IRON FROM DEFICIENCY TO OVERLOAD DR MOHAMED EL











































- Slides: 52

IRON: FROM DEFICIENCY TO OVERLOAD DR. MOHAMED EL FAKI OSMAN, MD Assistant Professor & Consultant Paediatric Haematologist/Oncologist Department of Paediatrics & King Khalid University Hospital College of Medicine King Saud University Riyadh, KSA Page 1


q Iron lacks the glitter of gold and the sparkle of silver but outshines both in biologic importance* * Nancy C Andrews, in Hematology of Infancy and Childhood Page 3

Iron Metabolism/Facts q Iron exists in two stable oxidation states: § Ferrous (Fe+2) § Ferric (Fe+3) q Can act as a redox catalyst i. e. reversibly donating or accepting electrons Page 4

Iron Metabolism/Facts q Adult body iron contents is 3 -5 gm (3545 mg/kg) § 75% (60% - 80%) in Hb § 25% stored in liver, RES macrophages, in haem containing proteins e. g myoglobin, cytochrome P-450, Myeloperoxidase…etc. Page 5

Iron Metabolism/Facts q Haemoglobin structure § § Hb molecule is a tetramer (4 polypeptide chains) 2 α chains (141 ά ά) 2 β chains (146 ά ά) Each chain attached to a prosthetic group (haem) § Haem→ protoporphyrin IX + iron molecule (Fe+2) Page 6

Iron Metabolism/Homeostasis q The daily requirement 10 -20 mg q The daily absorption 1 -2 mg(10%) q The daily excretion 1 -2 mg q The daily utilization in bone marrow for Erythropoiesis 20 mg q Excess iron stored in liver and RES Page 7

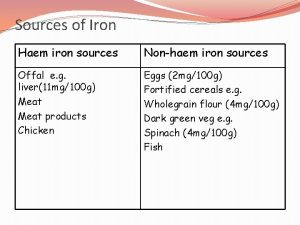
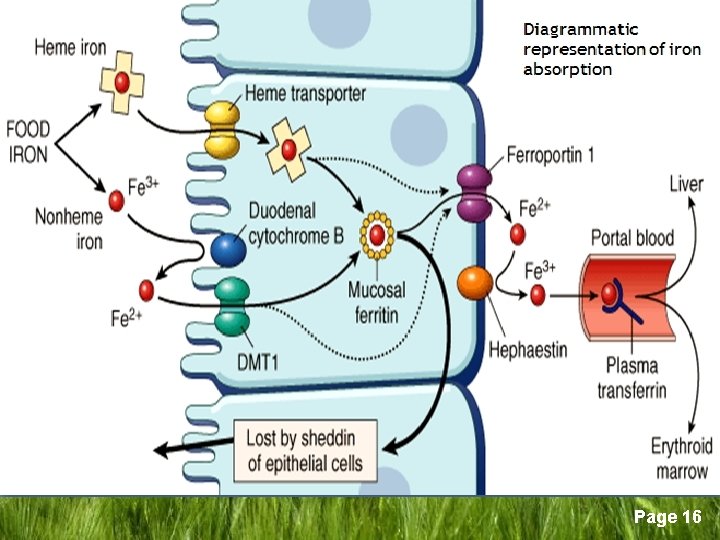
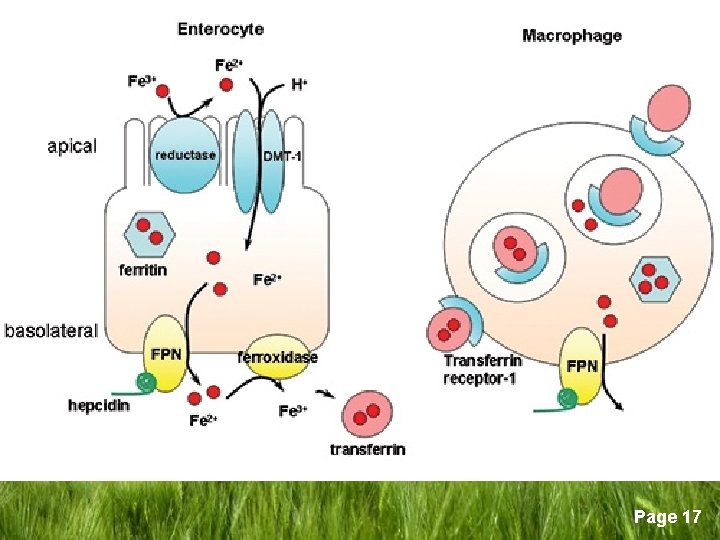
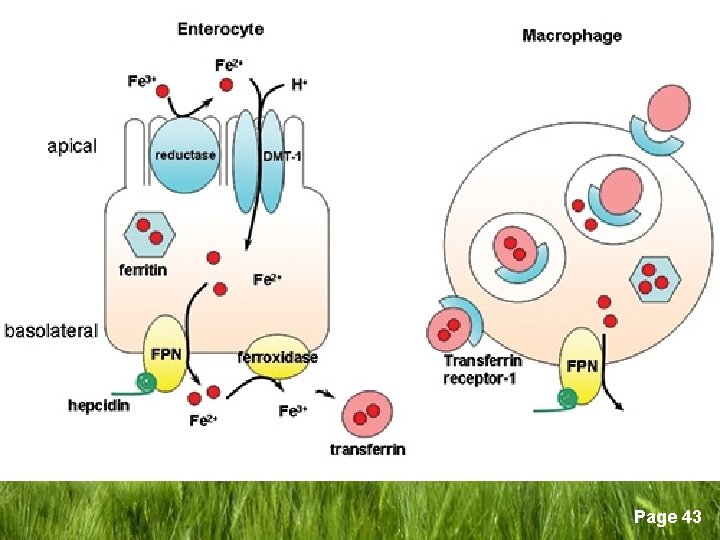
Iron Metabolism/Homeostasis q Iron Absorption § Mostly in the upper GIT § Haem iron is readily absorbed § Non-haem iron (inorganic) → duodenum entrocyte as Ferric (Fe+3) brush border Ferrous (Fe+2) Ferric reductase DMT 1* entrocyte *DMT-1 = Divalent Metal Transporter 1 Page 8

Iron Metabolism/Homeostasis q Duodenal entrocyte iron § Retained by the cell and lost when the cell dies and sloughed (excretion) § Transported through cell to enter the body by → Ferroportin § Binds to Transferrin in plasma Page 9

Iron Metabolism/Homeostasis q Role of Transferrin § The main iron carrying glycoprotein in plasma § Synthesized in the liver (sertoli cells, oligodendrocytes) § Renders iron more soluble § Prevents iron-mediated free radical toxicity § Facilitates transport into the cells Page 10

Iron Metabolism/Homeostasis q Role of transferrin § 1 apotransferrin molecule + 2 iron atoms → differic transferrin → transferrin receptor on cell surface → iron release to the cell. § Non transferrin bound iron (NTBI) is toxic to tissues § Mitochondria for Hb synthesis § Ferritin for storage Page 11

Iron Metabolism/Homeostasis q Ferritin § § § § Complex protein sub units Sphere with a central cavity Several thousands atoms of crystalline iron Metabolically inactive and non-toxic Mostly intracellular Measurable amount in serum Circulating Ferritin is iron-poor Page 12

Iron Metabolism/Homeostasis q Role of Hepcidin: § Hepcidin is the main hormone regulating iron homeostasis(2000) § 25 - α a glycoprotein synthesized in the liver § Binds to ferriportin leading to its degradation → suppression of iron release from intestine and from macrophages, stable iron levels Page 13

Iron Metabolism/Homeostasis q Role of Hepcidin § Levels increase in : • Inflammation/infection • Iron overload § Reduced in: • Hypoxia • Anaemia Page 14

Iron Metabolism/Homeostasis q Hemosiderin § Ferritin molecules aggregate → clusters § Engulfed and degraded by lysosomes § Denaturated protein and lipid interspered with iron oxide molecules Page 15

Page 16

Page 17

Iron Metabolism/Homeostasis q Non-Transferrin-Bound Iron § Level increases in complete transferrin saturation states eg hypotransferrinaemia and in iron overload § Weakly complexes with albumin, citrate, amino acids and sugars § Preferentially taken by non-hematopoetic cells e. g. liver, endocrine organs, kidneys and heart § Highly toxic to cells § Potentiates formations of free radicals → cell membrane damage and death Page 18

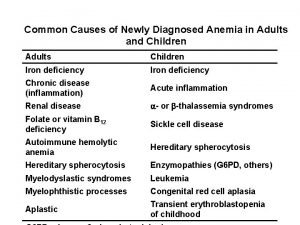
Extremes of Iron Balance q 1. Iron deficiency § Causes 1. Inadequate absorption – – – Poor bio availability (absorption of haem fe >Fe 2+ > Fe 3+) Antacid therapy/high gastric ph Bran, tannins, phytates, starch Other metals e. g. cobalt, lead Loss /dysfunction of absorptive enterocytes 2. Insufficient iron stores – Bleeding; GIT, urinary, pulmonary 3. Inadequate presentation to erythroid precursors – Atransferrinemia – Anti-transferrin receptors antibodies Page 19

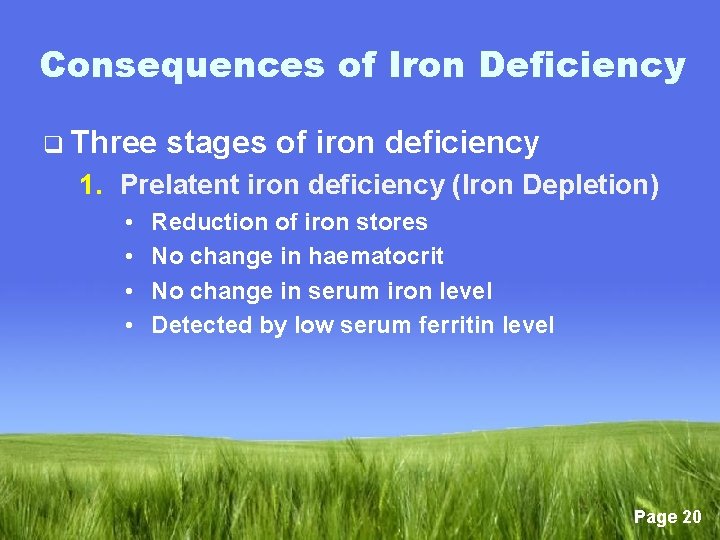
Consequences of Iron Deficiency q Three stages of iron deficiency 1. Prelatent iron deficiency (Iron Depletion) • • Reduction of iron stores No change in haematocrit No change in serum iron level Detected by low serum ferritin level Page 20

Consequences of Iron Deficiency q Three stages of iron deficiency (Continue) 2. Latent Iron Deficiency (Iron Deficient Erythropoiesis) • • • Shortage of iron available for Hb synthesis No change in haematocrit Low serum iron and ferritin levels Increase total iron binding capacity (TIBC) Detected by low early morning transferrin saturation Increase in free erythrocyte zinc protoporphyrin Page 21

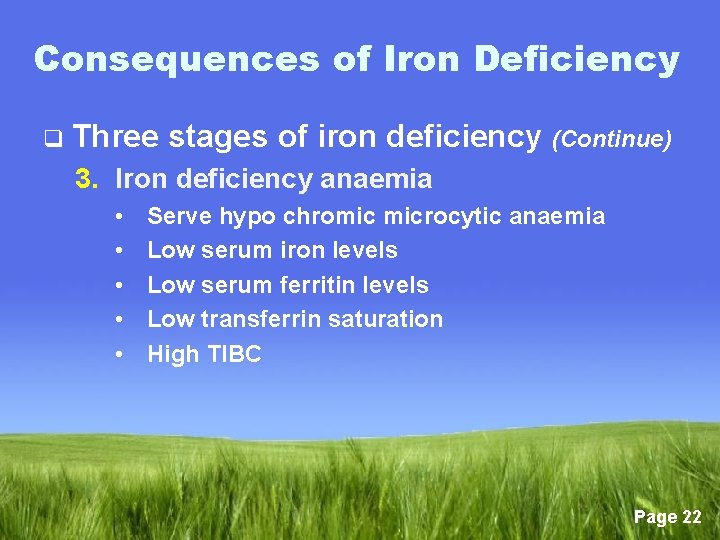
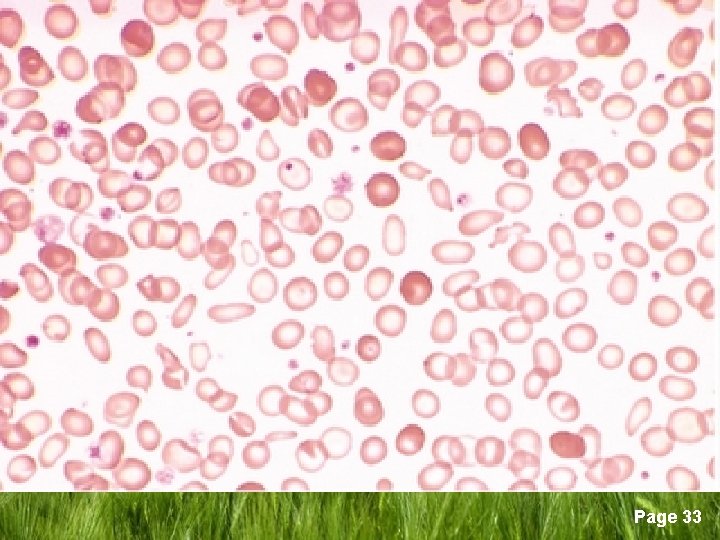
Consequences of Iron Deficiency q Three stages of iron deficiency (Continue) 3. Iron deficiency anaemia • • • Serve hypo chromic microcytic anaemia Low serum iron levels Low serum ferritin levels Low transferrin saturation High TIBC Page 22

Urinary hepcidin level as an early predictor of iron deficiency in children: A case control study Mohammed Sanad 1* and Amal F Gharib 2 25 normal children q 25 children with stage I ID q 25 children with stage 2 ID q 25 children with stage 3 ID q Page 23

Urinary hepcidin level as an early predictor of iron deficiency in children: A case control study Mohammed Sanad 1* and Amal F Gharib 2 q q q Urinary hepcidin level is normal in all 25 normal children (control group) Urinary hepcidin levels were low in all three stages of iron deficiency More significantly low with the progression of iron deficiency Page 24

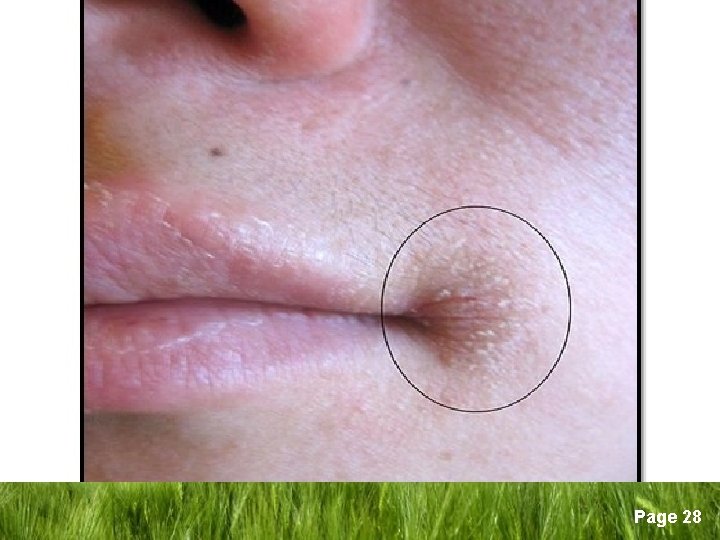
q Signs and symptoms of ID § No symptoms § Findings of anaemia; weakness, fatigue, palpitations, lightheadedness § Epithelial changes; angular stomatitis, glossitis, smooth tongue, gastric atrophy, koilonychia (spoon nails) § Plummer-Vinson syndrome (oesophageal web syndrome) § Pica (compulsive consumption of nonnutritive materials) Page 25

Page 26

Page 27

Page 28

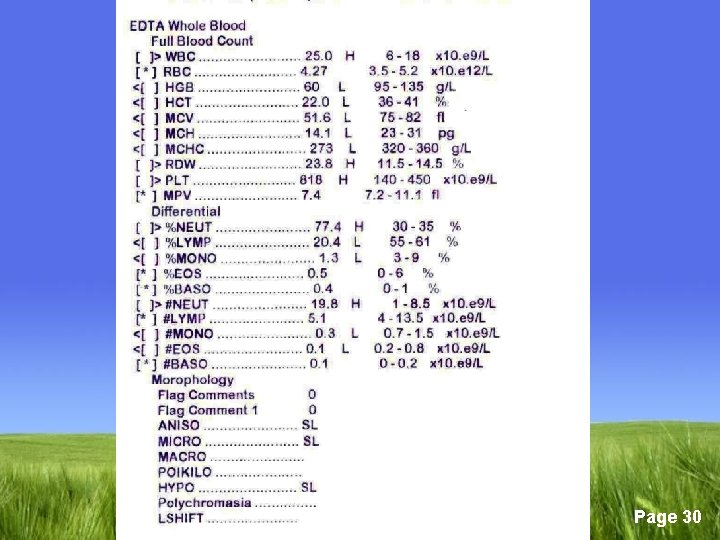
q Diagnosis; § Bone marrow smear shows no stainable iron is the only definitive diagnosis. § Low serum iron level § Low ferritin level § High TIBC. are considered diagnostic § Transferrin saturation should be less than 10% Page 29

Page 30

Page 31

Page 32

Page 33

q Treatment of ID § Treat the cause? § Oral iron supplementation: - Ferrous sulfate is recommended 6 mg/kg/day - Ascorbic acid may enhance intestinal absorption Page 34

q Response to iron therapy § Reticulocytosis by day 4 § Maximum by 7 -10 days § Hb may rise significantly by 2 -4 weeks § Complete Hb correction by 6 -8 weeks § Continue 2 -3 months after correction of the anaemia to replenish the body stores Page 35

q Causes § § § of poor response to iron therapy Noncompliance Ongoing blood loss Insufficient duration of therapy High gastric p. H; antacids Inhibitors of iron absorption/utilization • Lead • Chronic inflammation § Incorrect diagnosis • Thalassemia • Sideoblastic anaemia Page 36

q Parenteral iron replacement § Three forms: • Iron dextran • Iron gluconate • Iron sucrose § Indications: • Oral iron is poorly tolerated • Rapid replacement of iron stores • GIT absorption is compromised • Erythropoietin therapy is needed e. g. patients on dialysis Page 37

q Iron replacement in infants § Term babies has iron stores adequate for about 6 months § Preterm babies deplete iron stores by third -fourth month § Human milk contains high amount of iron which decreases by the 5 th month of lactation § Iron from human milk is 20 -50% absorbed Page 38

q Recommendations § Infants who are exclusively breast fed; give iron 1 mg/kg/day after 6 months § Non-breast milk fed infants should be on iron-supplemental formula (12 mg/L) till the end of the first year § Start iron-enriched cereals with solid food § Avoid cow’s milk during the first year. May chelate iron and may cause GIT hemorrhage Page 39

Extremes of Iron Balance q 2. Iron overload § Primary (hereditary) § Secondary (acquired) Page 40

q Secondary iron overload § Blood/PRBC transfusion § Erythroid hyperplasia • B+-thalassemia (Thalassemia intermedia) • X-linked sideroblastic anaemia • both condition lead to increase iron absorption • MDS Page 41

q Primary Iron Overload § Hepcidin feedback mechanism Page 42

Page 43

§ Primary Iron Overload (continue) • Levels of circulating iron control hepatic hepcidin production • Hepcidin production increases at times of high iron levels → degradation of ferriportin → less iron absorption • Hepcidin deficiency →increase iron level leading to haemochromatosis Page 44

q Consequences of Iron Overload § Iron reacts with reactive oxygen intermediates; oxygen superoxide (O 2) and hydrogen peroxide (H 2 O 2) → free radicals, hydroxyl radicals (HO) → severe tissue damage Page 45

q Consequences of Iron Overload (continue) § Liver → hepatomegaly (early) → fibrosis and micronodular cirrhosis. Diagnosed by MRI, liver biopsy § Heart → congestive cardiomyopathy, rarely pericarditis. Conduction defects → sudden death. Page 46

q Consequences of Iron Overload (continue) § Endocrine organs • Endocrine pancreatic dysfunction → diabetes mellitus • Pituitary dysfunction → several endocrine problems e. g. delayed sexual maturation - Hypoparathyroidism • Thyroid is usually preserved § Skin and joints Page 47

q Laboratory findings § High iron § High ferritin § Low TIBC § Transferrin saturation is increased § MRI/liver biopsy are diagnostic Page 48

q Management of primary haemochromatosis § Venesection • Induction phase; 7 ml/kg weekly • Follow up by ferritin level aiming to < 50 µg/d and Hb = 11 gm/dl • Avoid vitamin C • ? Oral chelation • Hepcidin correction may be a future therapy Page 49

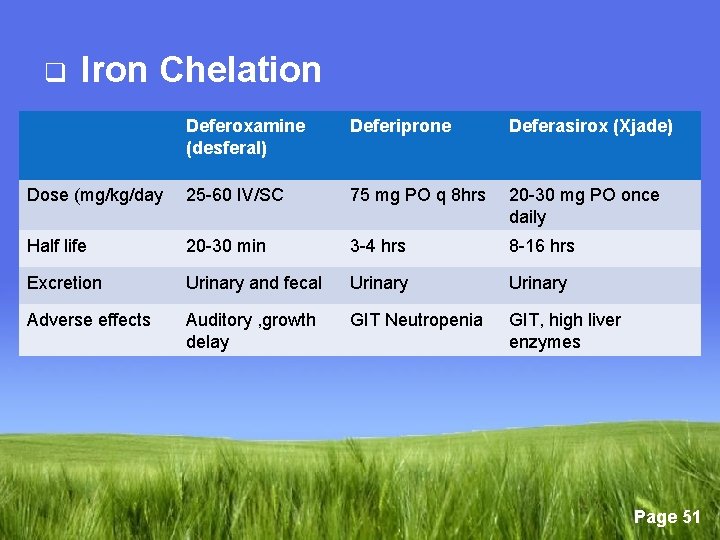
q Iron Chelation Bind non-transferrin-bound iron (NTBI) → soluble → urinary excretion/intestinal Page 50

q Iron Chelation Deferoxamine (desferal) Deferiprone Deferasirox (Xjade) Dose (mg/kg/day 25 -60 IV/SC 75 mg PO q 8 hrs 20 -30 mg PO once daily Half life 20 -30 min 3 -4 hrs 8 -16 hrs Excretion Urinary and fecal Urinary Adverse effects Auditory , growth delay GIT Neutropenia GIT, high liver enzymes Page 51

Page 52
 Serum ferritin in iron deficiency anemia
Serum ferritin in iron deficiency anemia Minerals are inorganic elements that the body
Minerals are inorganic elements that the body Microcytic hypochromic anemia
Microcytic hypochromic anemia Iron deficiency anemia smear
Iron deficiency anemia smear Elemental iron dose
Elemental iron dose Iron deficiency anemia labs
Iron deficiency anemia labs Sickle cell
Sickle cell Hypochromasia slight
Hypochromasia slight Rakhi naik
Rakhi naik Iron studies interpretation
Iron studies interpretation L
L Causes of macrocytic anaemia
Causes of macrocytic anaemia Nutrient deficiency
Nutrient deficiency Iron deficiency chart
Iron deficiency chart Symptoms of iron deficiency
Symptoms of iron deficiency Mass of iron in an iron tablet
Mass of iron in an iron tablet Iron sharpens iron friendship
Iron sharpens iron friendship Information overload
Information overload Biasnn
Biasnn Progressive overload
Progressive overload Barker's ecological theory of environmental psychology
Barker's ecological theory of environmental psychology Spc switching system overload control
Spc switching system overload control Yang dimaksud dengan information overload adalah
Yang dimaksud dengan information overload adalah Urban overload hypothesis
Urban overload hypothesis Prinsip overload adalah
Prinsip overload adalah Cisco nat overload
Cisco nat overload Information overload
Information overload Overload symbol
Overload symbol Techniques for avoiding resource overload? *
Techniques for avoiding resource overload? * Information overload syndrome
Information overload syndrome C++ overload arrow operator
C++ overload arrow operator Borg rating of perceived exertion (rpe)
Borg rating of perceived exertion (rpe) Information overload research group
Information overload research group Contactor symbol electrical
Contactor symbol electrical 7 principles of training
7 principles of training Chapter 6 training for fitness
Chapter 6 training for fitness Anne pemberton uncw death
Anne pemberton uncw death Fear of superiors in communication
Fear of superiors in communication Simbol thermal overload relay
Simbol thermal overload relay Fail safe circuit with overload protection
Fail safe circuit with overload protection Udp header
Udp header C++ operator overloading template
C++ operator overloading template Contactor coil symbol
Contactor coil symbol Bracket operator overloading c++
Bracket operator overloading c++ Discovering computer
Discovering computer Mohamed younis umbc
Mohamed younis umbc Mohamed hassoun
Mohamed hassoun Hassan fareed physics
Hassan fareed physics Mohamed akel
Mohamed akel Dr qazi omar
Dr qazi omar Lodacain
Lodacain Mohamed younis umbc
Mohamed younis umbc Sheikh abdul qadir jilani family tree
Sheikh abdul qadir jilani family tree