Iron Overload in NTDT 3 rd PanEuropean Conference


- Slides: 22

Iron Overload in NTDT 3 rd Pan-European Conference on Haemoglobinopathies & Rare Anaemias Limassol, 24 – 26 October 2012 Khaled M. Musallam, MD, Ph. D American University of Beirut, Lebanon University of Milan, Italy

Excess Iron in NTDT ● Primary: Increased intestinal absorption ● Secondary: Blood transfusions Musallam KM et al. Blood Rev 2012; 26: 16 -9.

Transfusions in NTDT ● Occasional • • • Infection Pregnancy Surgery ● More regular • • Poor growth and development Specific complications (advanced age) v Leg ulcer, pulmonary hypertension, extramedullary hematopoietic psuedotumors, thrombotic disease Musallam KM et al. Cold Spring Harb Perspect Med 2012; 2: a 013482.

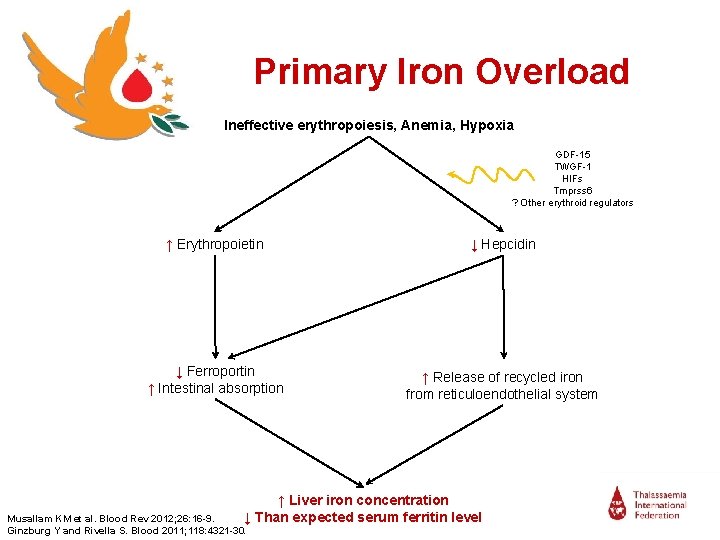
Primary Iron Overload Ineffective erythropoiesis, Anemia, Hypoxia GDF-15 TWGF-1 HIFs Tmprss 6 ? Other erythroid regulators ↑ Erythropoietin ↓ Hepcidin ↓ Ferroportin ↑ Intestinal absorption ↑ Release of recycled iron from reticuloendothelial system ↑ Liver iron concentration ↓ Than expected serum ferritin level Musallam KM et al. Blood Rev 2012; 26: 16 -9. Ginzburg Y and Rivella S. Blood 2011; 118: 4321 -30.

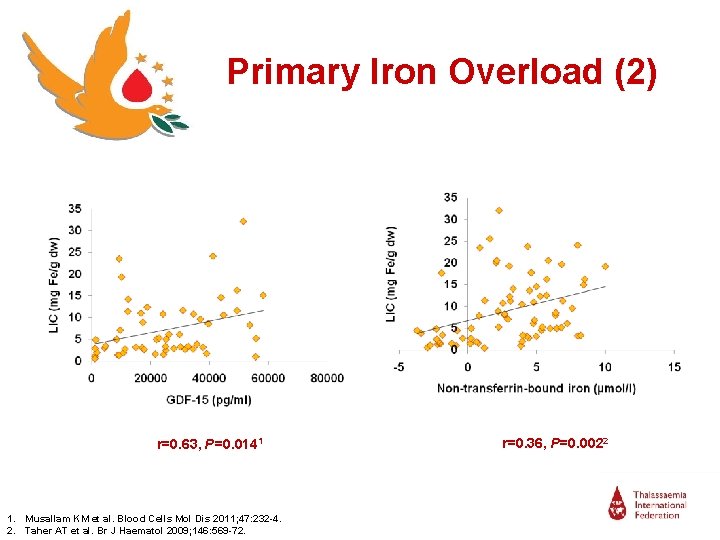
Primary Iron Overload (2) r=0. 63, P=0. 0141 1. Musallam KM et al. Blood Cells Mol Dis 2011; 47: 232 -4. 2. Taher AT et al. Br J Haematol 2009; 146: 569 -72. r=0. 36, P=0. 0022

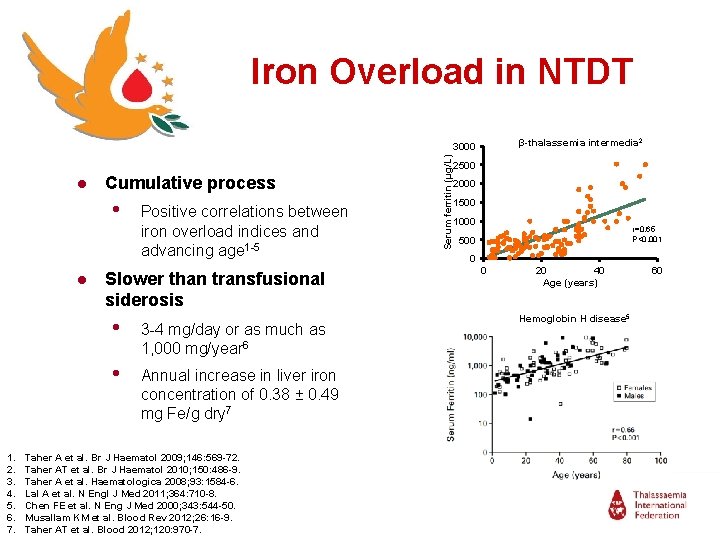
Iron Overload in NTDT β-thalassemia intermedia 2 ● Cumulative process • Positive correlations between iron overload indices and advancing age 1 -5 ● Slower than transfusional siderosis 1. 2. 3. 4. 5. 6. 7. • 3 -4 mg/day or as much as 1, 000 mg/year 6 • Annual increase in liver iron concentration of 0. 38 ± 0. 49 mg Fe/g dry 7 Taher A et al. Br J Haematol 2009; 146: 569 -72. Taher AT et al. Br J Haematol 2010; 150: 486 -9. Taher A et al. Haematologica 2008; 93: 1584 -6. Lal A et al. N Engl J Med 2011; 364: 710 -8. Chen FE et al. N Eng J Med 2000; 343: 544 -50. Musallam KM et al. Blood Rev 2012; 26: 16 -9. Taher AT et al. Blood 2012; 120: 970 -7. Serum ferritin (µg/L) 3000 2500 2000 1500 1000 r=0. 65 P<0. 001 500 0 0 20 40 Age (years) Hemoglobin H disease 5 60

Considerable iron overload warranting concern? ● Iron overload as early as 5 years 1 ● Iron-related morbidities beyond 10 years 2 ● Mean LIC values at cross-sectional assessment of NTDT cohorts with a mean age in early-mid adulthood range between 7 and 15 mg Fe/g dw 3 -7 1. 2. 3. 4. 5. 6. 7. Cossu P et al. Eur J Pediatr 1981; 137: 267 -71. Taher AT et al. Br J Haematol 2010; 150: 486 -9. Origa R et al. Haematologica 2007; 92: 583 -8. Musallam KM et al. Haematologica 2011; 96: 1605 -12. Taher AT et al. Blood 2012; 120: 970 -7. Lal A et al. N Engl J Med 2011; 364: 710 -8. Pakbaz Z et al. Pediatr Blood Cancer 2007; 49: 329 -32.

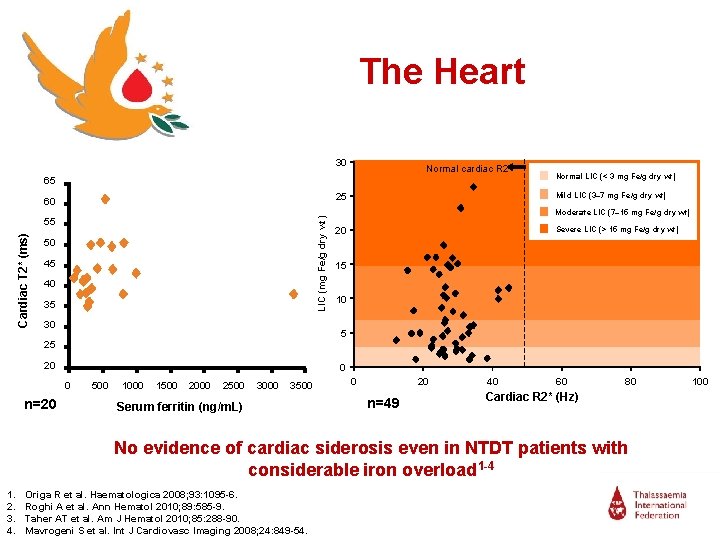
The Heart 30 Normal cardiac R 2* 65 LIC (mg Fe/g dry wt) 55 Cardiac T 2* (ms) Mild LIC (3– 7 mg Fe/g dry wt) 25 60 50 45 40 35 30 Normal LIC (< 3 mg Fe/g dry wt) Moderate LIC (7– 15 mg Fe/g dry wt) Severe LIC (> 15 mg Fe/g dry wt) 20 15 10 5 25 20 0 0 n=20 500 1000 1500 2000 2500 3000 3500 Serum ferritin (ng/m. L) 0 20 n=49 40 60 80 Cardiac R 2* (Hz) No evidence of cardiac siderosis even in NTDT patients with considerable iron overload 1 -4 1. 2. 3. 4. Origa R et al. Haematologica 2008; 93: 1095 -6. Roghi A et al. Ann Hematol 2010; 89: 585 -9. Taher AT et al. Am J Hematol 2010; 85: 288 -90. Mavrogeni S et al. Int J Cardiovasc Imaging 2008; 24: 849 -54. 100

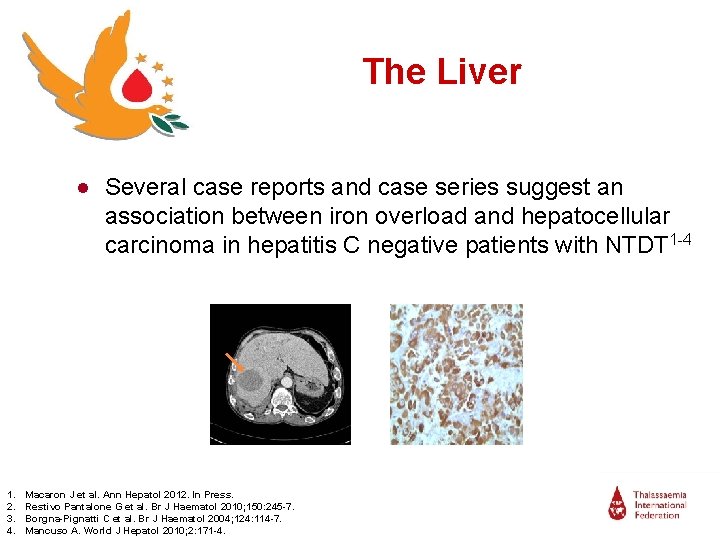
The Liver ● Several case reports and case series suggest an association between iron overload and hepatocellular carcinoma in hepatitis C negative patients with NTDT 1 -4 1. 2. 3. 4. Macaron J et al. Ann Hepatol 2012. In Press. Restivo Pantalone G et al. Br J Haematol 2010; 150: 245 -7. Borgna-Pignatti C et al. Br J Haematol 2004; 124: 114 -7. Mancuso A. World J Hepatol 2010; 2: 171 -4.

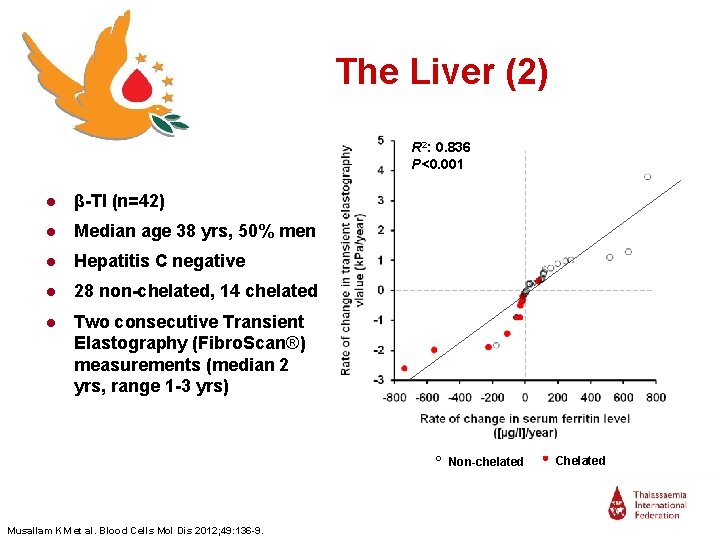
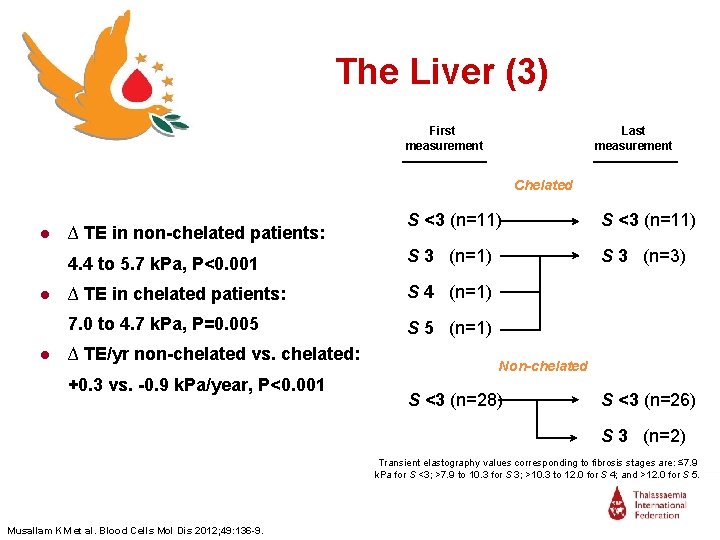
The Liver (2) R 2: 0. 836 P<0. 001 ● β-TI (n=42) ● Median age 38 yrs, 50% men ● Hepatitis C negative ● 28 non-chelated, 14 chelated ● Two consecutive Transient Elastography (Fibro. Scan®) measurements (median 2 yrs, range 1 -3 yrs) Non-chelated Musallam KM et al. Blood Cells Mol Dis 2012; 49: 136 -9. Chelated

The Liver (3) First measurement Last measurement Chelated S <3 (n=11) 4. 4 to 5. 7 k. Pa, P<0. 001 S 3 (n=1) S 3 (n=3) ● ∆ TE in chelated patients: S 4 (n=1) 7. 0 to 4. 7 k. Pa, P=0. 005 S 5 (n=1) ● ∆ TE in non-chelated patients: ● ∆ TE/yr non-chelated vs. chelated: +0. 3 vs. -0. 9 k. Pa/year, P<0. 001 Non-chelated S <3 (n=28) S <3 (n=26) S 3 (n=2) Transient elastography values corresponding to fibrosis stages are: ≤ 7. 9 k. Pa for S <3; >7. 9 to 10. 3 for S 3; >10. 3 to 12. 0 for S 4; and >12. 0 for S 5. Musallam KM et al. Blood Cells Mol Dis 2012; 49: 136 -9.

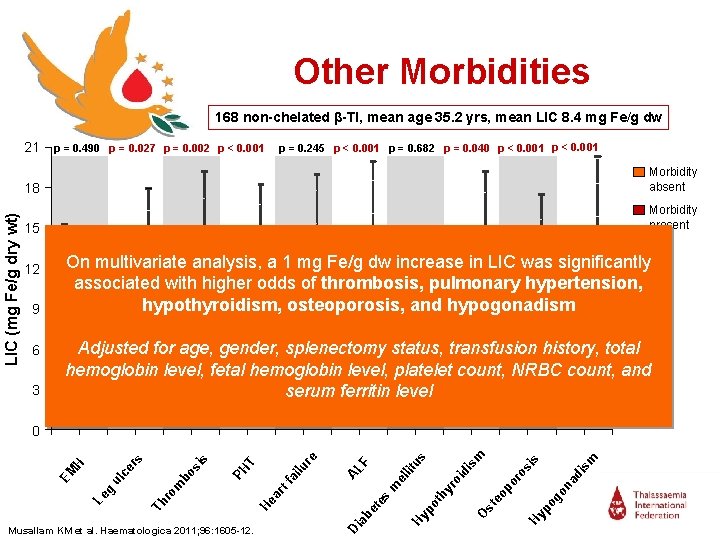
Other Morbidities 168 non-chelated β-TI, mean age 35. 2 yrs, mean LIC 8. 4 mg Fe/g dw LIC (mg Fe/g dry wt) 21 p = 0. 245 p < 0. 001 p = 0. 682 p = 0. 040 p < 0. 001 p = 0. 490 p = 0. 027 p = 0. 002 p < 0. 001 18 Morbidity absent 15 Morbidity present 12 9 6 3 On multivariate analysis, a 1 mg Fe/g dw increase in LIC was significantly associated with higher odds of thrombosis, pulmonary hypertension, hypothyroidism, osteoporosis, and hypogonadism Adjusted for age, gender, splenectomy status, transfusion history, total hemoglobin level, fetal hemoglobin level, platelet count, NRBC count, and serum ferritin level m s is si na d ro Hy po go po O st yr th po Hy eo oi di lit el m Di ab et es ar sm us F tf ai AL re lu T Musallam KM et al. Haematologica 2011; 96: 1605 -12. He bo Th ro m PH rs Le g ul ce H EM si s 0

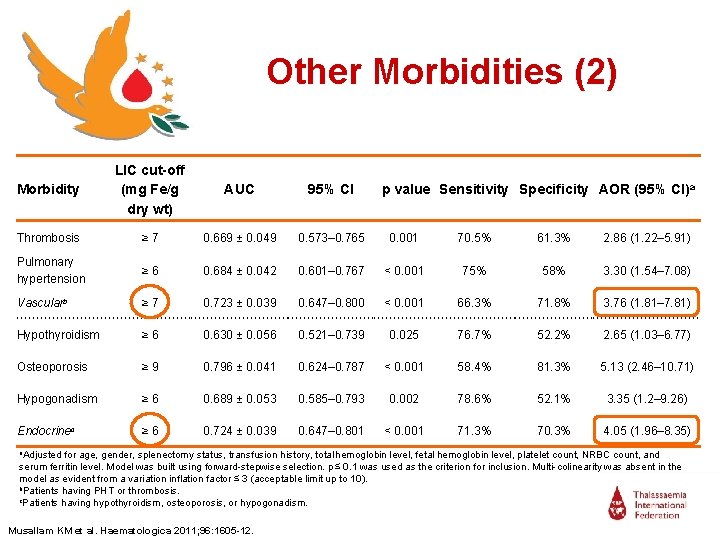
Other Morbidities (2) Morbidity LIC cut-off (mg Fe/g dry wt) AUC 95% CI Thrombosis ≥ 7 0. 669 ± 0. 049 0. 573– 0. 765 0. 001 70. 5% 61. 3% 2. 86 (1. 22– 5. 91) Pulmonary hypertension ≥ 6 0. 684 ± 0. 042 0. 601– 0. 767 < 0. 001 75% 58% 3. 30 (1. 54– 7. 08) Vascularb ≥ 7 0. 723 ± 0. 039 0. 647– 0. 800 < 0. 001 66. 3% 71. 8% 3. 76 (1. 81– 7. 81) Hypothyroidism ≥ 6 0. 630 ± 0. 056 0. 521– 0. 739 0. 025 76. 7% 52. 2% 2. 65 (1. 03– 6. 77) Osteoporosis ≥ 9 0. 796 ± 0. 041 0. 624– 0. 787 < 0. 001 58. 4% 81. 3% 5. 13 (2. 46– 10. 71) Hypogonadism ≥ 6 0. 689 ± 0. 053 0. 585– 0. 793 0. 002 78. 6% 52. 1% 3. 35 (1. 2– 9. 26) Endocrinec ≥ 6 0. 724 ± 0. 039 0. 647– 0. 801 < 0. 001 71. 3% 70. 3% 4. 05 (1. 96– 8. 35) a. Adjusted p value Sensitivity Specificity AOR (95% CI)a for age, gender, splenectomy status, transfusion history, total hemoglobin level, fetal hemoglobin level, platelet count, NRBC count, and serum ferritin level. Model was built using forward-stepwise selection. p ≤ 0. 1 was used as the criterion for inclusion. Multi-colinearity was absent in the model as evident from a variation inflation factor ≤ 3 (acceptable limit up to 10). b. Patients having PHT or thrombosis. c. Patients having hypothyroidism, osteoporosis, or hypogonadism. Musallam KM et al. Haematologica 2011; 96: 1605 -12.

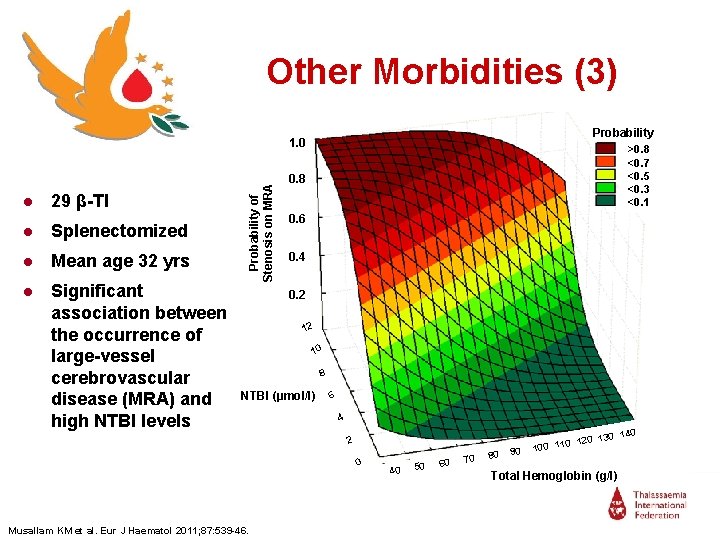
Other Morbidities (3) Probability ● 29 β-TI ● Splenectomized ● Mean age 32 yrs ● Significant association between the occurrence of large-vessel cerebrovascular disease (MRA) and high NTBI levels Probability of Stenosis on MRA 1. 0 >0. 8 <0. 7 <0. 5 <0. 3 <0. 1 0. 8 0. 6 0. 4 0. 2 12 12 0 1 10 88 NTBI (µmol/l) 66 44 22 00 Musallam KM et al. Eur J Haematol 2011; 87: 539 -46. 40 50 60 70 80 90 0 130 14 0 120 100 11 Total Hemoglobin (g/l)

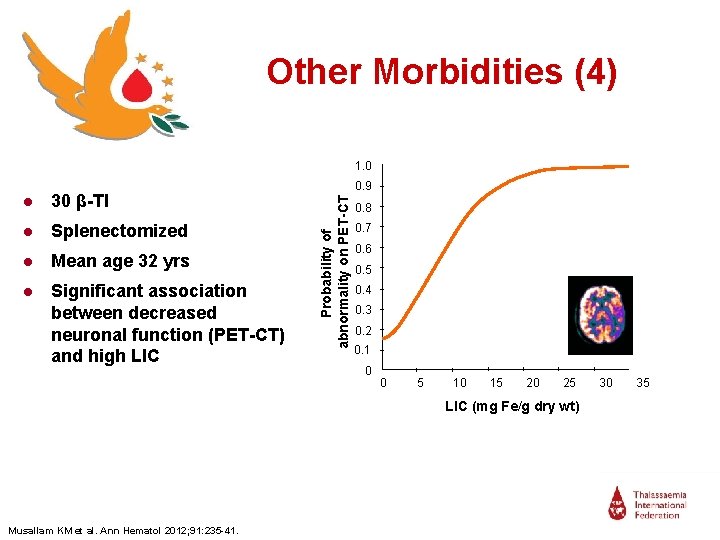
Other Morbidities (4) 0. 6 I 0. 5 I 0. 4 I 0. 3 I 0. 2 I 0. 1 I ● Significant association between decreased neuronal function (PET-CT) and high LIC 0. 7 I ● Mean age 32 yrs 0. 8 I ● Splenectomized 0. 9 I ● 30 β-TI Probability of abnormality on PET-CT 1. 0 0 0 I I 5 10 I I 15 I 20 I 25 LIC (mg Fe/g dry wt) Musallam KM et al. Ann Hematol 2012; 91: 235 -41. 30 35

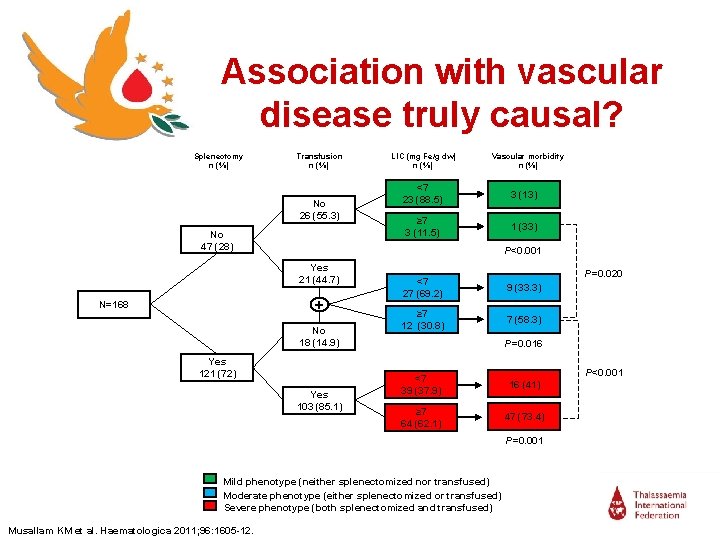
Association with vascular disease truly causal? Splenectomy n (%) Transfusion n (%) No 26 (55. 3) No 47 (28) Vascular morbidity n (%) <7 23 (88. 5) 3 (13) ≥ 7 3 (11. 5) 1 (33) P<0. 001 Yes 21 (44. 7) + N=168 LIC (mg Fe/g dw) n (%) No 18 (14. 9) Yes 121 (72) Yes 103 (85. 1) P=0. 020 <7 27 (69. 2) 9 (33. 3) ≥ 7 12 (30. 8) 7 (58. 3) P=0. 016 <7 39 (37. 9) 16 (41) ≥ 7 64 (62. 1) 47 (73. 4) P=0. 001 Mild phenotype (neither splenectomized nor transfused) Moderate phenotype (either splenectomized or transfused) Severe phenotype (both splenectomized and transfused) Musallam KM et al. Haematologica 2011; 96: 1605 -12. P<0. 001

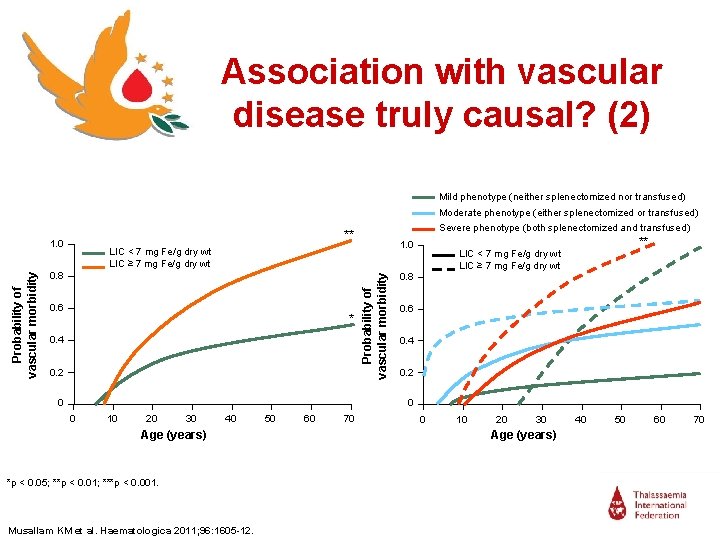
Association with vascular disease truly causal? (2) Mild phenotype (neither splenectomized nor transfused) Moderate phenotype (either splenectomized or transfused) 1. 0 LIC < 7 mg Fe/g dry wt LIC ≥ 7 mg Fe/g dry wt 0. 8 0. 6 * 0. 4 0. 2 0 Probability of vascular morbidity Severe phenotype (both splenectomized and transfused) ** ** LIC < 7 mg Fe/g dry wt LIC ≥ 7 mg Fe/g dry wt 0. 8 0. 6 0. 4 0. 2 0 0 10 20 30 40 Age (years) *p < 0. 05; **p < 0. 01; ***p < 0. 001. Musallam KM et al. Haematologica 2011; 96: 1605 -12. 50 60 70 0 10 20 30 Age (years) 40 50 60 70

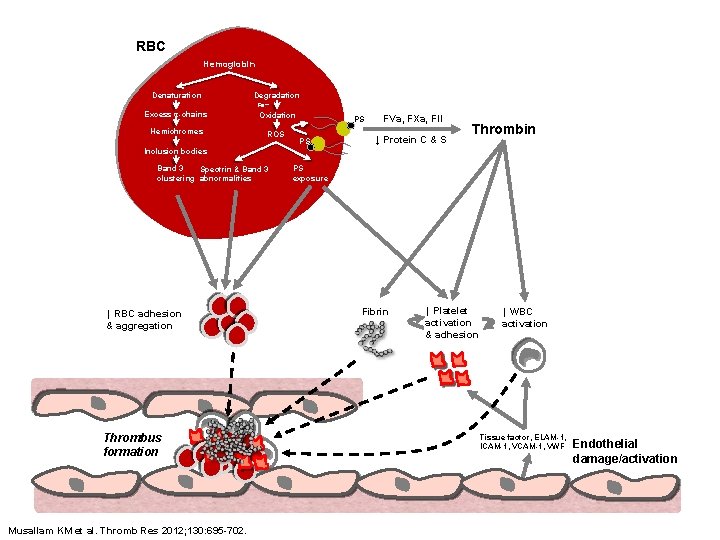
RBC Hemoglobin Denaturation Degradation Fe++ Excess α-chains Oxidation Hemichromes ROS PS PS FVa, FXa, FII ↓ Protein C & S Thrombin Inclusion bodies Band 3 Spectrin & Band 3 clustering abnormalities ↑ RBC adhesion & aggregation Thrombus formation Musallam KM et al. Thromb Res 2012; 130: 695 -702. PS exposure Fibrin ↑ Platelet activation & adhesion ↑ WBC activation Tissue factor, ELAM-1, ICAM-1, VWF Endothelial damage/activation

Assessment of Iron Overload ● Serum ferritin ● Liver iron concentration ● SQUID ● MRI ● Biopsy ● Cardiac MRI? ● Other markers (NTBI, Transferrin Sat)? Musallam KM et al. Blood Rev 2012; 26: 16 -9.

Spot Serum Ferritin Measurement ● Caution with interpreting spot serum ferritin values to tailor iron chelation therapy in NTDT ● The 1000 and 2500 ng/ml thresholds are used in thalassemia major patients as they predict survival and cardiac outcomes -> less relevant in NTDT and no similar predictive assessment exists ● Although serum ferritin correlates with LIC in NTDT 1 -4, the ratio of serum ferritin to LIC is lower relative to patients with β-thalassemia major 1, 4 -6 1. 2. 3. 4. 5. 6. Taher A et al. Haematologica 2008; 93: 1584 -6. Lal A et al. N Engl J Med 2011; 364: 710 -8. Taher AT et al. Blood 2012; 120: 970 -7. Pakbaz et al. Pediatr Blood Cancer 2007; 49: 329 -32. Origa R et al. Haematologica 2007; 92: 583 -8. Taher AT et al. Am J Hematol 2010; 85: 288 -90.

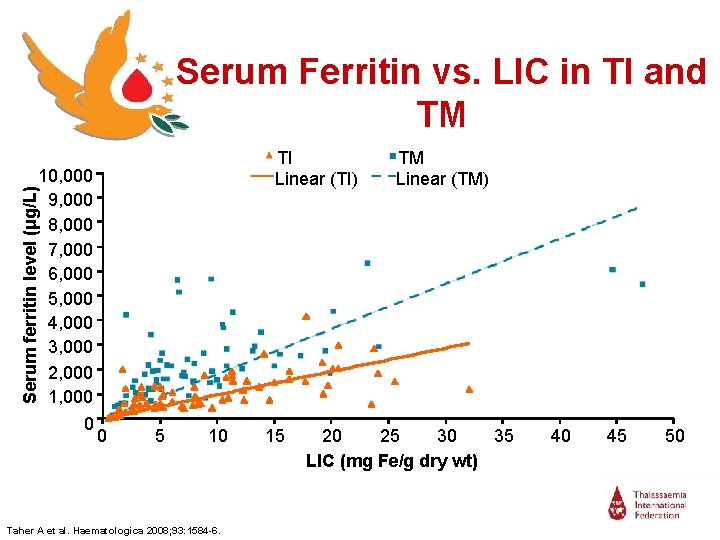
Serum Ferritin vs. LIC in TI and TM Serum ferritin level (μg/L) 10, 000 9, 000 8, 000 7, 000 6, 000 5, 000 4, 000 3, 000 2, 000 1, 000 0 TI Linear (TI) 0 5 10 Taher A et al. Haematologica 2008; 93: 1584 -6. 15 TM Linear (TM) 20 25 30 35 LIC (mg Fe/g dry wt) 40 45 50

Conclusions ● NTDT patients show considerable iron overload despite their transfusion-independence ● Ineffective erythropoiesis leading to hepcidin suppression and increased intestinal iron absorption is the primary implicated mechanism ● Iron overload in this patient population is associated with morbidities involving several organs and organ systems ● Timely detection is warranted, and caution regarding serum ferritin level interpretation is essential
 Mass of iron in an iron tablet
Mass of iron in an iron tablet Iron sharpens iron friendship
Iron sharpens iron friendship Cisco nat overload
Cisco nat overload Double pump hydraulic system
Double pump hydraulic system Information overload
Information overload Thermal overload relay electrical symbol
Thermal overload relay electrical symbol Hr department structure
Hr department structure C++ operator bracket
C++ operator bracket Information overload syndrome
Information overload syndrome Progressive overload
Progressive overload Information overload research group
Information overload research group Barker's ecological theory of environmental psychology
Barker's ecological theory of environmental psychology Contactor symbol electrical
Contactor symbol electrical Determinants of prosocial behaviour
Determinants of prosocial behaviour Anne pemberton uncw death
Anne pemberton uncw death Prinsip overload adalah
Prinsip overload adalah Too many transfer stations are barriers in
Too many transfer stations are barriers in Udp header
Udp header Electrical symbol for thermostat
Electrical symbol for thermostat Overload stream insertion operator c++ template
Overload stream insertion operator c++ template C++ overload arrow operator
C++ overload arrow operator Information overload
Information overload Progressive overload principle
Progressive overload principle