Early tumor shrinkage ETS and depth of response









- Slides: 34

Early tumor shrinkage (ETS) and depth of response (Dp. R) to anti-EGFR in (m)CRC helpful surrogates or meaningless endpoints? Marc Peeters MD, Ph. D Coordinator Multidisciplinary Oncological Center Antwerpen (MOCA) Head of the Oncology Department UZA, Professor in Oncology UA

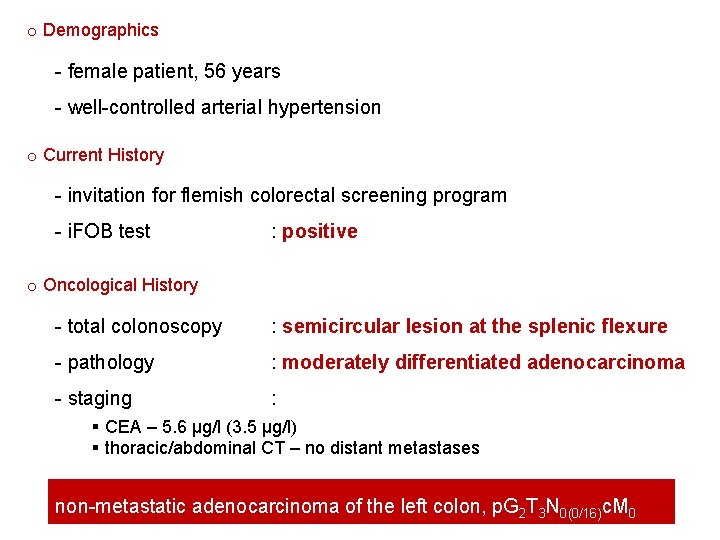
o Demographics - female patient, 56 years - well-controlled arterial hypertension o Current History - invitation for flemish colorectal screening program - i. FOB test : positive o Oncological History - total colonoscopy : semicircular lesion at the splenic flexure - pathology : moderately differentiated adenocarcinoma - staging : § CEA – 5. 6 µg/l (3. 5 µg/l) § thoracic/abdominal CT – no distant metastases non-metastatic adenocarcinoma of the left colon, p. G 2 T 3 N 0(0/16)c. M 0

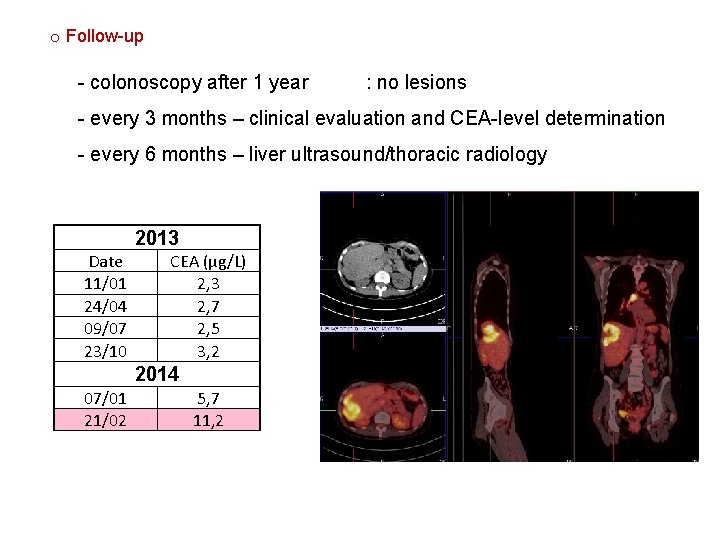
o Follow-up - colonoscopy after 1 year : no lesions - every 3 months – clinical evaluation and CEA-level determination - every 6 months – liver ultrasound/thoracic radiology 2013 Date 11/01 24/04 09/07 23/10 CEA (µg/L) 2, 3 2, 7 2, 5 3, 2 2014 07/01 21/02 5, 7 11, 2

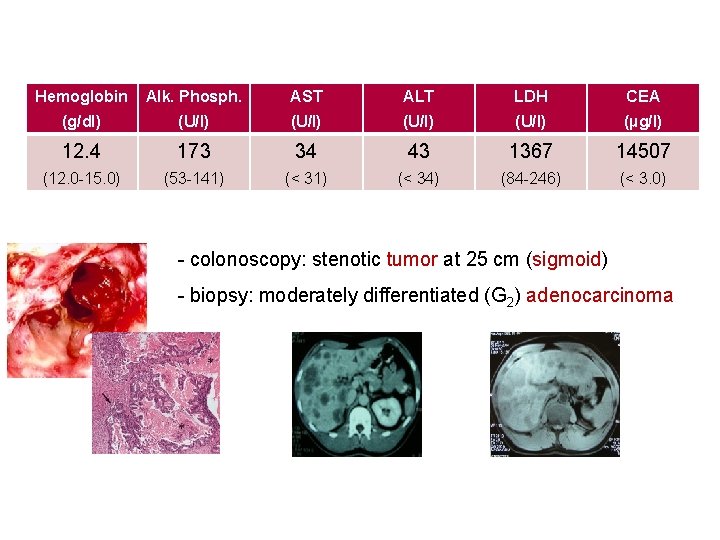
Hemoglobin Alk. Phosph. AST ALT LDH CEA (g/dl) (U/l) (µg/l) 12. 4 173 34 43 1367 14507 (12. 0 -15. 0) (53 -141) (< 34) (84 -246) (< 3. 0) - colonoscopy: stenotic tumor at 25 cm (sigmoid) - biopsy: moderately differentiated (G 2) adenocarcinoma

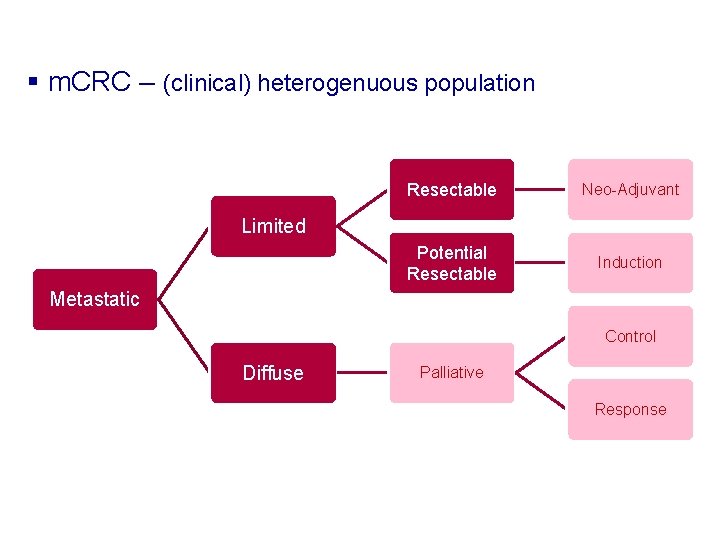
§ m. CRC – (clinical) heterogenuous population Resectable Neo-Adjuvant Potential Resectable Induction Limited Metastatic Control Diffuse Palliative Response

§ EGFR inhibitors in m. CRC – combination therapy Treatment PFS, mo OS, mo Chemo + bevacizumab 9. 4 -10. 6 20. 3 -21. 3 Chemo + anti-EGFR 7. 9 -11. 4 17. 0 -28. 4 FIRE-3 Chemo + bev vs Chemo + anti-EGFR 10. 2 10. 4 25. 6 33. 1 CALGB 80405 Chemo + bev vs Chemo + anti-EGFR 11. 3 11. 4 31. 2 32. 0 Adapted from Cremolini et al. Nat Rev Clin Oncol. 2015; doi: 10. 1038/nrclinonc. 2015. 129

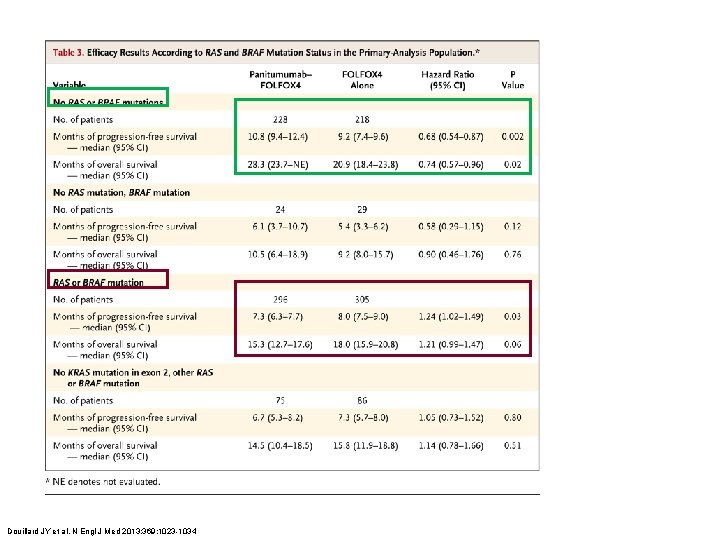
Douillard JY et al. N Engl J Med 2013; 369: 1023 -1034

§ Assessment of Efficacy in First line m. CRC - PFS • Influenced by toxicity of the regimen and size of the tumour lesion, favouring larger lesions - OS • Influenced by 2 nd and subsequent lines of treatment • With longer post-progression survival, the influence of further treatment lines becomes greater - ORR: • Influenced by RECIST measurements • Used as an endpoint in Phase 2 studies Slide courtesy of S. Stintzing


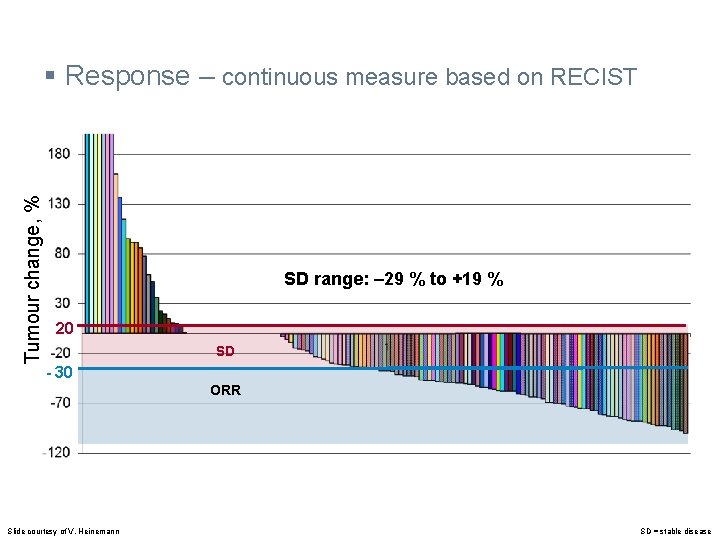
Tumour change, % § Response – continuous measure based on RECIST SD range: ‒ 29 % to +19 % 20 SD - 30 ORR Slide courtesy of V. Heinemann SD = stable disease

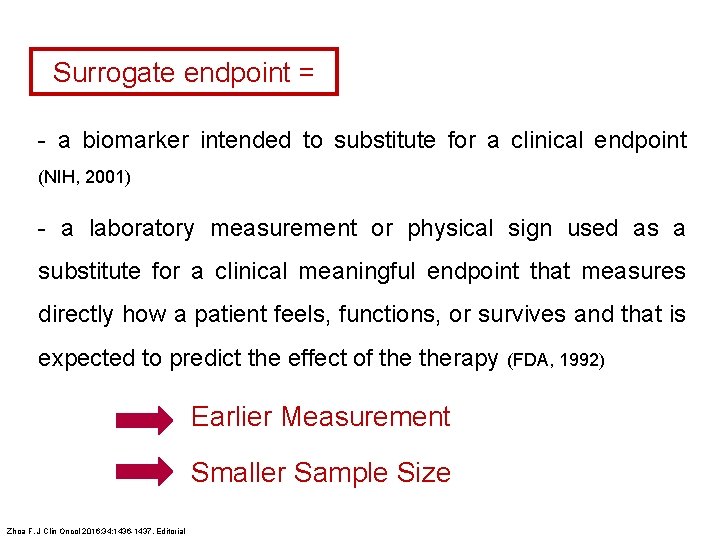
Surrogate endpoint = - a biomarker intended to substitute for a clinical endpoint (NIH, 2001) - a laboratory measurement or physical sign used as a substitute for a clinical meaningful endpoint that measures directly how a patient feels, functions, or survives and that is expected to predict the effect of therapy (FDA, 1992) Earlier Measurement Smaller Sample Size Zhoa F. J Clin Oncol 2016; 34: 1436 -1437. Editorial

§ ETS – % decrease in tumour load at a given time Baseline = 100% ETS = % decrease (8 weeks) 8 weeks With a 20% cut-off, this results in a categorical decision: Early tumour shrinkage (ETS) Non-early tumour shrinkage (non-ETS) Heinemann V et al. Eur J Cancer 2015; 51: 1927 -36 Time

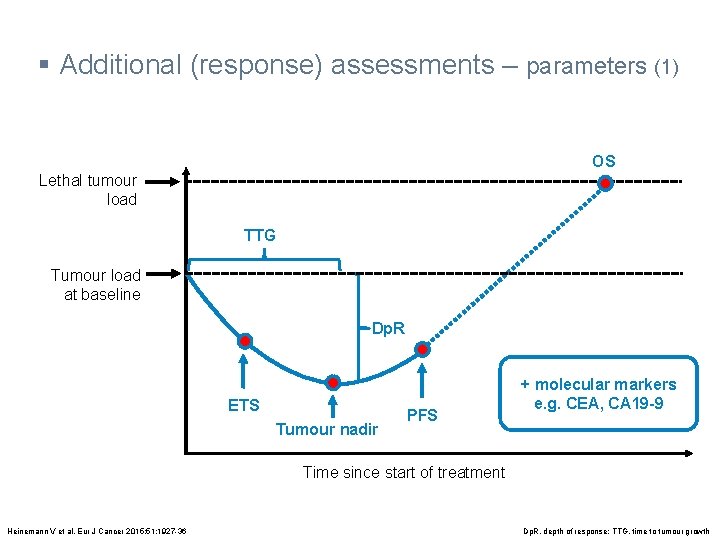
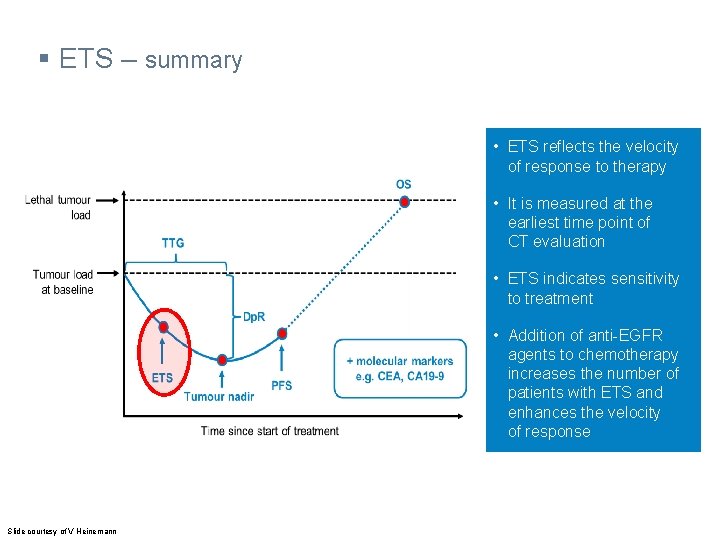
§ Additional (response) assessments – parameters (1) OS Lethal tumour load TTG Tumour load at baseline Dp. R ETS Tumour nadir PFS + molecular markers e. g. CEA, CA 19 -9 Time since start of treatment Heinemann V et al. Eur J Cancer 2015; 51: 1927 -36 Dp. R, depth of response; TTG, time to tumour growth

§ Additional (response) assessments – clinical (2) ETS: § Rapidly predicts sensitivity to treatment 1− 5 § Potential to relieve tumour-related symptoms 5 § May increase possibility of resection/chance of cure in some patients with initially unresectable metastases 5 ETS, Dp. R: § Potentially prognostic for PFS/OS 4− 6 1. Giessen C, et al. Cancer Sci 2013; 104: 718− 24; 2. Piessevaux H, et al. Ann Oncol 2009; 20: 1375– 82; 3. Piessevaux H, et al. J Clin Oncol 2013; 31: 3764− 75; 4. Stintzing S, et al. Ann Oncol 2014; 25(Suppl 5): v 1–v 41: abstract LBA 11 (and oral presentation); 5. Douillard JY, et al. Eur J Cancer 2015 ; 51: 1231− 42; 6. Petrelli F, et al. Eur J Cancer 2015; 51: 800− 7

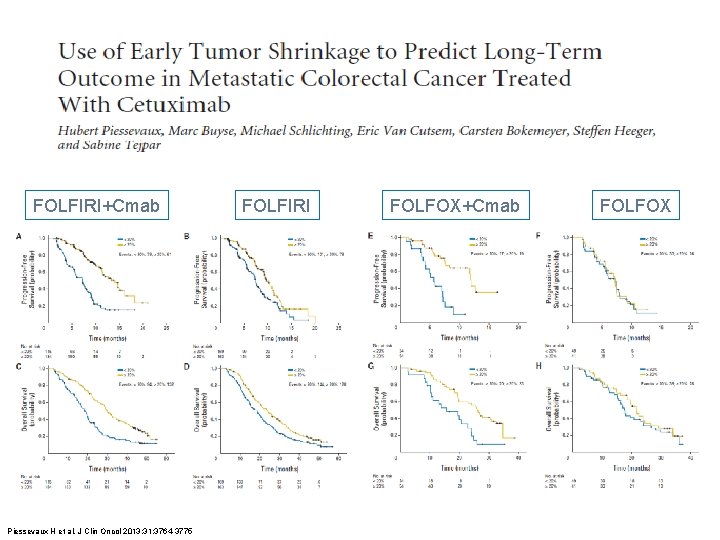
FOLFIRI+Cmab Piessevaux H et al. J Clin Oncol 2013; 31: 3764 -3775 FOLFIRI FOLFOX+Cmab FOLFOX

Piessevaux H et al. J Clin Oncol 2013; 31: 3764 -3775

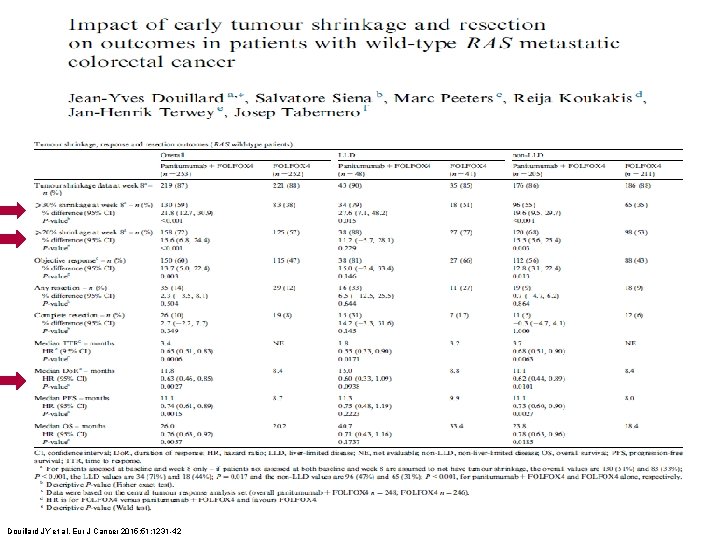
Douillard JY et al. Eur J Cancer 2015; 51: 1231 -42

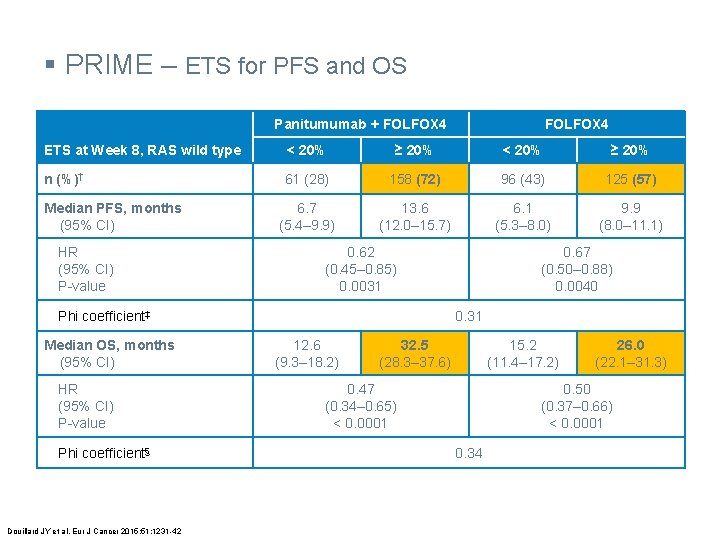
§ PRIME – ETS for PFS and OS Panitumumab + FOLFOX 4 ETS at Week 8, RAS wild type < 20% ≥ 20% n (%)† 61 (28) 158 (72) 96 (43) 125 (57) 6. 7 (5. 4– 9. 9) 13. 6 (12. 0– 15. 7) 6. 1 (5. 3– 8. 0) 9. 9 (8. 0– 11. 1) Median PFS, months (95% CI) HR (95% CI) P-value 0. 62 (0. 45– 0. 85) 0. 0031 Phi coefficient‡ Median OS, months (95% CI) HR (95% CI) P-value Phi coefficient§ Douillard JY et al. Eur J Cancer 2015; 51: 1231 -42 0. 67 (0. 50– 0. 88) 0. 0040 0. 31 12. 6 (9. 3– 18. 2) 32. 5 (28. 3– 37. 6) 15. 2 (11. 4– 17. 2) 0. 47 (0. 34– 0. 65) < 0. 0001 26. 0 (22. 1– 31. 3) 0. 50 (0. 37– 0. 66) < 0. 0001 0. 34

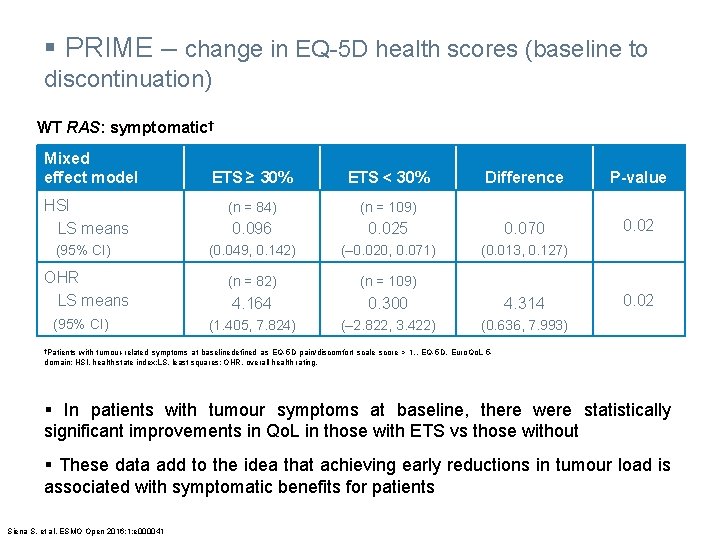
§ PRIME – change in EQ-5 D health scores (baseline to discontinuation) WT RAS: symptomatic† Mixed effect model Difference P-value 0. 025 0. 070 0. 02 (0. 049, 0. 142) (‒ 0. 020, 0. 071) (0. 013, 0. 127) (n = 82) (n = 109) 4. 164 0. 300 4. 314 (1. 405, 7. 824) (‒ 2. 822, 3. 422) (0. 636, 7. 993) ETS ≥ 30% ETS < 30% HSI LS means (n = 84) (n = 109) 0. 096 (95% CI) OHR LS means (95% CI) 0. 02 †Patients with tumour-related symptoms at baselinedefined as EQ-5 D pain/discomfort scale score > 1. . EQ-5 D, Euro. Qo. L 5 domain; HSI, health state index; LS, least squares; OHR, overall health rating. § In patients with tumour symptoms at baseline, there were statistically significant improvements in Qo. L in those with ETS vs those without § These data add to the idea that achieving early reductions in tumour load is associated with symptomatic benefits for patients Siena S, et al. ESMO Open 2016; 1: e 000041

§ PRIME – ETS, conclusions - Whole population vs. Individuals - ETS in symptomatic patients & in conversion Douillard JY et al. Eur J Cancer 2015; 51: 1231 -42

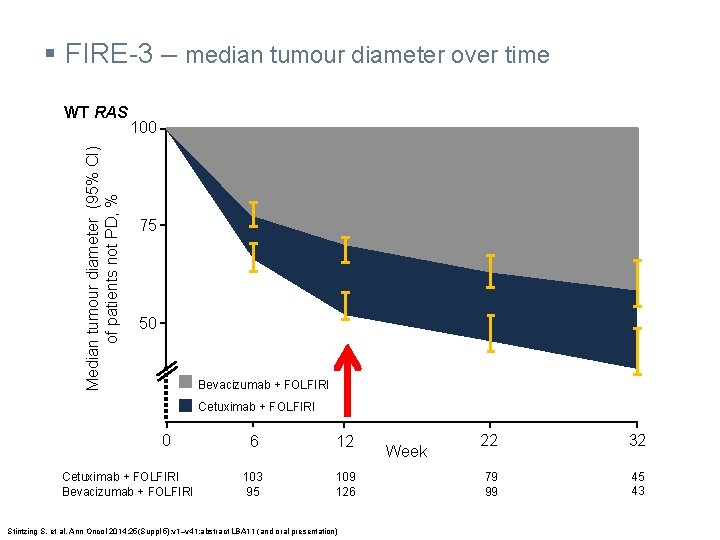
§ FIRE-3 – median tumour diameter over time Median tumour diameter (95% CI) of patients not PD, % WT RAS 100 75 50 Bevacizumab + FOLFIRI Cetuximab + FOLFIRI 0 Cetuximab + FOLFIRI Bevacizumab + FOLFIRI 6 12 103 95 109 126 Stintzing S, et al. Ann Oncol 2014; 25(Suppl 5): v 1–v 41: abstract LBA 11 (and oral presentation) Week 22 32 79 99 45 43

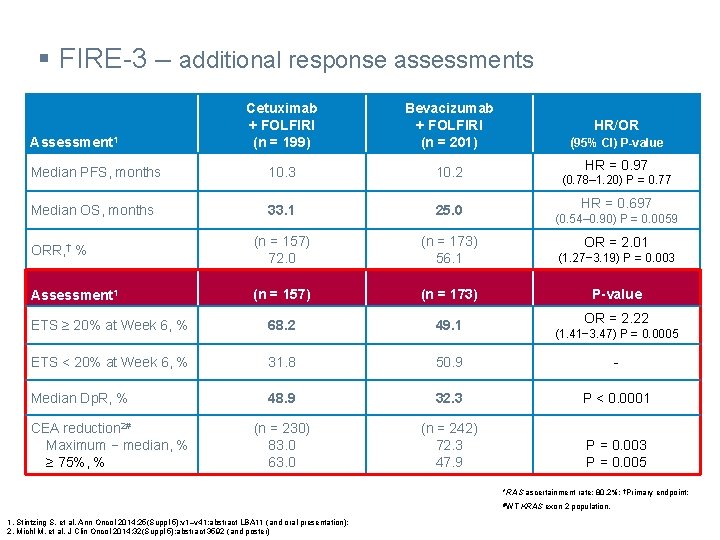
§ FIRE-3 – additional response assessments Cetuximab + FOLFIRI (n = 199) Bevacizumab + FOLFIRI (n = 201) Median PFS, months 10. 3 10. 2 Median OS, months 33. 1 25. 0 ORR, † % (n = 157) 72. 0 (n = 173) 56. 1 (1. 27− 3. 19) P = 0. 003 Assessment 1 (n = 157) (n = 173) P-value ETS ≥ 20% at Week 6, % 68. 2 49. 1 ETS < 20% at Week 6, % 31. 8 50. 9 - Median Dp. R, % 48. 9 32. 3 P < 0. 0001 (n = 230) 83. 0 63. 0 (n = 242) 72. 3 47. 9 P = 0. 003 P = 0. 005 Assessment 1 CEA reduction 2# Maximum − median, % ≥ 75%, % HR/OR (95% CI) P-value HR = 0. 97 (0. 78– 1. 20) P = 0. 77 HR = 0. 697 (0. 54– 0. 90) P = 0. 0059 OR = 2. 01 OR = 2. 22 (1. 41− 3. 47) P = 0. 0005 *RAS ascertainment rate: 80. 2%; †Primary endpoint; #WT 1. Stintzing S, et al. Ann Oncol 2014; 25(Suppl 5): v 1–v 41: abstract LBA 11 (and oral presentation); 2. Michl M, et al. J Clin Oncol 2014; 32(Suppl 5): abstract 3592 (and poster) KRAS exon 2 population.

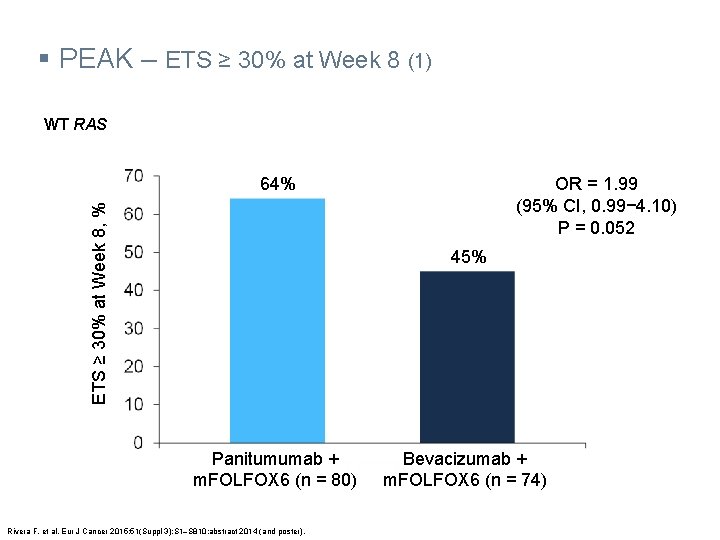
§ PEAK – ETS ≥ 30% at Week 8 (1) WT RAS ETS ≥ 30% at Week 8, % 64% OR = 1. 99 (95% CI, 0. 99− 4. 10) P = 0. 052 45% Panitumumab + m. FOLFOX 6 (n = 80) Rivera F, et al. Eur J Cancer 2015; 51(Suppl 3): S 1‒S 810: abstract 2014 (and poster). Bevacizumab + m. FOLFOX 6 (n = 74)

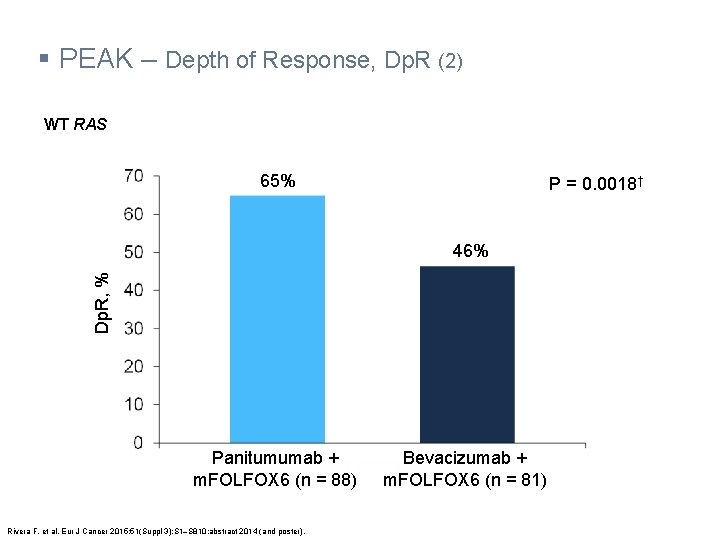
§ PEAK – Depth of Response, Dp. R (2) WT RAS 65% P = 0. 0018† Dp. R, % 46% Panitumumab + m. FOLFOX 6 (n = 88) Rivera F, et al. Eur J Cancer 2015; 51(Suppl 3): S 1‒S 810: abstract 2014 (and poster). Bevacizumab + m. FOLFOX 6 (n = 81)

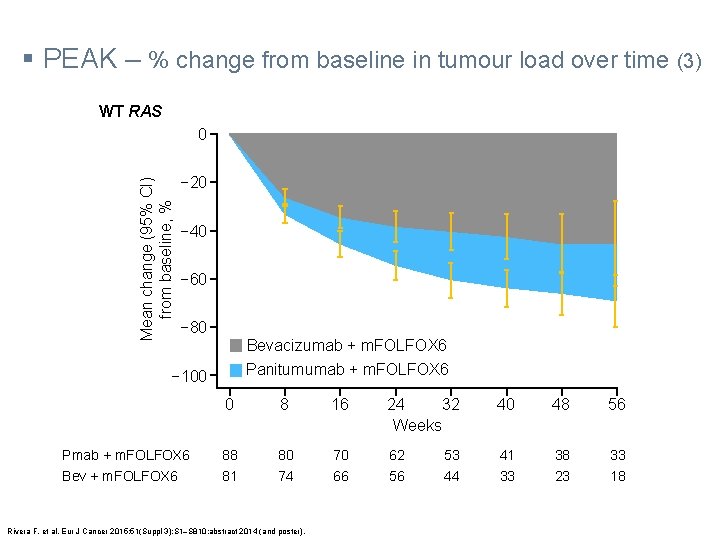
§ PEAK – % change from baseline in tumour load over time (3) WT RAS Mean change (95% CI) from baseline, % 0 − 20 − 40 − 60 − 80 Bevacizumab + m. FOLFOX 6 Panitumumab + m. FOLFOX 6 − 100 Pmab + m. FOLFOX 6 Bev + m. FOLFOX 6 0 8 16 24 32 Weeks 40 48 56 88 81 80 74 70 66 62 56 41 33 38 23 33 18 Rivera F, et al. Eur J Cancer 2015; 51(Suppl 3): S 1‒S 810: abstract 2014 (and poster). 53 44

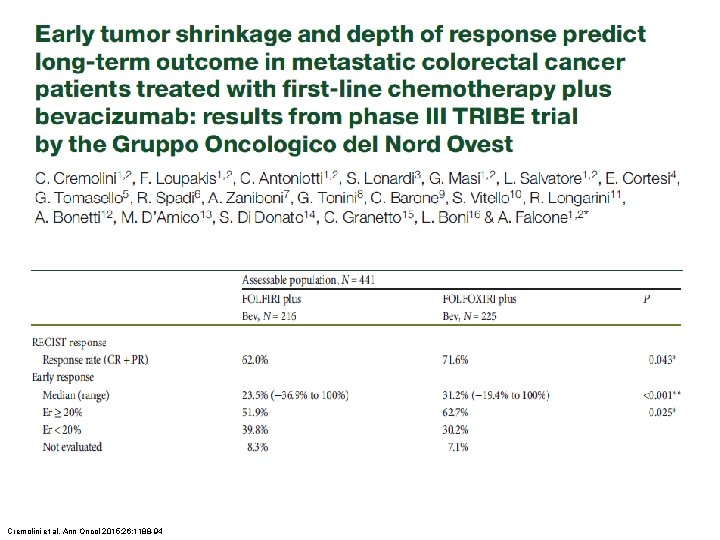
Cremolini et al. Ann Oncol 2015; 26: 1188 -94

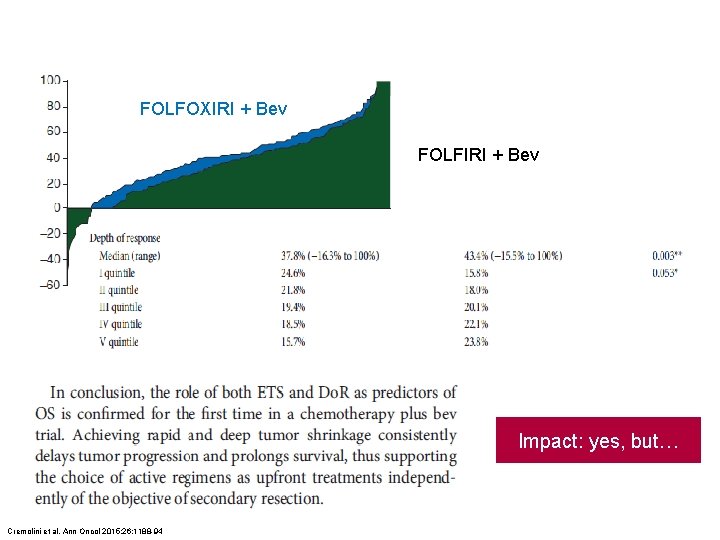
FOLFOXIRI + Bev FOLFIRI + Bev Impact: yes, but… Cremolini et al. Ann Oncol 2015; 26: 1188 -94

§ ETS & PFS, OS – overview of the literature (1) Heinemann V et al. Eur J Cancer 2015; 51: 1927 -36

§ ETS & PFS, OS – overview of the literature (2) Heinemann V et al. Eur J Cancer 2015; 51: 1927 -36

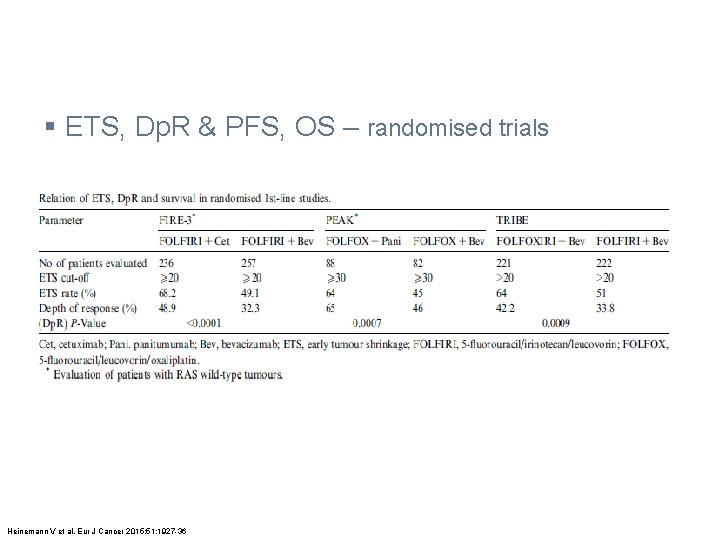
§ ETS, Dp. R & PFS, OS – randomised trials Heinemann V et al. Eur J Cancer 2015; 51: 1927 -36

§ ETS – summary • ETS reflects the velocity of response to therapy • It is measured at the earliest time point of CT evaluation • ETS indicates sensitivity to treatment • Addition of anti-EGFR agents to chemotherapy increases the number of patients with ETS and enhances the velocity of response Slide courtesy of V Heinemann

§ Dp. R – summary Δ OS Lethal tumour load Tumour size, % Δ OS 100% 70% SD PR • ETS is an early predictor of sensitivity to treatment PR • Dp. R correlates with OS Deepest response Time since start of treatment Adapted from: Stintzing S, et al. Ann Oncol 2014; 25 (Suppl 5): v 1–v 41: abstract LBA 11 (and oral presentation).

§ Additional response assessments – conclusions - ETS is associated with prolonged survival and is an early predictor of sensitivity to treatment in m. CRC - Based on ETS and Dp. R, anti-EGFR therapy may show a benefit over anti-VEGF therapy in 1 st-line WT RAS m. CRC - knowledge of the radiologist in standard setting - global population vs. individual decision

UZA DIAMOND DESIGN HARBOR I wish to thank The Multidisciplinary Oncology Team at Antwerp University Hospital
 Real depth and apparent depth
Real depth and apparent depth Regression shrinkage and selection via the lasso
Regression shrinkage and selection via the lasso Concrete crack comparator card
Concrete crack comparator card Shrinkage formula
Shrinkage formula Sprue casting junction
Sprue casting junction Conduit bending shrinkage
Conduit bending shrinkage Warehouse inventory shrinkage statistics
Warehouse inventory shrinkage statistics Shrinkage control in retail ppt
Shrinkage control in retail ppt Early cpr and early defibrillation can: *
Early cpr and early defibrillation can: * Natural response and forced response
Natural response and forced response First order system transfer function
First order system transfer function A subsequent
A subsequent Difference between proto oncogene and oncogene
Difference between proto oncogene and oncogene Benign and malignant tumor
Benign and malignant tumor Ets-nocv
Ets-nocv Criterion online writing evaluation service
Criterion online writing evaluation service Safirh ets
Safirh ets Http //pisa.ets.org/school questionnaire
Http //pisa.ets.org/school questionnaire Ets venipuncture materials
Ets venipuncture materials Ets
Ets Kompleks 3
Kompleks 3 Como se clasifican las ets
Como se clasifican las ets Toef exam
Toef exam Safirh
Safirh Gti350 ets
Gti350 ets Kemosentetik bakteriler
Kemosentetik bakteriler Ets licence
Ets licence Ets 4
Ets 4 Safirh
Safirh Ets success navigator
Ets success navigator Krebse hazırlık evresi
Krebse hazırlık evresi Ets
Ets Ets
Ets Ets
Ets Mytoms ets
Mytoms ets