. 5 mos Not reached NR NR ORR, n/N (%) Median PFS Median OS NR = not reported. 1. Brahmer J, et al. Poster presented at ASCO 2014 (Abstract 8112); 2. Gettinger S, et al. Poster presented at ASCO 2014 (Abstract 8024); 3. Garon E, et al. Poster presented at ASCO 2014 (Abstract 8020); 4. Rizvi N, et al. Oral presentation at ASCO 2014 (Abstract 8007); 5. Brahmer J, et al. Poster presented at ASCO 2014 (Abstract 8021); 6. Soria J, et al. Oral presentation at ECC 2013 (Abstract 3408); 7. Soria J‐C, et al. Poster presented at ESMO 2014 (Abstract 1322 P).

PD-L 1 expression and response to anti-PD-1/anti-PD-L 1 agents in NSCLC

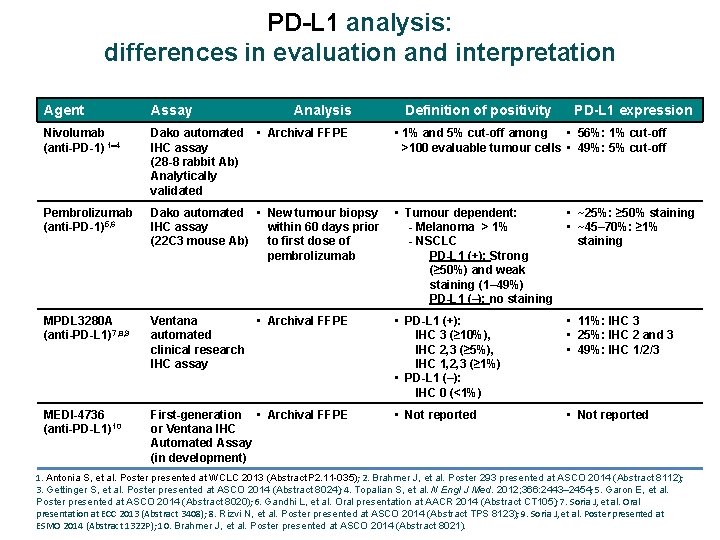
PD-L 1 analysis: differences in evaluation and interpretation Agent Assay Analysis Nivolumab (anti-PD-1) 1 4 Dako automated IHC assay (28 -8 rabbit Ab) Analytically validated Pembrolizumab (anti-PD-1)5, 6 Dako automated • New tumour biopsy IHC assay within 60 days prior (22 C 3 mouse Ab) to first dose of pembrolizumab • Tumour dependent: - Melanoma > 1% - NSCLC PD-L 1 (+): Strong (≥ 50%) and weak staining (1– 49%) PD-L 1 (–): no staining • ~25%: ≥ 50% staining • ~45– 70%: ≥ 1% staining MPDL 3280 A (anti-PD-L 1)7, 8, 9 Ventana • Archival FFPE automated clinical research IHC assay • PD-L 1 (+): IHC 3 (≥ 10%), IHC 2, 3 (≥ 5%), IHC 1, 2, 3 (≥ 1%) • PD-L 1 (–): IHC 0 (<1%) • 11%: IHC 3 • 25%: IHC 2 and 3 • 49%: IHC 1/2/3 MEDI-4736 (anti-PD-L 1)10 First-generation • Archival FFPE or Ventana IHC Automated Assay (in development) • Not reported • Archival FFPE Definition of positivity PD-L 1 expression • 1% and 5% cut-off among • 56%: 1% cut-off >100 evaluable tumour cells • 49%: 5% cut-off 1. Antonia S, et al. Poster presented at WCLC 2013 (Abstract P 2. 11 -035); 2. Brahmer J, et al. Poster 293 presented at ASCO 2014 (Abstract 8112); 3. Gettinger S, et al. Poster presented at ASCO 2014 (Abstract 8024); 4. Topalian S, et al. N Engl J Med. 2012; 366: 2443– 2454; 5. Garon E, et al. Poster presented at ASCO 2014 (Abstract 8020); 6. Gandhi L, et al. Oral presentation at AACR 2014 (Abstract CT 105); 7. Soria J, et al. Oral presentation at ECC 2013 (Abstract 3408); 8. Rizvi N, et al. Poster presented at ASCO 2014 (Abstract TPS 8123); 9. Soria J, et al. Poster presented at ESMO 2014 (Abstract 1322 P); 10. Brahmer J, et al. Poster presented at ASCO 2014 (Abstract 8021).

Limitations associated with the assessment of tumour PD-L 1 expression Tumour heterogeneity Tissue acquisition Role of fresh vs archived samples PD-L 1 expression • Numerous histological subtypes and diverse genomic aberrations in patients with NSCLC • Intratumour heterogeneity with markers for both poor and good prognosis within the same tumour • Limited sample size • Difficult to retain tissue architecture and tumour microenvironment • Aging of FFPE tissue sections causes degradation of epitopes and DNA • Age, gender, histology and stage of disease appear associated with PD-L 1 expression in patients with NSCLC • May change after exposure to treatment FFPE = formalin fixed, paraffin‐embedded. Li T, et al. J Clin Oncol. 2013; 31: 1039– 1049; Thomas A, et al. Ann Oncol. 2013; 24: 577– 585; Gerlinger M, et al. N Engl J Med. 2012; 366: 883– 889; Kerr K, et al. Ann Oncol. 2014 Apr 8. [Epub ahead of print]; Sun J‐M, et al. Poster presented at ASCO 2014 (Abstract 8066).

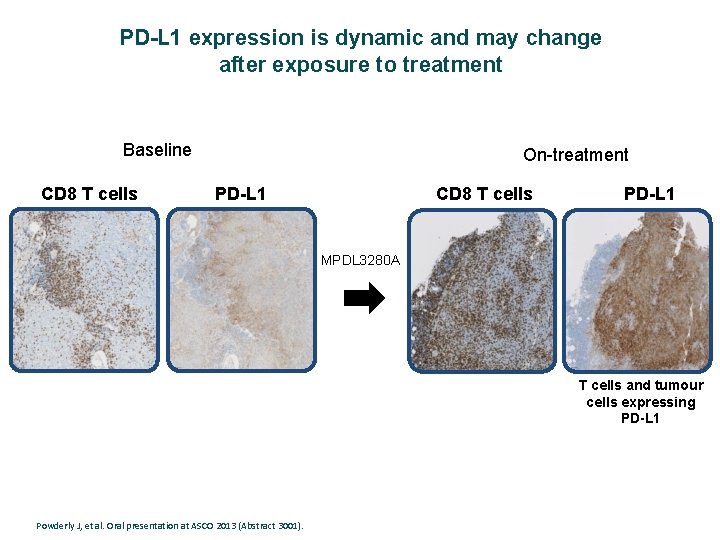
PD-L 1 expression is dynamic and may change after exposure to treatment Baseline CD 8 T cells On-treatment PD-L 1 CD 8 T cells PD-L 1 MPDL 3280 A T cells and tumour cells expressing PD-L 1 Powderly J, et al. Oral presentation at ASCO 2013 (Abstract 3001).

Objetivos de la Charla: Ø Revisar los fundamentos científicos de la inmuno‐oncología en el cáncer de pulmón y en qué se diferencia de otras modalidades de tratamiento. Ø Discutir los últimos avances en inmunoterapia para el tratamiento del cáncer de pulmón. Ø Analizar el problema de la identificación de biomarcadores para las terapias inumono‐oncológicas y su potencial valor predictivo. Ø Valorar el potencial de la inmunoterapia sola o en combinación con otras modalidades de tratamiento en la práctica clínica.

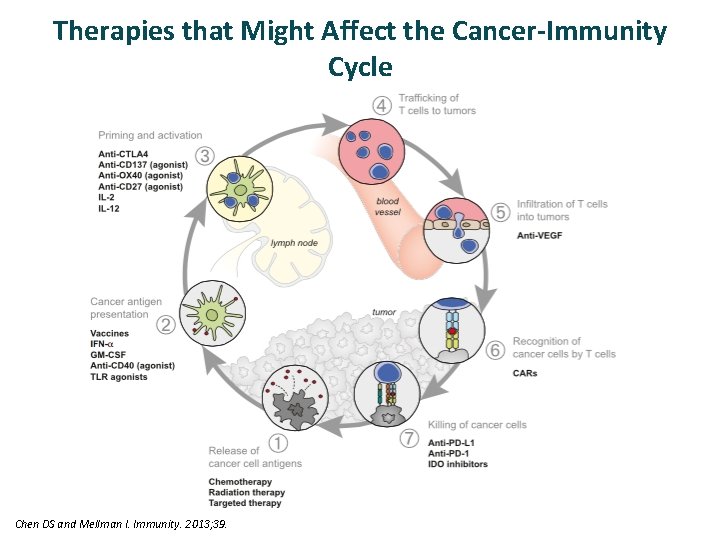
Therapies that Might Affect the Cancer-Immunity Cycle Chen DS and Mellman I. Immunity. 2013; 39.

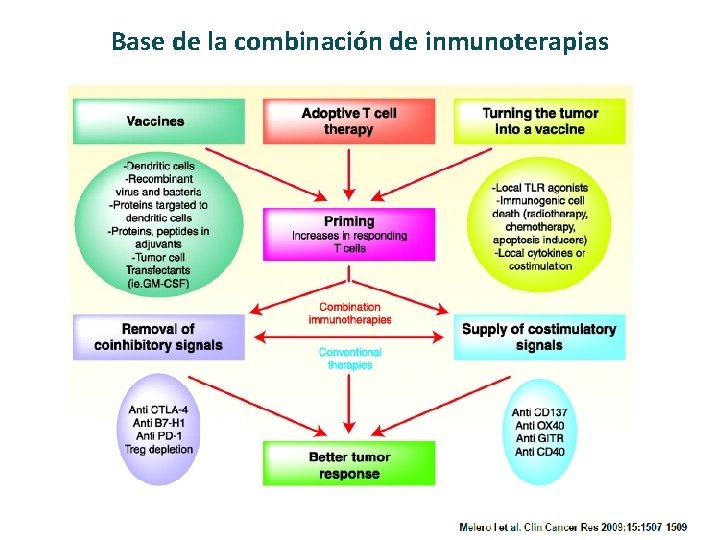
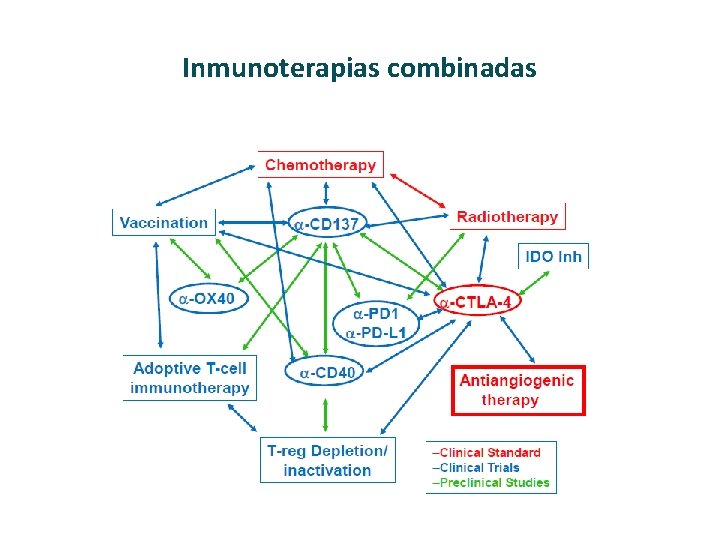
Base de la combinación de inmunoterapias

Inmunoterapias combinadas

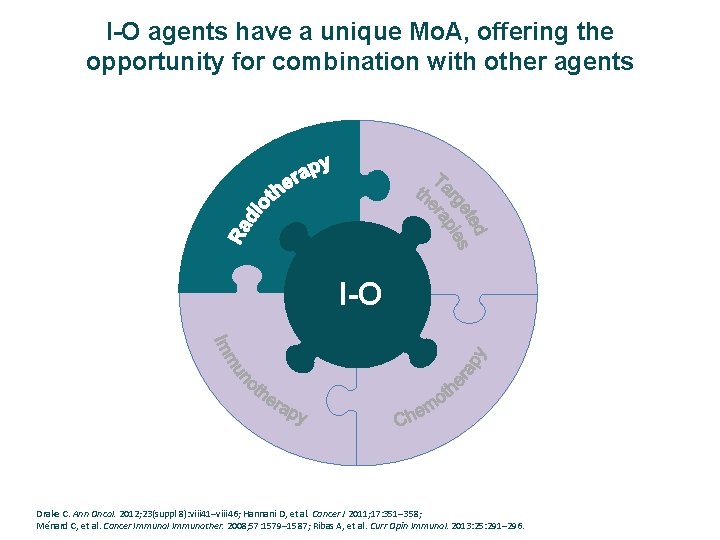
I-O agents have a unique Mo. A, offering the opportunity for combination with other agents I-O Drake C. Ann Oncol. 2012; 23(suppl 8): viii 41–viii 46; Hannani D, et al. Cancer J 2011; 17: 351– 358; Ménard C, et al. Cancer Immunol Immunother. 2008; 57: 1579– 1587; Ribas A, et al. Curr Opin Immunol. 2013: 25: 291– 296.

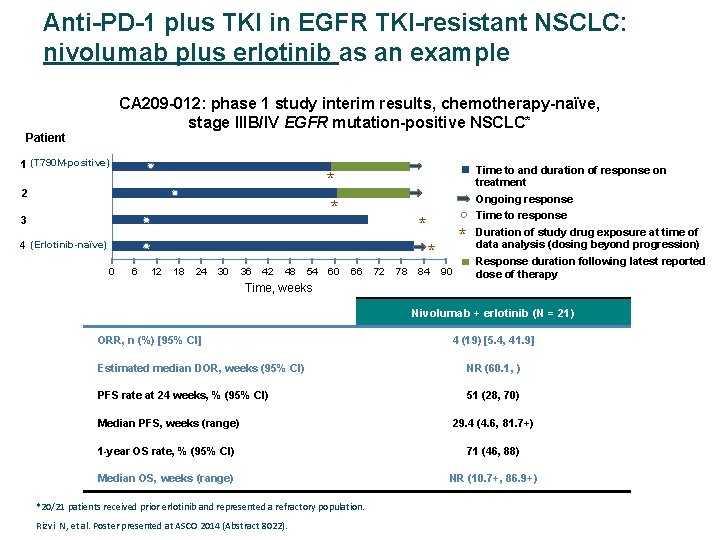
Anti-PD-1 plus TKI in EGFR TKI-resistant NSCLC: nivolumab plus erlotinib as an example CA 209 -012: phase 1 study interim results, chemotherapy-naïve, stage IIIB/IV EGFR mutation-positive NSCLC* Patient 1 (T 790 M-positive) Time to and duration of response on treatment * * 2 3 Ongoing response Time to response * 4 (Erlotinib-naïve) * * 0 6 12 18 24 30 36 42 48 54 60 66 72 78 84 90 Duration of study drug exposure at time of data analysis (dosing beyond progression) Response duration following latest reported dose of therapy Time, weeks Nivolumab + erlotinib (N = 21) ORR, n (%) [95% CI] 4 (19) [5. 4, 41. 9] Estimated median DOR, weeks (95% CI) NR (60. 1, ) PFS rate at 24 weeks, % (95% CI) 51 (28, 70) Median PFS, weeks (range) 29. 4 (4. 6, 81. 7+) 1 -year OS rate, % (95% CI) 71 (46, 88) Median OS, weeks (range) NR (10. 7+, 86. 9+) *20/21 patients received prior erlotinib and represented a refractory population. Rizvi N, et al. Poster presented at ASCO 2014 (Abstract 8022).

I-O agents have a unique Mo. A, offering the opportunity for combination with other agents I-O Drake C. Ann Oncol. 2012; 23(suppl 8): viii 41–viii 46; Hannani D, et al. Cancer J 2011; 17: 351– 358; Ménard C, et al. Cancer Immunol Immunother. 2008; 57: 1579– 1587; Ribas A, et al. Curr Opin Immunol. 2013: 25: 291– 296.

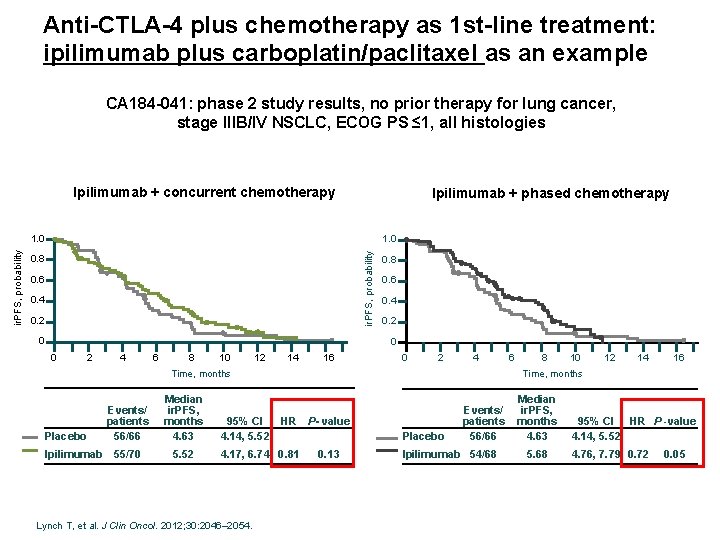
Anti-CTLA-4 plus chemotherapy as 1 st-line treatment: ipilimumab plus carboplatin/paclitaxel as an example CA 184 -041: phase 2 study results, no prior therapy for lung cancer, stage IIIB/IV NSCLC, ECOG PS ≤ 1, all histologies Ipilimumab + concurrent chemotherapy Ipilimumab + phased chemotherapy 1. 0 ir. PFS, probability 1. 0 0. 8 0. 6 0. 4 0. 2 0 0 2 4 6 8 10 12 14 16 0 2 4 Placebo Ipilimumab Median ir. PFS, months 4. 63 55/70 5. 52 95% CI 4. 14, 5. 52 HR P- value 4. 17, 6. 74 0. 81 Lynch T, et al. J Clin Oncol. 2012; 30: 2046– 2054. 8 10 12 14 16 Time, months Events/ patients 56/66 6 Placebo 0. 13 Events/ patients 56/66 Ipilimumab 54/68 Median ir. PFS, months 4. 63 5. 68 95% CI HR P -value 4. 14, 5. 52 4. 76, 7. 79 0. 72 0. 05

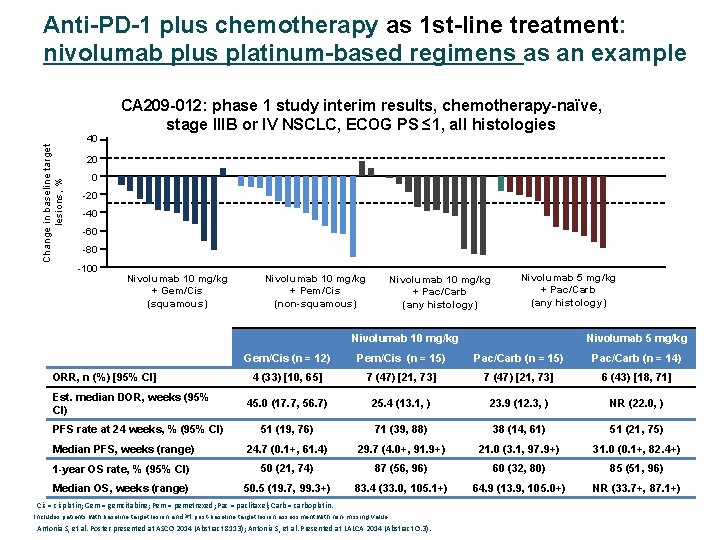
Anti-PD-1 plus chemotherapy as 1 st-line treatment: nivolumab plus platinum-based regimens as an example Change in baseline target lesions, % 40 CA 209 -012: phase 1 study interim results, chemotherapy-naïve, stage IIIB or IV NSCLC, ECOG PS ≤ 1, all histologies 20 0 -20 -40 -60 -80 -100 Nivolumab 10 mg/kg + Gem/Cis (squamous) Nivolumab 10 mg/kg + Pem/Cis (non-squamous) Nivolumab 10 mg/kg + Pac/Carb (any histology) Nivolumab 5 mg/kg + Pac/Carb (any histology) Nivolumab 10 mg/kg Nivolumab 5 mg/kg Gem/Cis (n = 12) Pem/Cis (n = 15) Pac/Carb (n = 14) 4 (33) [10, 65] 7 (47) [21, 73] 6 (43) [18, 71] 45. 0 (17. 7, 56. 7) 25. 4 (13. 1, ) 23. 9 (12. 3, ) NR (22. 0, ) 51 (19, 76) 71 (39, 88) 38 (14, 61) 51 (21, 75) Median PFS, weeks (range) 24. 7 (0. 1+, 61. 4) 29. 7 (4. 0+, 91. 9+) 21. 0 (3. 1, 97. 9+) 31. 0 (0. 1+, 82. 4+) 1 -year OS rate, % (95% CI) 50 (21, 74) 87 (56, 96) 60 (32, 80) 85 (51, 96) Median OS, weeks (range) 50. 5 (19. 7, 99. 3+) 83. 4 (33. 0, 105. 1+) 64. 9 (13. 9, 105. 0+) NR (33. 7+, 87. 1+) ORR, n (%) [95% CI] Est. median DOR, weeks (95% CI) PFS rate at 24 weeks, % (95% CI) Cis = cisplatin; Gem = gemcitabine; Pem = pemetrexed; Pac = paclitaxel; Carb = carboplatin. Includes patients with baseline target lesion and ≥ 1 post-baseline target lesion assessment with non-missing value. Antonia S, et al. Poster presented at ASCO 2014 (Abstract 8113); Antonia S, et al. Presented at LALCA 2014 (Abstract O. 3).

I-O agents have a unique Mo. A, offering the opportunity for combination with other agents I-O Drake C. Ann Oncol. 2012; 23(suppl 8): viii 41–viii 46; Hannani D, et al. Cancer J 2011; 17: 351– 358; Ménard C, et al. Cancer Immunol Immunother. 2008; 57: 1579– 1587; Ribas A, et al. Curr Opin Immunol. 2013: 25: 291– 296.

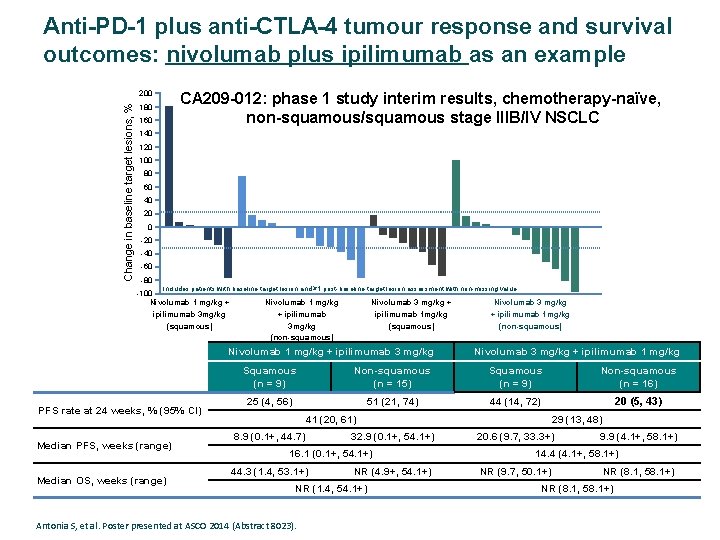
Anti-PD-1 plus anti-CTLA-4 tumour response and survival outcomes: nivolumab plus ipilimumab as an example CA 209 -012: phase 1 study interim results, chemotherapy-naïve, non-squamous/squamous stage IIIB/IV NSCLC Change in baseline target lesions, % 200 180 160 140 120 100 80 60 40 20 0 -20 -40 -60 -80 Includes patients with baseline target lesion and ≥ 1 post-baseline target lesion assessment with non-missing value. -100 Nivolumab 1 mg/kg + ipilimumab 3 mg/kg (squamous) Nivolumab 1 mg/kg + ipilimumab 3 mg/kg (non-squamous) Nivolumab 3 mg/kg + ipilimumab 1 mg/kg (squamous) Nivolumab 1 mg/kg + ipilimumab 3 mg/kg PFS rate at 24 weeks, % (95% CI) Median PFS, weeks (range) Median OS, weeks (range) Nivolumab 3 mg/kg + ipilimumab 1 mg/kg (non-squamous) Nivolumab 3 mg/kg + ipilimumab 1 mg/kg Squamous (n = 9) Non-squamous (n = 15) Squamous (n = 9) Non-squamous (n = 16) 25 (4, 56) 51 (21, 74) 44 (14, 72) 20 (5, 43) 41 (20, 61) 8. 9 (0. 1+, 44. 7) 32. 9 (0. 1+, 54. 1+) 16. 1 (0. 1+, 54. 1+) 44. 3 (1. 4, 53. 1+) NR (4. 9+, 54. 1+) NR (1. 4, 54. 1+) Antonia S, et al. Poster presented at ASCO 2014 (Abstract 8023). 29 (13, 48) 20. 6 (9. 7, 33. 3+) 9. 9 (4. 1+, 58. 1+) 14. 4 (4. 1+, 58. 1+) NR (9. 7, 50. 1+) NR (8. 1, 58. 1+)

I-O agents have a unique Mo. A, offering the opportunity for combination with other agents I-O Drake C. Ann Oncol. 2012; 23(suppl 8): viii 41–viii 46; Hannani D, et al. Cancer J 2011; 17: 351– 358; Ménard C, et al. Cancer Immunol Immunother. 2008; 57: 1579– 1587; Ribas A, et al. Curr Opin Immunol. 2013: 25: 291– 296.

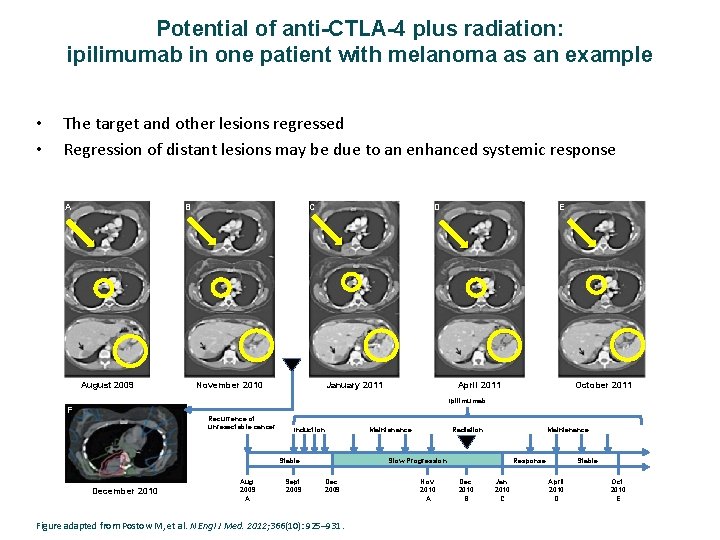
Potential of anti-CTLA-4 plus radiation: ipilimumab in one patient with melanoma as an example • • The target and other lesions regressed Regression of distant lesions may be due to an enhanced systemic response A B August 2009 C D November 2010 January 2011 E April 2011 October 2011 Ipilimumab F Recurrence of unresectable cancer Induction Aug. 2009 A Sept. 2009 Radiation Maintenance Slow Progression Stable December 2010 Maintenance Dec. 2009 Figure adapted from Postow M, et al. N Engl J Med. 2012; 366(10): 925– 931. Nov. 2010 A Response Dec. 2010 B Jan. 2010 C Stable April 2010 D Oct. 2010 E

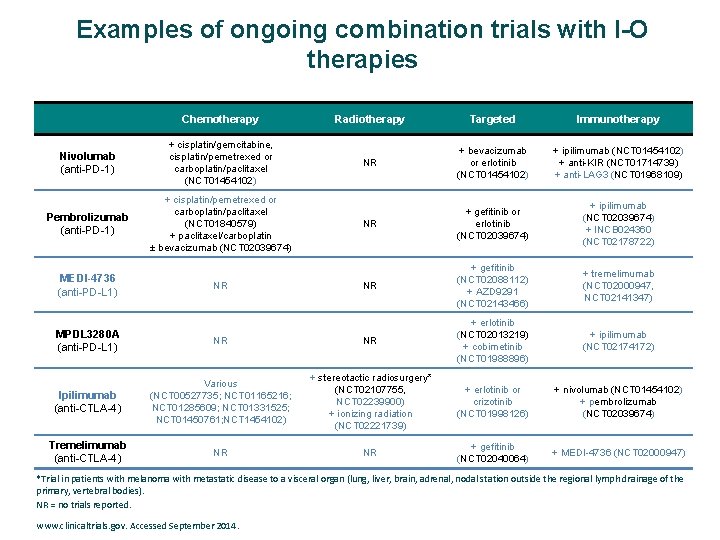
Examples of ongoing combination trials with I-O therapies Chemotherapy Radiotherapy Targeted Immunotherapy Nivolumab (anti-PD-1) + cisplatin/gemcitabine, cisplatin/pemetrexed or carboplatin/paclitaxel (NCT 01454102) NR + bevacizumab or erlotinib (NCT 01454102) + ipilimumab (NCT 01454102) + anti-KIR (NCT 01714739) + anti-LAG 3 (NCT 01968109) Pembrolizumab (anti-PD-1) + cisplatin/pemetrexed or carboplatin/paclitaxel (NCT 01840579) + paclitaxel/carboplatin ± bevacizumab (NCT 02039674) NR + gefitinib or erlotinib (NCT 02039674) + ipilimumab (NCT 02039674) + INCB 024360 (NCT 02178722) NR + gefitinib (NCT 02088112) + AZD 9291 (NCT 02143466) + tremelimumab (NCT 02000947, NCT 02141347) + ipilimumab (NCT 02174172) MEDI-4736 (anti-PD-L 1) NR MPDL 3280 A (anti-PD-L 1) NR NR + erlotinib (NCT 02013219) + cobimetinib (NCT 01988896) Ipilimumab (anti-CTLA-4) Various (NCT 00527735; NCT 01165216; NCT 01285609; NCT 01331525; NCT 01450761; NCT 1454102) + stereotactic radiosurgery* (NCT 02107755, NCT 02239900) + ionizing radiation (NCT 02221739) + erlotinib or crizotinib (NCT 01998126) + nivolumab (NCT 01454102) + pembrolizumab (NCT 02039674) Tremelimumab (anti-CTLA-4) NR NR + gefitinib (NCT 02040064) + MEDI-4736 (NCT 02000947) *Trial in patients with melanoma with metastatic disease to a visceral organ (lung, liver, brain, adrenal, nodal station outside the regional lymph drainage of the primary, vertebral bodies). NR = no trials reported. www. clinicaltrials. gov. Accessed September 2014.

In summary Ø Los agentes bloqueantes de los estímulos inhibitorios Inmunológicos han demostrao un potencial en respuestas duraderas a través de diferentes histologías, diferentes líneas de tratamiento e independiente de las mutaciones oncogénicas. Ø La identificación de biomarcadores para terapias IO es compleja. Los futuros datos de los ensayos clínicos y el uso de métodos estandarizados de evaluación ayudarán a clarificar su papel pronóstico y predictivo en CPNM. Ø Los Ac. Mo IO ofrecen la oportunidad de tratamientos combinados con otros agentes IO y con otras modalidades de tratamiento, para aumentar aún más el potencial de beneficio clínico. Ø La IO puede representar la base de los futuros tratamientos de los diversos tumores malignos sólidos o hematológicos, ofreciendo una posibilidad para la supervivencia a largo plazo.