Iridium Ruthenium and Osmium in Gold Jewellery 13














- Slides: 34

Iridium, Ruthenium and Osmium in Gold Jewellery 13 th August 2016 An MKS PAMP Group Company

Objectives An MKS PAMP Group Company 2

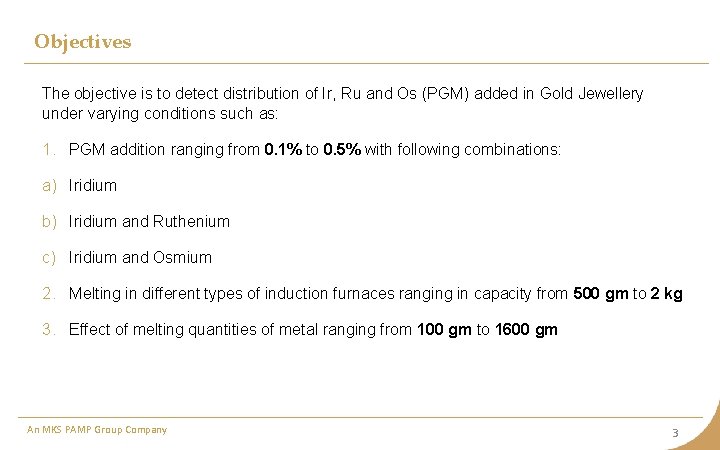
Objectives The objective is to detect distribution of Ir, Ru and Os (PGM) added in Gold Jewellery under varying conditions such as: 1. PGM addition ranging from 0. 1% to 0. 5% with following combinations: a) Iridium b) Iridium and Ruthenium c) Iridium and Osmium 2. Melting in different types of induction furnaces ranging in capacity from 500 gm to 2 kg 3. Effect of melting quantities of metal ranging from 100 gm to 1600 gm An MKS PAMP Group Company 3

Advantage-Disadvantage of adding Iridium, Ruthenium and Osmium in gold alloys/ Jewellery An MKS PAMP Group Company 4

Advantage and Disadvantage Advantage Use in Jewellery fabrication • The process of Jewellery fabrication by cold working involves multiple, intermediate annealing. • The purpose of which is to restore alloy ductility by recrystallization of the workhardened structure. • Annealing leads to coarse grained structure and addition of 0. 01 to 0. 1% iridium, ruthenium or osmium restricts grain growth. The finer the grains higher is ductility. • Out of 3 metals, iridium gives strong and reproducible grain refinement of the as-cast structure even with multiple processing and so Iridium is predominantly used for the purpose. An MKS PAMP Group Company 5

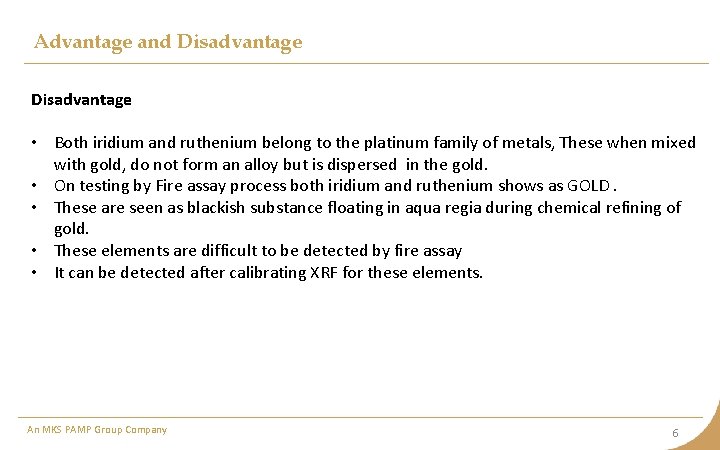
Advantage and Disadvantage • Both iridium and ruthenium belong to the platinum family of metals, These when mixed with gold, do not form an alloy but is dispersed in the gold. • On testing by Fire assay process both iridium and ruthenium shows as GOLD. • These are seen as blackish substance floating in aqua regia during chemical refining of gold. • These elements are difficult to be detected by fire assay • It can be detected after calibrating XRF for these elements. An MKS PAMP Group Company 6

Properties of Ir, Ru , Os An MKS PAMP Group Company 7

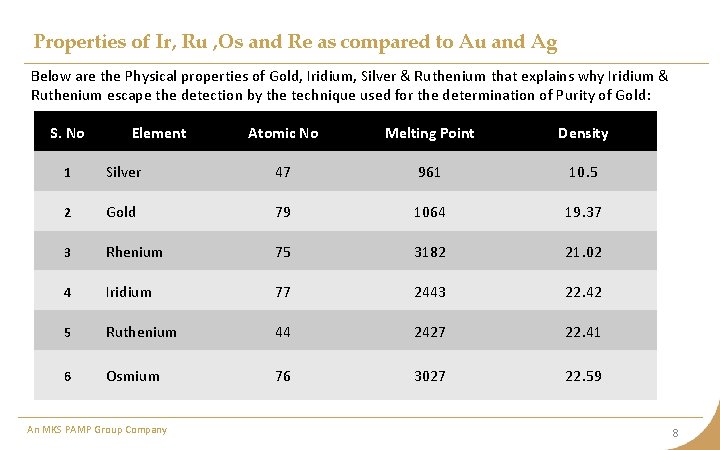
Properties of Ir, Ru , Os and Re as compared to Au and Ag Below are the Physical properties of Gold, Iridium, Silver & Ruthenium that explains why Iridium & Ruthenium escape the detection by the technique used for the determination of Purity of Gold: S. No Element Atomic No Melting Point Density 1 Silver 47 961 10. 5 2 Gold 79 1064 19. 37 3 Rhenium 75 3182 21. 02 4 Iridium 77 2443 22. 42 5 Ruthenium 44 2427 22. 41 6 Osmium 76 3027 22. 59 An MKS PAMP Group Company 8

Properties of Ir, Ru , Os and PGM The platinum group of metals namely Ir, Ru, Os and Re has the following salient features; • Very high melting temperature (>2000 ºC) compared to gold and hence does not melt when gold is melted ( MP 1063 ºC). • Very low solubility in gold and never goes into solid solution (alloying). - Floats as black particles on molten metal surface while melting and can be observed visually. • No reaction with Aqua Regia – remains unreacted during Aqua Regia treatment or during fire assay. • Higher specific gravity than gold An MKS PAMP Group Company 9

X-RAY vs FIRE ASSAY Salient points of both techniques An MKS PAMP Group Company 10

X-RAY vs FIRE ASSAY X-ray Fluorescence Spectrometry (XRF) • Well suited technique for determination of trace refractory elements as assays are carried out directly on the solid sample and do not rely on acid digestion. • When evaluating XRF results it is essential to distinguish between accuracy and precision. The precision of the XRF technique is normally very high and is demonstrated by evaluation of replicate results. • Accuracy on the other hand depends heavily on two factors: the attenuation-enhancement correction procedure used to correct matrix effects caused by concomitant elements, and calibration procedures. • Laboratories analyzing samples with variable matrices require a high skilled professional along with proficiency in the XRF technique to produce accurate data. • XRF Spectrometer can be used to check/detect the presence of Iridium and Ruthenium impurities in Gold Alloys. The resolution of Semi-conductor Detector is 4 times (approx. ) of the Gas-filled Proportional counter, and is thus able to resolve /separate the peaks of interest. An MKS PAMP Group Company 11

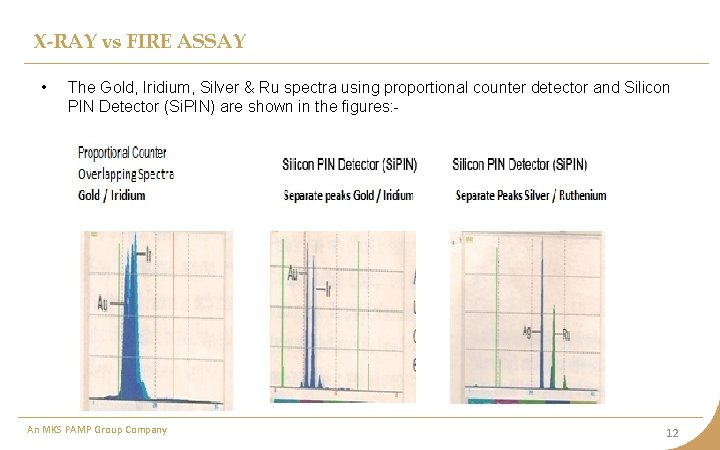
X-RAY vs FIRE ASSAY • The Gold, Iridium, Silver & Ru spectra using proportional counter detector and Silicon PIN Detector (Si. PIN) are shown in the figures: - An MKS PAMP Group Company 12

X-RAY vs FIRE ASSAY Fire assay • Fire assaying is used for the determination of gold, silver and PGM in all types of materials, ranging from bullion, jewelry and ores to concentrates and electronic scrap. • This process leaves the precious metal bead on the cupel, which is weighed accurately to obtain total precious metal weight. This bead is then treated further using nitric acid to determine the metals in the bead, usually silver and gold (gravimetrically), platinum and palladium (ICP). • Due to their high melting points, the detection of Iridium & Ruthenium by Fire Assay method is difficult as most of the Labs have furnace up to 1150 °C coupled with the fact that when mixed with Gold, both Iridium & Ruthenium do not form an alloy but is dispersed in the Yellow metal and are available in scattered form as fine particles. An MKS PAMP Group Company 13

X-RAY vs FIRE ASSAY Fire assay • By visual inspection of the cornet after the fire assay process, the presence of Iridium & Ruthenium may be found as tiny black particles (as shown in figures of cornets) by skilled/experienced assayers. • This is only qualitative detection and in order to ascertain the exact % of PGM the cornet has to be dissolved in Aqua regia and the impurities separated out. An MKS PAMP Group Company 14

X-RAY vs FIRE ASSAY Why XRF? Why Not Fire Assay? • Fire Assay gives only qualitative evaluation for PGM and for Quantitative detection aqua regia process is required additionally. • In case of XRF after calibration the spectrometer gives accurate percentage of PGM with the tolerance level ± 0. 1% Sampling • For Fire assay sampling, drilling is done at few spots and then 0. 25 gm of drill is taken for analysis. The probability of detecting PGM thus gets limited. • XRF on other hand gives the purity at each spot and is not limited to only 0. 25 gm of drill chips, thereby increasing the probability of detection of these PGM which is very unevenly distributed in the ingot/sample. An MKS PAMP Group Company 15

Various trials to determine the presence of Ru, Ir and Os using XRF as testing method An MKS PAMP Group Company 16

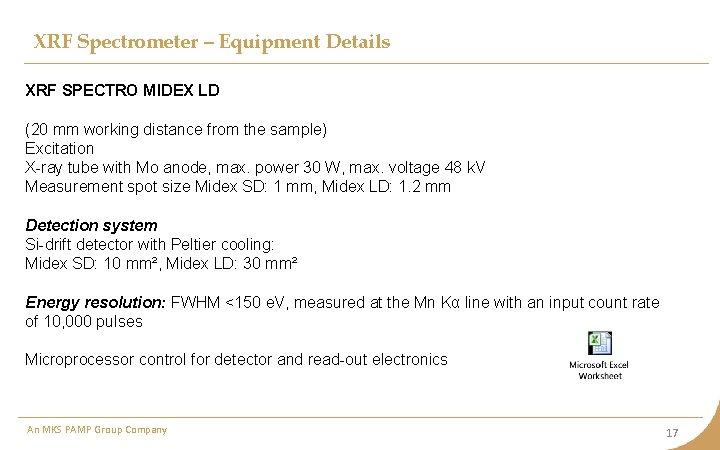
XRF Spectrometer – Equipment Details XRF SPECTRO MIDEX LD (20 mm working distance from the sample) Excitation X-ray tube with Mo anode, max. power 30 W, max. voltage 48 k. V Measurement spot size Midex SD: 1 mm, Midex LD: 1. 2 mm Detection system Si-drift detector with Peltier cooling: Midex SD: 10 mm², Midex LD: 30 mm² Energy resolution: FWHM <150 e. V, measured at the Mn Kα line with an input count rate of 10, 000 pulses Microprocessor control for detector and read-out electronics An MKS PAMP Group Company 17

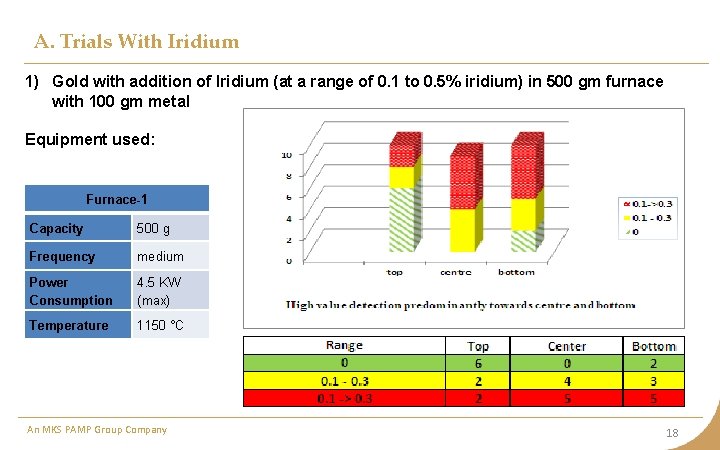
A. Trials With Iridium 1) Gold with addition of Iridium (at a range of 0. 1 to 0. 5% iridium) in 500 gm furnace with 100 gm metal Equipment used: Furnace-1 Capacity 500 g Frequency medium Power Consumption 4. 5 KW (max) Temperature 1150 °C An MKS PAMP Group Company 18

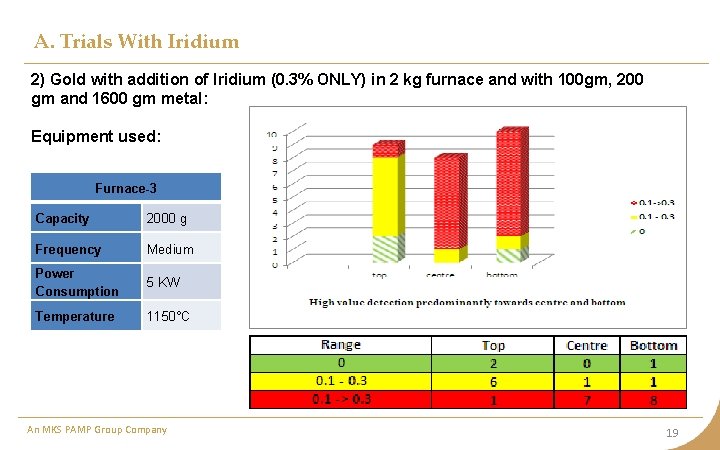
A. Trials With Iridium 2) Gold with addition of Iridium (0. 3% ONLY) in 2 kg furnace and with 100 gm, 200 gm and 1600 gm metal: Equipment used: Furnace-3 Capacity 2000 g Frequency Medium Power Consumption 5 KW Temperature 1150°C An MKS PAMP Group Company 19

A. Trials With Iridium Observation • With only Iridium the distribution pattern is from center to bottom. However, in all cases, the range of detection is seen to vary without any predictable pattern and deviating from the actual addition. • A qualitative vs quantitative comparison shows that it is relatively easier to predict the location with more reliability than the quantity. Inference : Hence, the rational approach to minimize the chances of error in detection of Iridium in karat gold , is to draw a sampling plan spreading predominantly at center and bottom at multiple location. An MKS PAMP Group Company 20

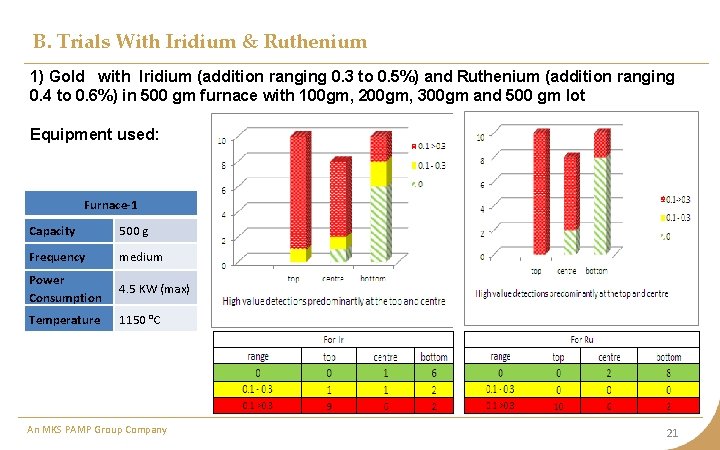
B. Trials With Iridium & Ruthenium 1) Gold with Iridium (addition ranging 0. 3 to 0. 5%) and Ruthenium (addition ranging 0. 4 to 0. 6%) in 500 gm furnace with 100 gm, 200 gm, 300 gm and 500 gm lot Equipment used: Furnace-1 Capacity 500 g Frequency medium Power Consumption 4. 5 KW (max) Temperature 1150 °C An MKS PAMP Group Company 21

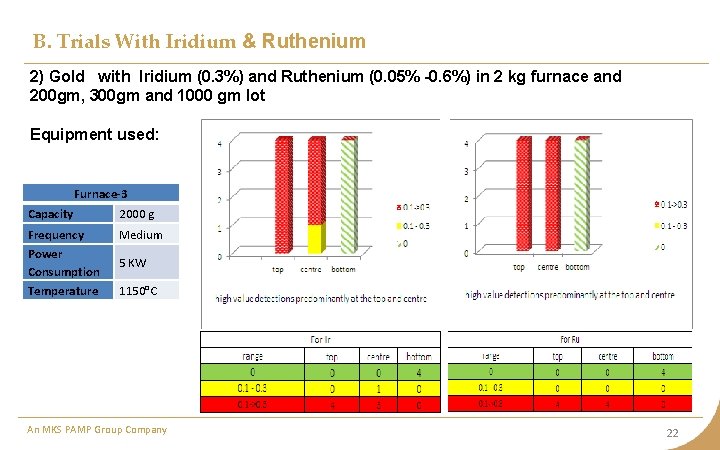
B. Trials With Iridium & Ruthenium 2) Gold with Iridium (0. 3%) and Ruthenium (0. 05% -0. 6%) in 2 kg furnace and 200 gm, 300 gm and 1000 gm lot Equipment used: Furnace-3 Capacity 2000 g Frequency Power Consumption Temperature Medium 5 KW 1150°C An MKS PAMP Group Company 22

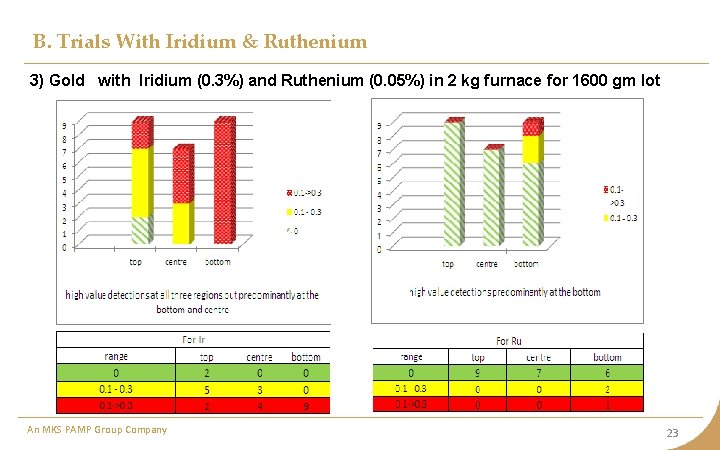
B. Trials With Iridium & Ruthenium 3) Gold with Iridium (0. 3%) and Ruthenium (0. 05%) in 2 kg furnace for 1600 gm lot An MKS PAMP Group Company 23

B. Trials With Iridium & Ruthenium Observation • With addition of Ruthenium, the distribution seems to reverse in comparison to previous case when only Iridium was added. In this case, ruthenium being lighter, is not allowing Iridium to settle towards bottom. • In case of large quantity (> 1600 gm), the bar thickness increases due to which the impurities do not get time to migrate upwards and freezing towards bottom • The quantity at various places is still very unpredictable. Inference The sampling spots should be more at top and center compared to bottom for metal quantity up to 1000 gm. An MKS PAMP Group Company 24

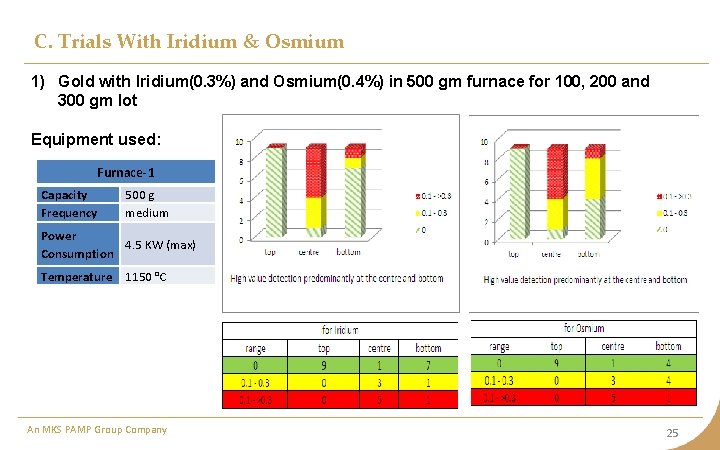
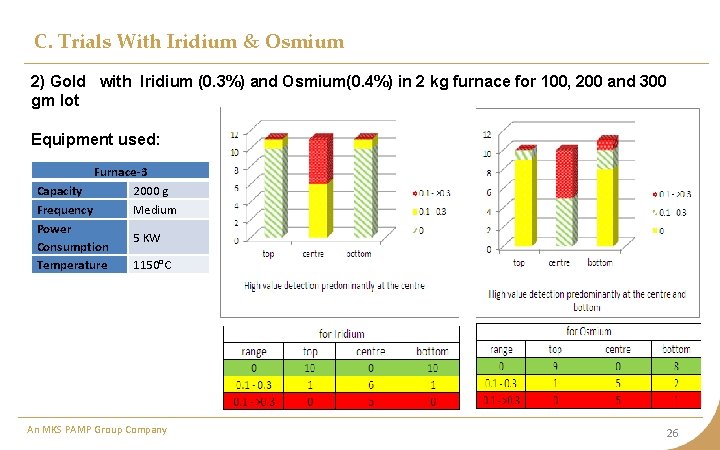
C. Trials With Iridium & Osmium 1) Gold with Iridium(0. 3%) and Osmium(0. 4%) in 500 gm furnace for 100, 200 and 300 gm lot Equipment used: Furnace-1 Capacity Frequency 500 g medium Power 4. 5 KW (max) Consumption Temperature 1150 °C An MKS PAMP Group Company 25

C. Trials With Iridium & Osmium 2) Gold with Iridium (0. 3%) and Osmium(0. 4%) in 2 kg furnace for 100, 200 and 300 gm lot Equipment used: Furnace-3 Capacity 2000 g Frequency Medium Power 5 KW Consumption Temperature 1150°C An MKS PAMP Group Company 26

C. Trials With Iridium & Osmium Observation • Iridium and Osmium being heavier settles at the bottom. • The possibility of detecting Iridium and Osmium quantity is still very un-predictable. • Osmium is surprisingly getting undetected in most of the cases. • Osmium is highly volatile and form tetraoxide at 800 C. • Osmium combines with Iridium to form Osmoiridium Inference Predominantly detection at bottom with some points at center An MKS PAMP Group Company 27

Conclusion An MKS PAMP Group Company 28

Conclusion XRF Spectrometric analysis – Need of the hour • Qualitatively or quantitatively it is not possible to catch or detect the presence of Iridium, Ruthenium and Osmium (being volatile forms tetraoxide) in Fire Assay method. The possibility of the catching these elements in sampling of Fire assay is very low. • Fire assay process, it required highly skilled assayer to identify the presence of these elements in cupellation or on beads or on the cornets. • One experienced and skilled assayer only can really identify the presence of these elements in cupellation or on beads or on cornet! But even after this, its tedious job to find out the concentration of these elements in sample by using chemical digestion and spectroscopic analysis! XRF spectrometric analysis is the solution to all the above points ! • All the analysis in the previous slides were conducted on XRF An MKS PAMP Group Company 29

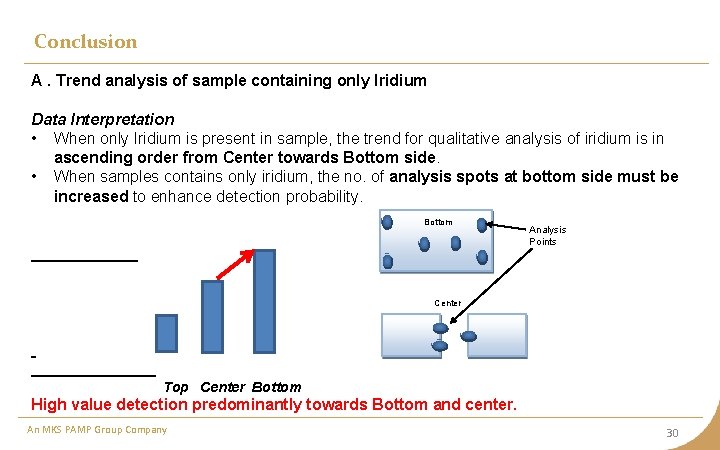
Conclusion A. Trend analysis of sample containing only Iridium Data Interpretation • When only Iridium is present in sample, the trend for qualitative analysis of iridium is in ascending order from Center towards Bottom side. • When samples contains only iridium, the no. of analysis spots at bottom side must be increased to enhance detection probability. Bottom Analysis Points Center Top Center Bottom High value detection predominantly towards Bottom and center. An MKS PAMP Group Company 30

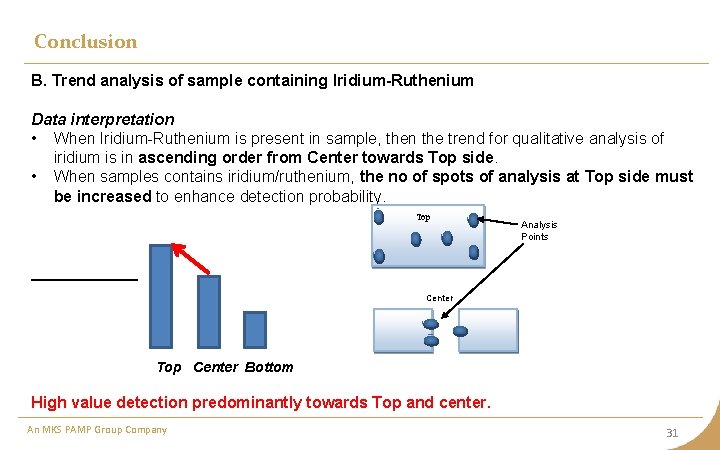
Conclusion B. Trend analysis of sample containing Iridium-Ruthenium Data interpretation • When Iridium-Ruthenium is present in sample, then the trend for qualitative analysis of iridium is in ascending order from Center towards Top side. • When samples contains iridium/ruthenium, the no of spots of analysis at Top side must be increased to enhance detection probability. Top Analysis Points Center Top Center Bottom High value detection predominantly towards Top and center. An MKS PAMP Group Company 31

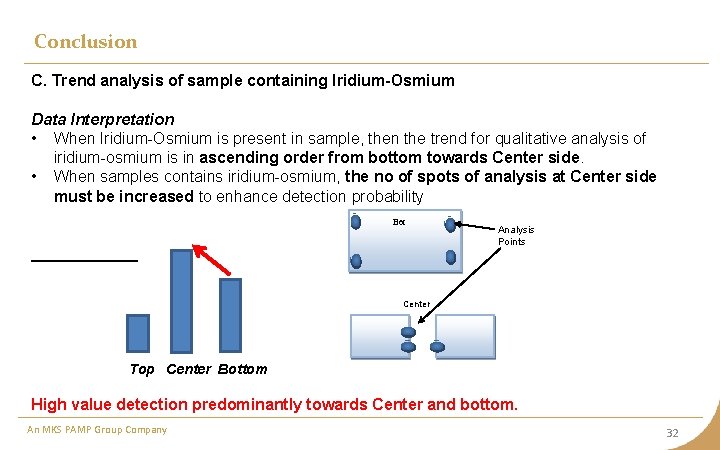
Conclusion C. Trend analysis of sample containing Iridium-Osmium Data Interpretation • When Iridium-Osmium is present in sample, then the trend for qualitative analysis of iridium-osmium is in ascending order from bottom towards Center side. • When samples contains iridium-osmium, the no of spots of analysis at Center side must be increased to enhance detection probability Bot Analysis Points Center Top Center Bottom High value detection predominantly towards Center and bottom. An MKS PAMP Group Company 32

Reasearch team Conceptualization of the paper Ankur Goyal : Metallurgist Debasish Bhatacharjee : Metallurgist Pankaj Deshmukh : Analytical chemistry Melting and Analysis by XRF Jaideep : Chemist Compilation of the data Praveen Kumar – Chemical Engineer Special Thanks to Mr Rajesh Khosla to have guided us for this Research An MKS PAMP Group Company 33

THANK YOU 34
 Osmium jewellery
Osmium jewellery Rhodium ruthenium
Rhodium ruthenium Ruthenium
Ruthenium Chmia
Chmia Icg iridium satcom
Icg iridium satcom Iridium layer
Iridium layer Caso iridium
Caso iridium Copyright
Copyright Iridium project failure
Iridium project failure Iridium(iii) nitride formula
Iridium(iii) nitride formula Intmit
Intmit Iridium kankernetwerk
Iridium kankernetwerk Iridium
Iridium Iridium ptt command center
Iridium ptt command center Silver electron configuration
Silver electron configuration Bridal jewellery west delhi
Bridal jewellery west delhi Jewellery accounting entries
Jewellery accounting entries Peter chang biography
Peter chang biography Digital jewellery ring
Digital jewellery ring Jewellery module in tally
Jewellery module in tally Alamkara
Alamkara Rene lalique jewellery
Rene lalique jewellery Digital jewellery earrings
Digital jewellery earrings Nora fok jewellery
Nora fok jewellery Ffa sentinel symbol
Ffa sentinel symbol Ffa emblem parts
Ffa emblem parts Salt and gold trade in west africa
Salt and gold trade in west africa When did the gold rush start and end in australia
When did the gold rush start and end in australia Salt and gold trade in west africa
Salt and gold trade in west africa For spain for glory for gold
For spain for glory for gold Facebook account hacked
Facebook account hacked Gold glory and god
Gold glory and god Fsu garnet and gold scholar society
Fsu garnet and gold scholar society Ffa study guide
Ffa study guide The ffa colors
The ffa colors