Inmunoterapia en cncer de ovario Dnde estamos Antonio







- Slides: 37

Inmunoterapia en cáncer de ovario : ¿Dónde estamos? Antonio Gonzalez Martin Clínica Universidad de Navarra, Madrid (Head Medical Oncology) GEICO (Grupo Español de Investigación en Cáncer de Ovario) (President) ENGOT (European Network of Gynecological Oncological Trials groups) (Chairman 2018 -2020) #SEOM 2019 1

Disclosure Information q Consultant or Advisory Role: AZ, TESARO-GSK, CLOVIS, ROCHE, PHARMAMAR, MSD, MERCK-PFIZER, GENMAB, INMUNOGEN, ONCOINVENT q Research Funding: ROCHE, TESARO-GSK q Speaking: AZ, ROCHE, TESARO-GSK, PHARMAMAR q Grant support (Travelling and accommodation): ROCKE, AZ, TESARO-GSK #SEOM 2019

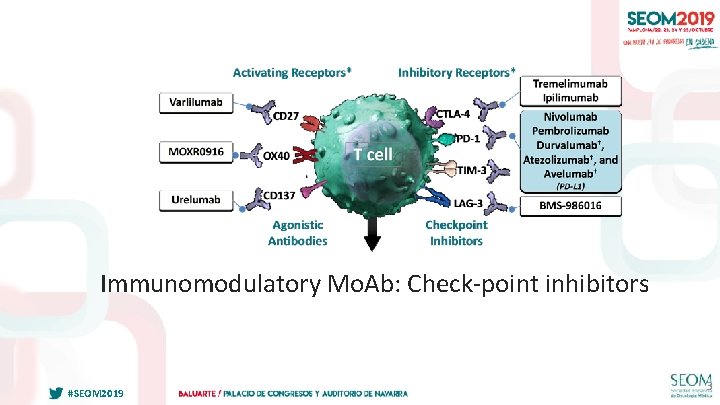
Immunomodulatory Mo. Ab: Check-point inhibitors #SEOM 2019 3

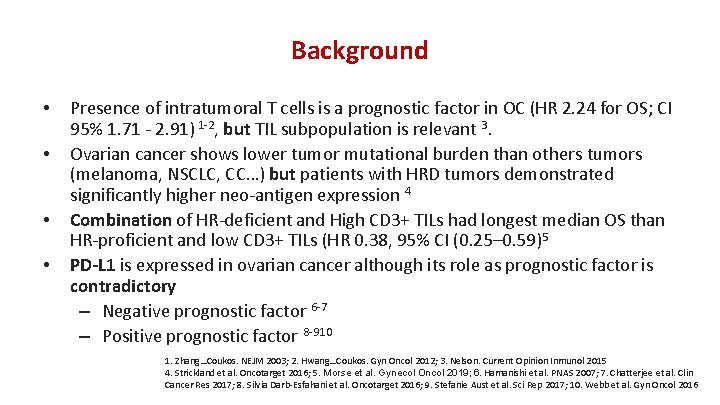
Background • • Presence of intratumoral T cells is a prognostic factor in OC (HR 2. 24 for OS; CI 95% 1. 71 - 2. 91) 1 -2, but TIL subpopulation is relevant 3. Ovarian cancer shows lower tumor mutational burden than others tumors (melanoma, NSCLC, CC…) but patients with HRD tumors demonstrated significantly higher neo-antigen expression 4 Combination of HR-deficient and High CD 3+ TILs had longest median OS than HR-proficient and low CD 3+ TILs (HR 0. 38, 95% CI (0. 25– 0. 59)5 PD-L 1 is expressed in ovarian cancer although its role as prognostic factor is contradictory – Negative prognostic factor 6 -7 – Positive prognostic factor 8 -910 1. Zhang…Coukos. NEJM 2003; 2. Hwang…Coukos. Gyn Oncol 2012; 3. Nelson. Current Opinion Inmunol 2015 4. Strickland et al. Oncotarget 2016; 5. Morse et al. Gynecol Oncol 2019; 6. Hamanishi et al. PNAS 2007; 7. Chatterjee et al. Clin Cancer Res 2017; 8. Silvia Darb-Esfahani et al. Oncotarget 2016; 9. Stefanie Aust et al. Sci Rep 2017; 10. Webb et al. Gyn Oncol 2016

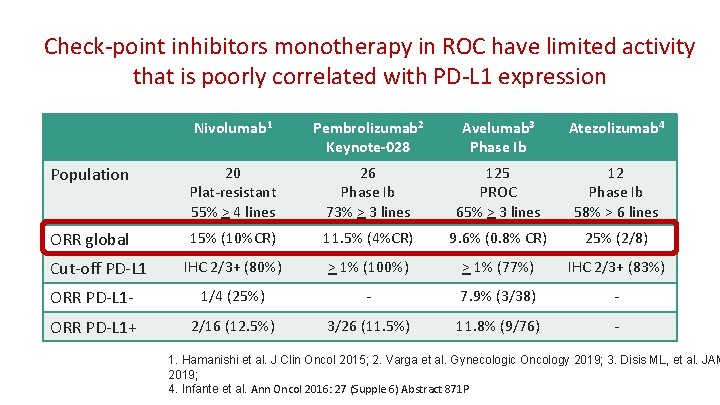
Check-point inhibitors monotherapy in ROC have limited activity that is poorly correlated with PD-L 1 expression Nivolumab 1 Pembrolizumab 2 Keynote-028 Avelumab 3 Phase Ib Atezolizumab 4 Population 20 Plat-resistant 55% > 4 lines 26 Phase Ib 73% > 3 lines 125 PROC 65% > 3 lines 12 Phase Ib 58% > 6 lines ORR global 15% (10%CR) 11. 5% (4%CR) 9. 6% (0. 8% CR) 25% (2/8) IHC 2/3+ (80%) > 1% (100%) > 1% (77%) IHC 2/3+ (83%) ORR PD-L 1 - 1/4 (25%) - 7. 9% (3/38) - ORR PD-L 1+ 2/16 (12. 5%) 3/26 (11. 5%) 11. 8% (9/76) - Cut-off PD-L 1 1. Hamanishi et al. J Clin Oncol 2015; 2. Varga et al. Gynecologic Oncology 2019; 3. Disis ML, et al. JAM 2019; 4. Infante et al. Ann Oncol 2016: 27 (Supple 6) Abstract 871 P

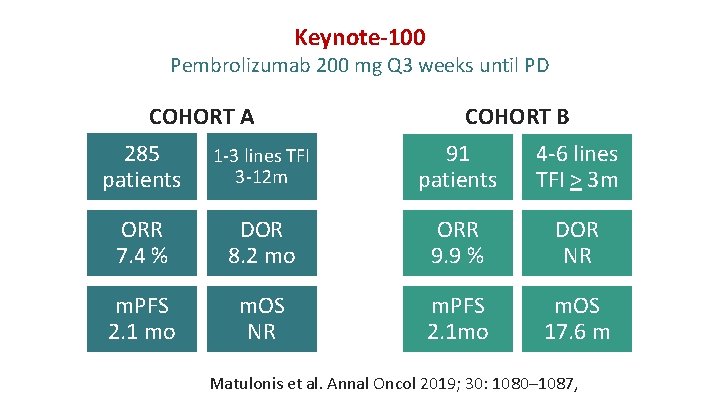
Keynote-100 Pembrolizumab 200 mg Q 3 weeks until PD COHORT A COHORT B 285 patients 1 -3 lines TFI 3 -12 m 91 patients 4 -6 lines TFI > 3 m ORR 7. 4 % DOR 8. 2 mo ORR 9. 9 % DOR NR m. PFS 2. 1 mo m. OS NR m. PFS 2. 1 mo m. OS 17. 6 m Matulonis et al. Annal Oncol 2019; 30: 1080– 1087,

WHY IS NOT WORKING?

Immune-desert absence of immune cells both in the tumor and stroma • No priming • Tolerance • Immunologic ignorance • No antigens • No presentation Chen and Mellman. Nature 2017

Check-point inhibitor combined with chemotherapy

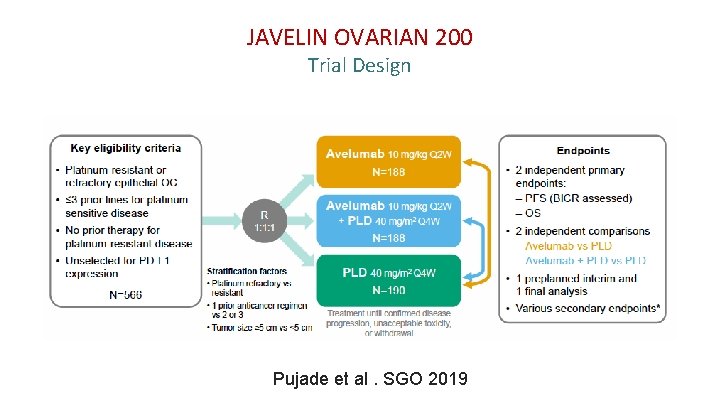
JAVELIN OVARIAN 200 Trial Design Pujade et al. SGO 2019

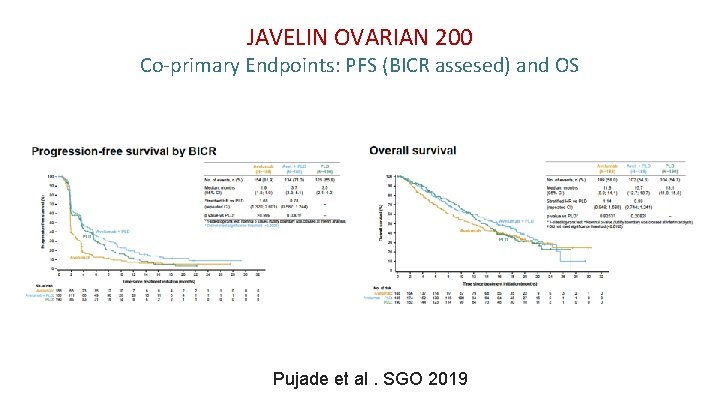
JAVELIN OVARIAN 200 Co-primary Endpoints: PFS (BICR assesed) and OS Pujade et al. SGO 2019

Optimizing check point inhibitors in AOC • Better patient selection: – Search for more efficient biomarker • Check-point inhibitors combination – Anti-angiogenic – PARPi – Multiple combinations Chemo +/- Bev +/- PARPi

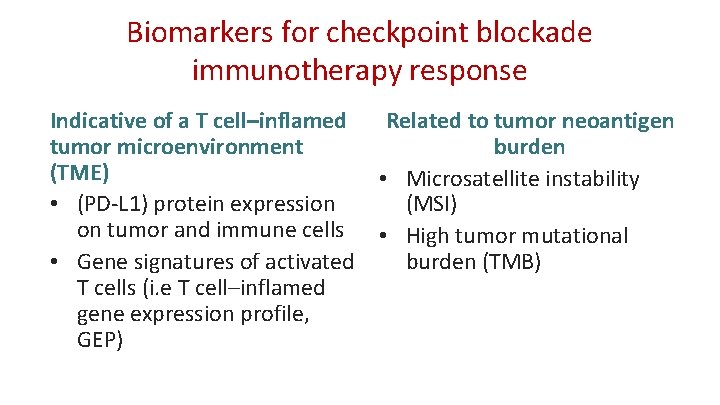
Biomarkers for checkpoint blockade immunotherapy response Indicative of a T cell–inflamed Related to tumor neoantigen tumor microenvironment burden (TME) • Microsatellite instability • (PD-L 1) protein expression (MSI) on tumor and immune cells • High tumor mutational burden (TMB) • Gene signatures of activated T cells (i. e T cell–inflamed gene expression profile, GEP)

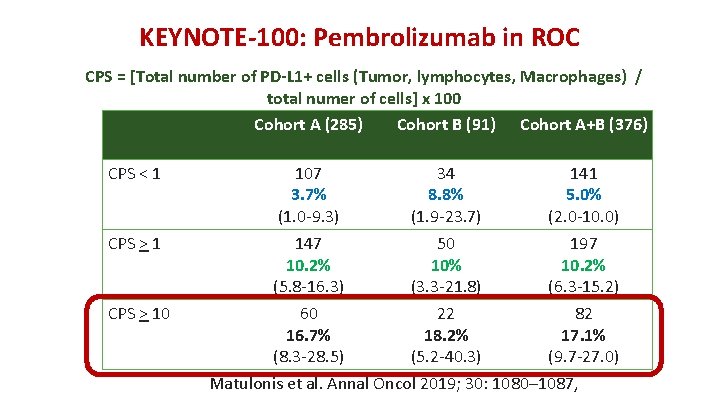
KEYNOTE-100: Pembrolizumab in ROC CPS = [Total number of PD-L 1+ cells (Tumor, lymphocytes, Macrophages) / total numer of cells] x 100 Cohort A (285) Cohort B (91) Cohort A+B (376) CPS < 1 107 3. 7% (1. 0 -9. 3) 34 8. 8% (1. 9 -23. 7) 141 5. 0% (2. 0 -10. 0) CPS > 1 147 10. 2% (5. 8 -16. 3) 60 16. 7% (8. 3 -28. 5) 50 10% (3. 3 -21. 8) 22 18. 2% (5. 2 -40. 3) 197 10. 2% (6. 3 -15. 2) 82 17. 1% (9. 7 -27. 0) CPS > 10 Matulonis et al. Annal Oncol 2019; 30: 1080– 1087,

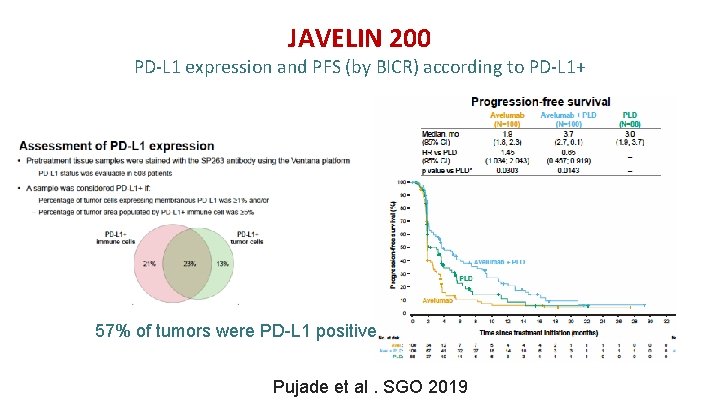
JAVELIN 200 PD-L 1 expression and PFS (by BICR) according to PD-L 1+ 57% of tumors were PD-L 1 positive Pujade et al. SGO 2019

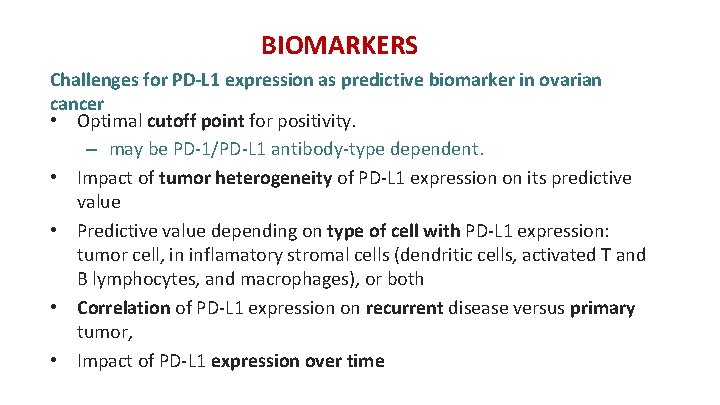
BIOMARKERS Challenges for PD-L 1 expression as predictive biomarker in ovarian cancer • Optimal cutoff point for positivity. – may be PD-1/PD-L 1 antibody-type dependent. • Impact of tumor heterogeneity of PD-L 1 expression on its predictive value • Predictive value depending on type of cell with PD-L 1 expression: tumor cell, in inflamatory stromal cells (dendritic cells, activated T and B lymphocytes, and macrophages), or both • Correlation of PD-L 1 expression on recurrent disease versus primary tumor, • Impact of PD-L 1 expression over time

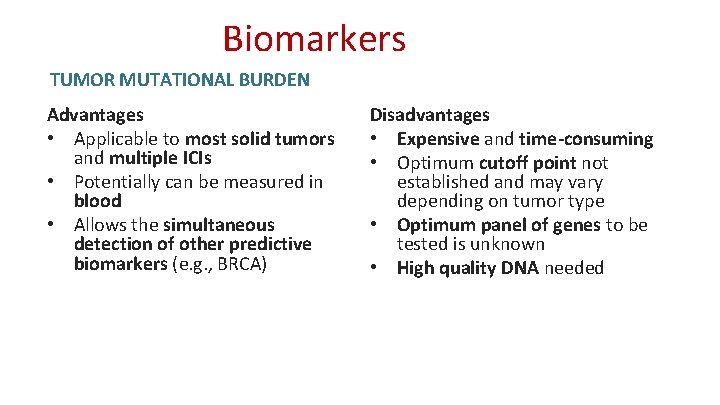
Biomarkers TUMOR MUTATIONAL BURDEN Advantages • Applicable to most solid tumors and multiple ICIs • Potentially can be measured in blood • Allows the simultaneous detection of other predictive biomarkers (e. g. , BRCA) Disadvantages • Expensive and time-consuming • Optimum cutoff point not established and may vary depending on tumor type • Optimum panel of genes to be tested is unknown • High quality DNA needed

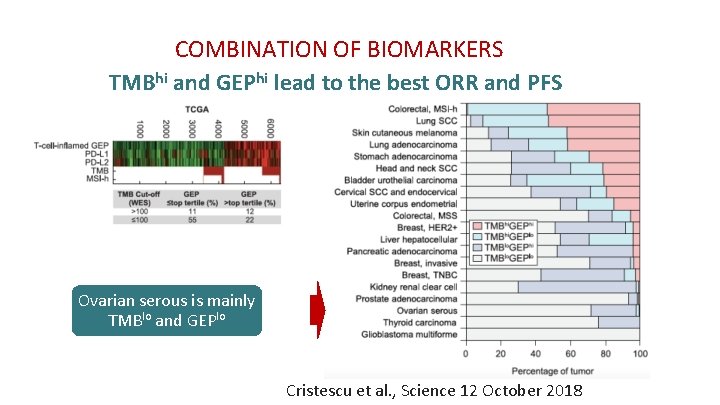
COMBINATION OF BIOMARKERS TMBhi and GEPhi lead to the best ORR and PFS Ovarian serous is mainly TMBlo and GEPlo Cristescu et al. , Science 12 October 2018

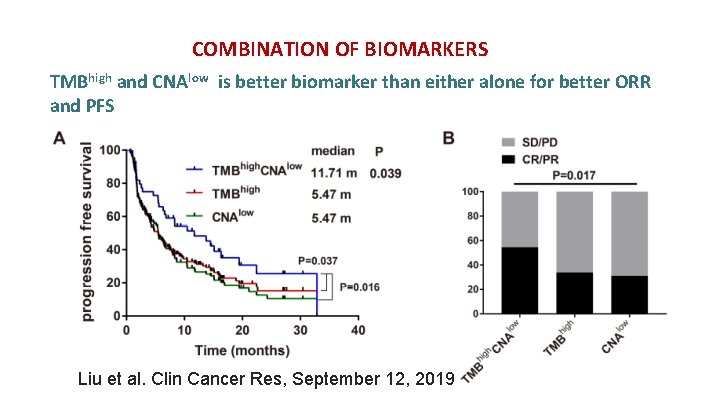
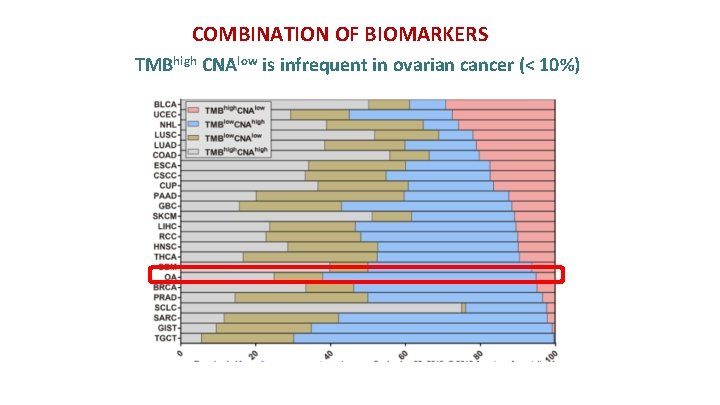
COMBINATION OF BIOMARKERS TMBhigh and CNAlow is better biomarker than either alone for better ORR and PFS Liu et al. Clin Cancer Res, September 12, 2019

COMBINATION OF BIOMARKERS TMBhigh CNAlow is infrequent in ovarian cancer (< 10%)

Optimizing check point inhibitors in AOC • • Better patient selection: – Search for more efficient biomarker Check-point inhibitors combination – Anti-angiogenic – PARPi – Multiple combinations Chemo +/- Bev +/- PARPi

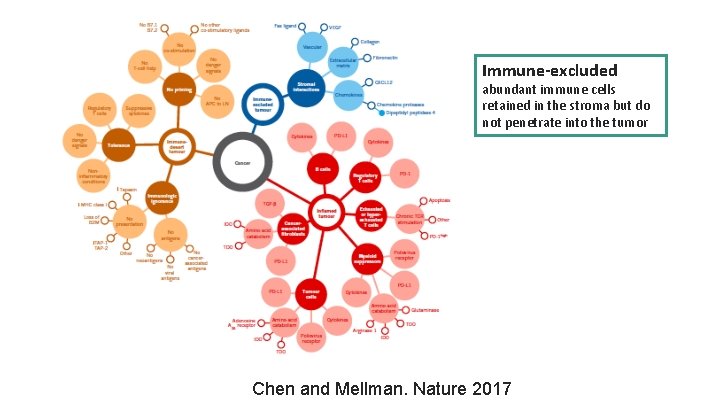
Immune-excluded abundant immune cells retained in the stroma but do not penetrate into the tumor Chen and Mellman. Nature 2017

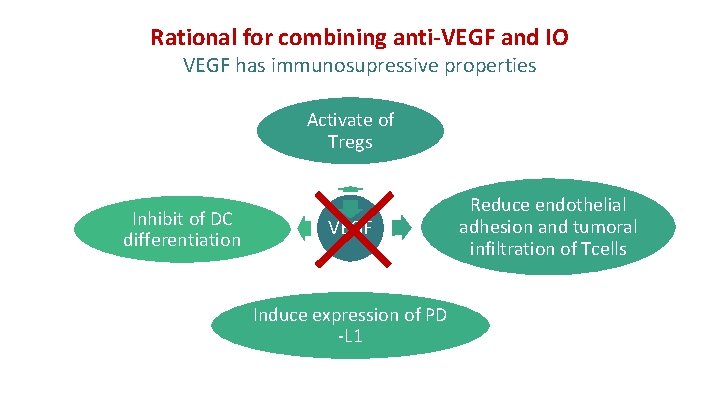
Rational for combining anti-VEGF and IO VEGF has immunosupressive properties Activate of Tregs Inhibit of DC differentiation VEGF Induce expression of PD -L 1 Reduce endothelial adhesion and tumoral infiltration of Tcells

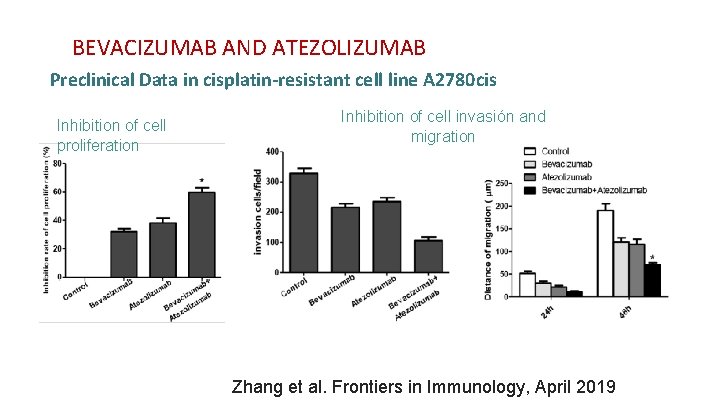
BEVACIZUMAB AND ATEZOLIZUMAB Preclinical Data in cisplatin-resistant cell line A 2780 cis Inhibition of cell proliferation Inhibition of cell invasión and migration Zhang et al. Frontiers in Immunology, April 2019

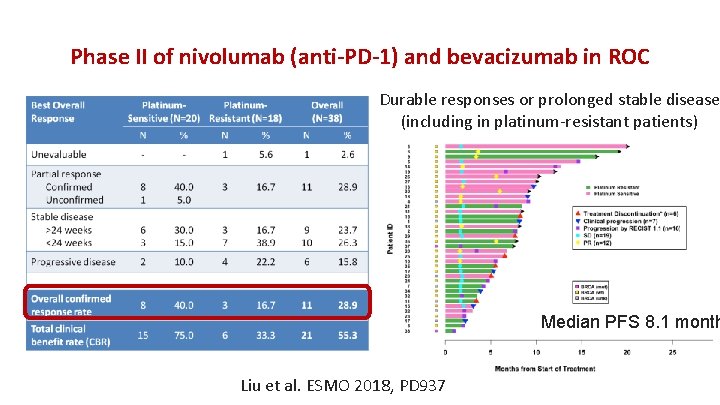
Phase II of nivolumab (anti-PD-1) and bevacizumab in ROC Durable responses or prolonged stable disease (including in platinum-resistant patients) Median PFS 8. 1 month Liu et al. ESMO 2018, PD 937

ENGOT ov 29 Principal Investigator: J. E. KURTZ Status: Closed : ATezolizumab and Avastin in LAte recurre. NT diseas. E ATALANTE DESIGN Sponsor ARCAGY-GINECO

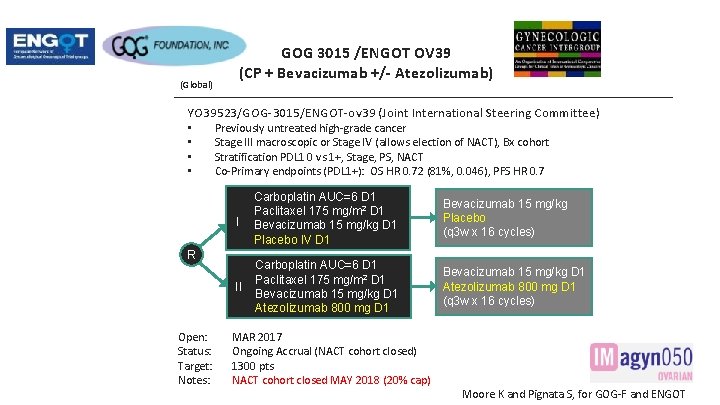
(Global) GOG 3015 /ENGOT OV 39 (CP + Bevacizumab +/- Atezolizumab) YO 39523/GOG-3015/ENGOT-ov 39 (Joint International Steering Committee) • • Previously untreated high-grade cancer Stage III macroscopic or Stage IV (allows election of NACT), Bx cohort Stratification PDL 1 0 vs 1+, Stage, PS, NACT Co-Primary endpoints (PDL 1+): OS HR 0. 72 (81%, 0. 046), PFS HR 0. 7 I Carboplatin AUC=6 D 1 Paclitaxel 175 mg/m 2 D 1 Bevacizumab 15 mg/kg D 1 Placebo IV D 1 Bevacizumab 15 mg/kg Placebo (q 3 w x 16 cycles) II Carboplatin AUC=6 D 1 Paclitaxel 175 mg/m 2 D 1 Bevacizumab 15 mg/kg D 1 Atezolizumab 800 mg D 1 (q 3 w x 16 cycles) R Open: Status: Target: Notes: MAR 2017 Ongoing Accrual (NACT cohort closed) 1300 pts NACT cohort closed MAY 2018 (20% cap) Moore K and Pignata S, for GOG-F and ENGOT

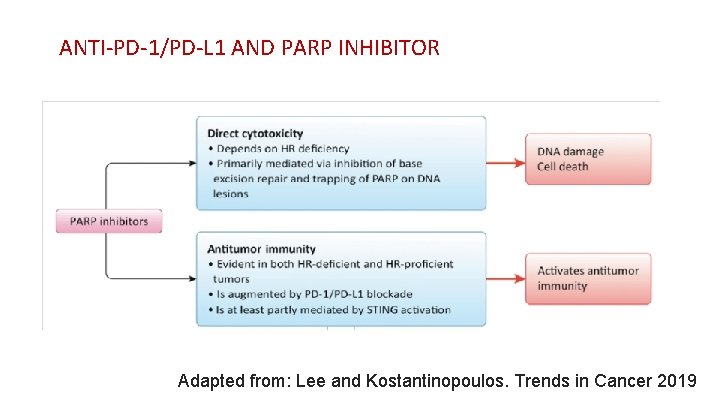
ANTI-PD-1/PD-L 1 AND PARP INHIBITOR Adapted from: Lee and Kostantinopoulos. Trends in Cancer 2019

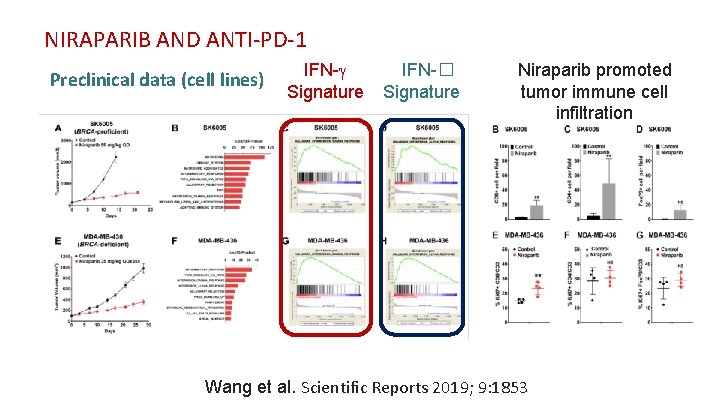
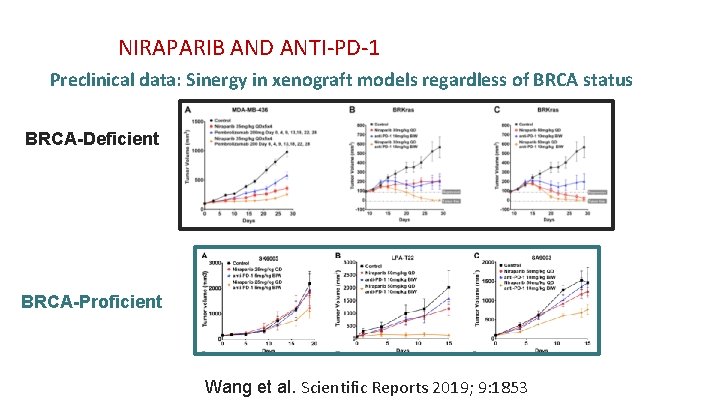
NIRAPARIB AND ANTI-PD-1 Preclinical data (cell lines) IFN-g Signature IFN-� Signature Niraparib promoted tumor immune cell infiltration Wang et al. Scientific Reports 2019; 9: 1853

NIRAPARIB AND ANTI-PD-1 Preclinical data: Sinergy in xenograft models regardless of BRCA status BRCA-Deficient BRCA-Proficient Wang et al. Scientific Reports 2019; 9: 1853

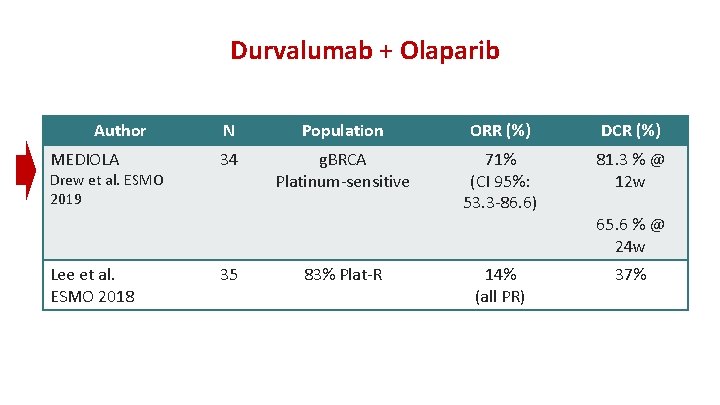
Durvalumab + Olaparib Author MEDIOLA Drew et al. ESMO 2019 Lee et al. ESMO 2018 N Population ORR (%) DCR (%) 34 g. BRCA Platinum-sensitive 71% (CI 95%: 53. 3 -86. 6) 81. 3 % @ 12 w 14% (all PR) 37% 35 83% Plat-R 65. 6 % @ 24 w

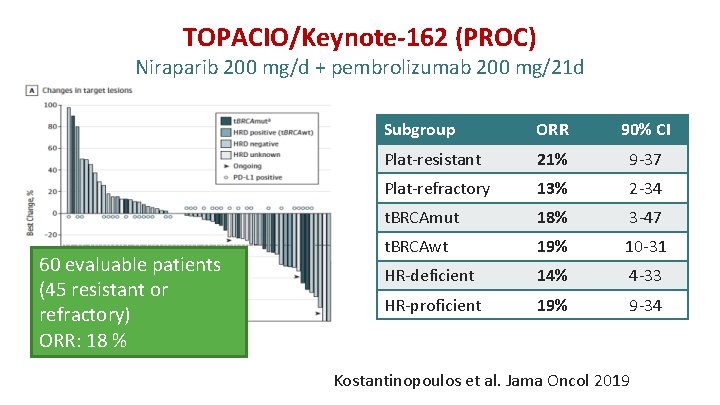
TOPACIO/Keynote-162 (PROC) Niraparib 200 mg/d + pembrolizumab 200 mg/21 d 60 evaluable patients (45 resistant or refractory) ORR: 18 % DCR: 65% Subgroup ORR 90% CI Plat-resistant 21% 9 -37 Plat-refractory 13% 2 -34 t. BRCAmut 18% 3 -47 t. BRCAwt 19% 10 -31 HR-deficient 14% 4 -33 HR-proficient 19% 9 -34 Kostantinopoulos et al. Jama Oncol 2019

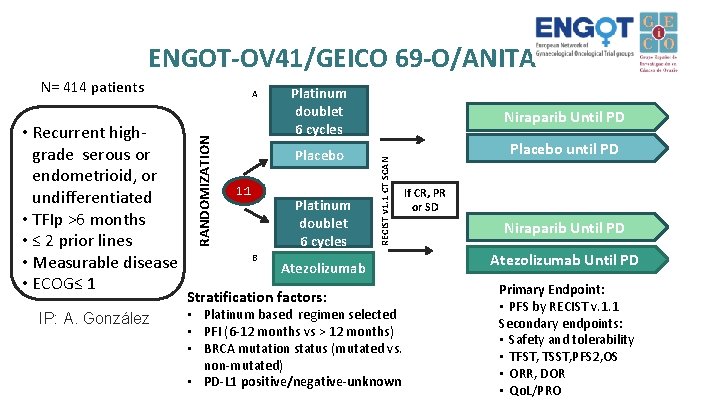
ENGOT-OV 41/GEICO 69 -O/ANITA IP: A. González RANDOMIZATION • Recurrent highgrade serous or endometrioid, or undifferentiated • TFIp >6 months • ≤ 2 prior lines • Measurable disease • ECOG≤ 1 A 1: 1 Platinum doublet 6 cycles Niraparib Until PD Placebo until PD Platinum doublet 6 cycles B RECIST v 1. 1 CT SCAN N= 414 patients Atezolizumab Stratification factors: • Platinum based regimen selected • PFI (6 -12 months vs > 12 months) • BRCA mutation status (mutated vs. non-mutated) • PD-L 1 positive/negative-unknown If CR, PR or SD Niraparib Until PD Atezolizumab Until PD Primary Endpoint: • PFS by RECIST v. 1. 1 Secondary endpoints: • Safety and tolerability • TFST, TSST, PFS 2, OS • ORR, DOR • Qo. L/PRO

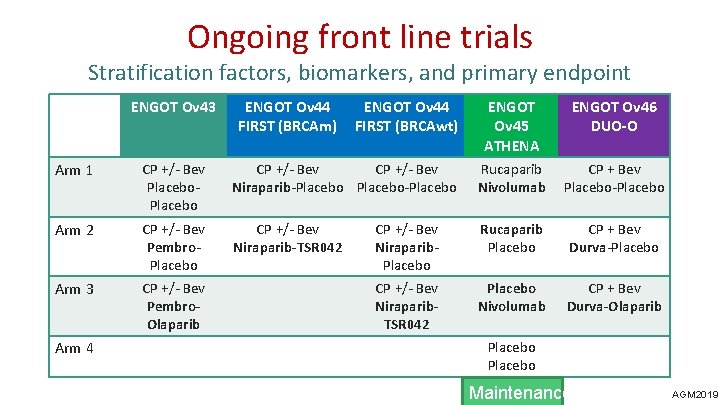
Ongoing front line trials Stratification factors, biomarkers, and primary endpoint ENGOT Ov 43 ENGOT Ov 44 FIRST (BRCAm) ENGOT Ov 44 FIRST (BRCAwt) ENGOT Ov 45 ATHENA ENGOT Ov 46 DUO-O Arm 1 CP +/- Bev Placebo CP +/- Bev Niraparib-Placebo-Placebo Rucaparib Nivolumab CP + Bev Placebo-Placebo Arm 2 CP +/- Bev Pembro. Placebo CP +/- Bev Niraparib-TSR 042 CP +/- Bev Niraparib. Placebo Rucaparib Placebo CP + Bev Durva-Placebo Arm 3 CP +/- Bev Pembro. Olaparib CP +/- Bev Niraparib. TSR 042 Placebo Nivolumab CP + Bev Durva-Olaparib Arm 4 Placebo Maintenance AGM 2019

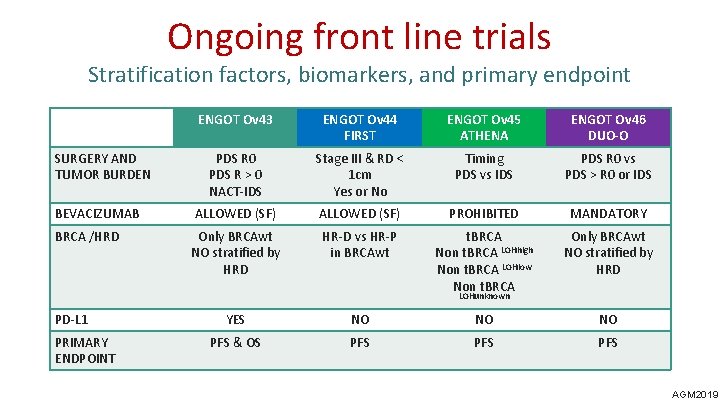
Ongoing front line trials Stratification factors, biomarkers, and primary endpoint ENGOT Ov 43 ENGOT Ov 44 FIRST ENGOT Ov 45 ATHENA ENGOT Ov 46 DUO-O PDS R 0 PDS R > 0 NACT-IDS Stage III & RD < 1 cm Yes or No Timing PDS vs IDS PDS R 0 vs PDS > R 0 or IDS BEVACIZUMAB ALLOWED (SF) PROHIBITED MANDATORY BRCA /HRD Only BRCAwt NO stratified by HRD HR-D vs HR-P in BRCAwt t. BRCA Non t. BRCA LOHhigh Non t. BRCA LOHlow Non t. BRCA Only BRCAwt NO stratified by HRD SURGERY AND TUMOR BURDEN LOHunknown PD-L 1 PRIMARY ENDPOINT YES NO NO NO PFS & OS PFS PFS AGM 2019

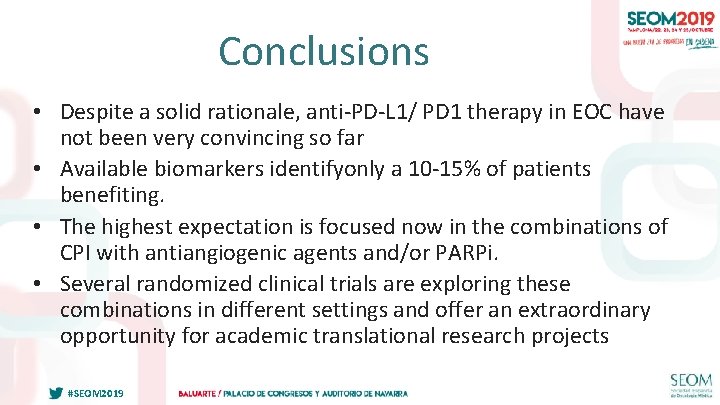
Conclusions • Despite a solid rationale, anti-PD-L 1/ PD 1 therapy in EOC have not been very convincing so far • Available biomarkers identifyonly a 10 -15% of patients benefiting. • The highest expectation is focused now in the combinations of CPI with antiangiogenic agents and/or PARPi. • Several randomized clinical trials are exploring these combinations in different settings and offer an extraordinary opportunity for academic translational research projects #SEOM 2019

Muchas Gracias Antonio Gonzalez Martin Clínica Universidad de Navarra, Madrid (Head Medical Oncology) GEICO (Grupo Español de Investigación en Cáncer de Ovario) (President) ENGOT (European Network of Gynecological Oncological Trials groups) (Chairman 2018 -2020) #SEOM 2019 41
 Dnde estoy
Dnde estoy Vall d'hebron institute of oncology
Vall d'hebron institute of oncology Gog 240
Gog 240 Investigacionales
Investigacionales Utero
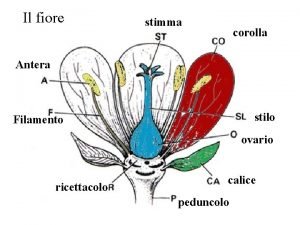
Utero Fiore
Fiore Actinomorfia
Actinomorfia Cumulo ooforo corona radiata
Cumulo ooforo corona radiata Tnm ovario
Tnm ovario Gotiera paracólica
Gotiera paracólica Fase proliferativa
Fase proliferativa Funções do utero
Funções do utero Intran3
Intran3 Tnm ovario
Tnm ovario Ovogenesis
Ovogenesis Monocotiledones
Monocotiledones Fascia de camper y scarpa
Fascia de camper y scarpa Ovulo saindo do ovario
Ovulo saindo do ovario Ovario que se desarrolla por debajo del caliz
Ovario que se desarrolla por debajo del caliz Qt neo
Qt neo Perfil hormonal completo
Perfil hormonal completo Quiste dermoide ovario ecografía
Quiste dermoide ovario ecografía Ligamento suspensorio del ovario
Ligamento suspensorio del ovario Ciclo hipotalamo hipofisis ovario
Ciclo hipotalamo hipofisis ovario Quiero brindar por mi gente sencilla
Quiero brindar por mi gente sencilla Que semana estamos del tiempo ordinario
Que semana estamos del tiempo ordinario Estamos aqui de passagem nada trouxemos e nada levaremos
Estamos aqui de passagem nada trouxemos e nada levaremos Atividade de projeto de vida 6 ano
Atividade de projeto de vida 6 ano En que semana de tiempo ordinario estamos
En que semana de tiempo ordinario estamos En que semana del tiempo ordinario estamos
En que semana del tiempo ordinario estamos De qué estamos rodeados
De qué estamos rodeados Verbo estar
Verbo estar Cuidar las avenidas del alma
Cuidar las avenidas del alma Nos estamos haciendo viejos
Nos estamos haciendo viejos Estamos cambiando
Estamos cambiando Estoy estamos
Estoy estamos Estamos a 28
Estamos a 28 Frases para pessoas que querem te prejudicar
Frases para pessoas que querem te prejudicar