Introductory Chemistry A Foundation 6 th Ed Introductory














![Acidic and Basic Solutions • Acidic solutions have a larger [H+] than [OH-] • Acidic and Basic Solutions • Acidic solutions have a larger [H+] than [OH-] •](https://slidetodoc.com/presentation_image_h2/74c22ddbcb8538e6bd29e947a2c22fe5/image-27.jpg)
![Example #2 Determine the [H+] and [OH-] in a 10. 0 M H+ solution Example #2 Determine the [H+] and [OH-] in a 10. 0 M H+ solution](https://slidetodoc.com/presentation_image_h2/74c22ddbcb8538e6bd29e947a2c22fe5/image-29.jpg)
![Example #2 (cont. ) Given [H+] = 10. 0 M = 1. 00 x Example #2 (cont. ) Given [H+] = 10. 0 M = 1. 00 x](https://slidetodoc.com/presentation_image_h2/74c22ddbcb8538e6bd29e947a2c22fe5/image-31.jpg)
![Self check p 497 • Calculate [H+] in a solution in which [OH-] = Self check p 497 • Calculate [H+] in a solution in which [OH-] =](https://slidetodoc.com/presentation_image_h2/74c22ddbcb8538e6bd29e947a2c22fe5/image-32.jpg)
![Example #3 Calculate the p. H of a solution with a [OH-] = 1. Example #3 Calculate the p. H of a solution with a [OH-] = 1.](https://slidetodoc.com/presentation_image_h2/74c22ddbcb8538e6bd29e947a2c22fe5/image-37.jpg)
![Example #3 (cont. ) • Find the concentration of [H+] Copyright © Houghton Mifflin Example #3 (cont. ) • Find the concentration of [H+] Copyright © Houghton Mifflin](https://slidetodoc.com/presentation_image_h2/74c22ddbcb8538e6bd29e947a2c22fe5/image-38.jpg)
![Example #3 (cont. ) • Enter the [H+] concentration into your calculator and press Example #3 (cont. ) • Enter the [H+] concentration into your calculator and press](https://slidetodoc.com/presentation_image_h2/74c22ddbcb8538e6bd29e947a2c22fe5/image-39.jpg)
![Solving concentration from p. H or p. OH Calculate the [OH-] of a solution Solving concentration from p. H or p. OH Calculate the [OH-] of a solution](https://slidetodoc.com/presentation_image_h2/74c22ddbcb8538e6bd29e947a2c22fe5/image-41.jpg)




![Example #6 (cont. ) • Determine the [H+] from the acid concentration HNO 3 Example #6 (cont. ) • Determine the [H+] from the acid concentration HNO 3](https://slidetodoc.com/presentation_image_h2/74c22ddbcb8538e6bd29e947a2c22fe5/image-46.jpg)




- Slides: 50

Introductory Chemistry: A Foundation, 6 th Ed. Introductory Chemistry, 6 th Ed. Basic Chemistry, 6 th Ed. by Steven S. Zumdahl & Donald J. De. Coste University of Illinois

Chapter 16 Acids and Bases



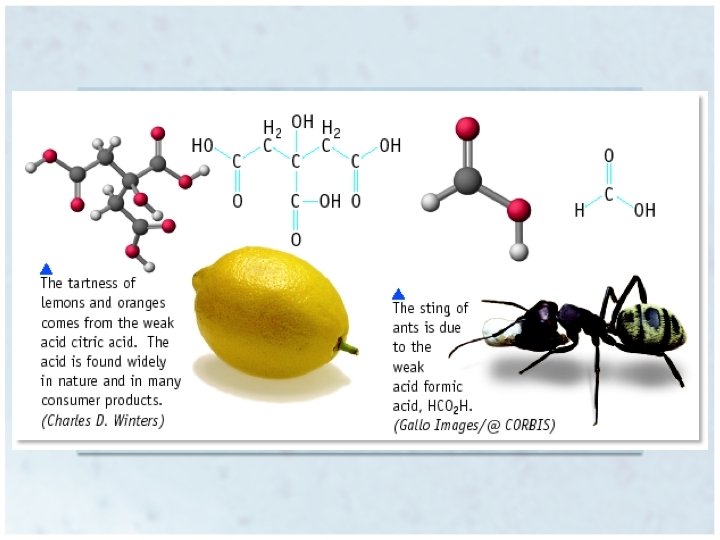
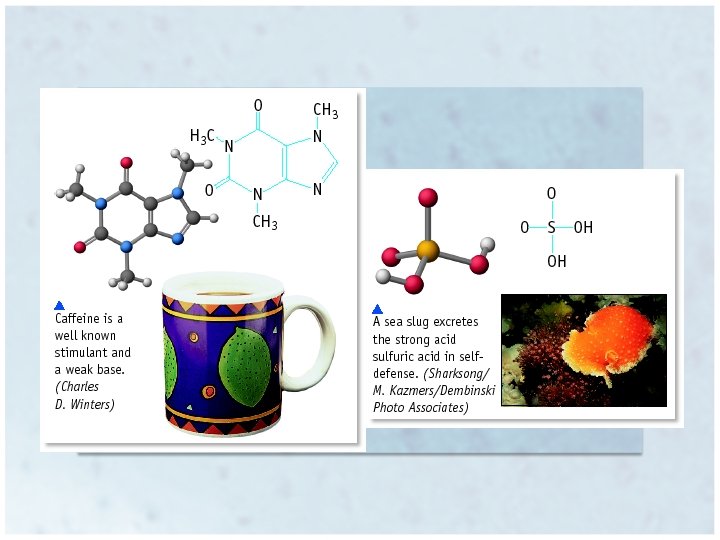
Properties of Acids • • Sour taste Turn blue litmus paper red Change color of vegetable dyes (red cabbage juice) React with “active” metals – Like Al, Zn, Fe, but not Cu, Ag or Au Zn + 2 HCl Zn. Cl 2 + H 2 • Corrosive • React with carbonates, producing CO 2 – Marble, baking soda, chalk Ca. CO 3 + 2 HCl Ca. Cl 2 + CO 2 + H 2 O • React with bases to form ionic salts, and often water Copyright © Houghton Mifflin Company. All rights reserved. 16 | 5

Properties of Bases • • Also known as alkalis Bitter Taste Feel slippery Change color of vegetable dyes – Different color than acid – Turn red litmus blue • React with acids to form ionic salts, and often water – Neutralization Copyright © Houghton Mifflin Company. All rights reserved. 16 | 6

Arrhenius Theory • Acids ionize in water to H+ ions and anions • Bases ionize in water to OH- ions and cations • Neutralization reaction involves H+ combining with OH- to make water • H+ ions are protons Copyright © Houghton Mifflin Company. All rights reserved. 16 | 7

Arrhenius Theory (cont. ) • Definition only good in water solution • Definition does not explain why ammonia solutions turn litmus blue – Basic without OH- ions Copyright © Houghton Mifflin Company. All rights reserved. 16 | 8

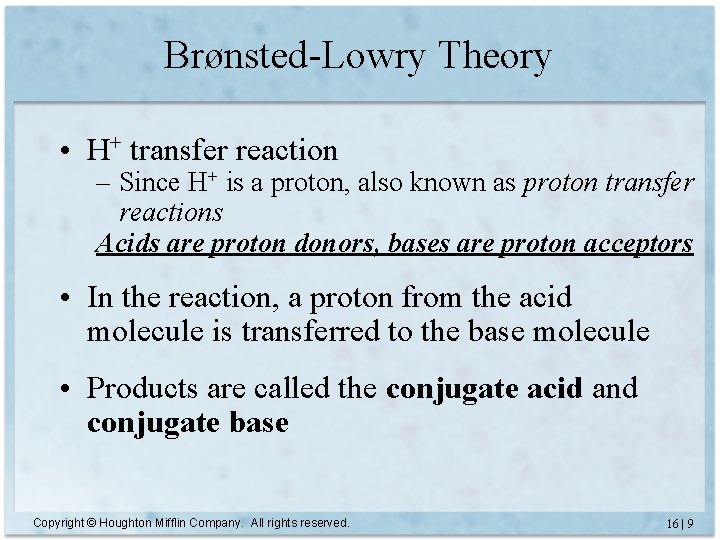
Brønsted-Lowry Theory • H+ transfer reaction – Since H+ is a proton, also known as proton transfer reactions Acids are proton donors, bases are proton acceptors • In the reaction, a proton from the acid molecule is transferred to the base molecule • Products are called the conjugate acid and conjugate base Copyright © Houghton Mifflin Company. All rights reserved. 16 | 9

Brønsted-Lowry Theory (cont. ) Copyright © Houghton Mifflin Company. All rights reserved. 16 | 10

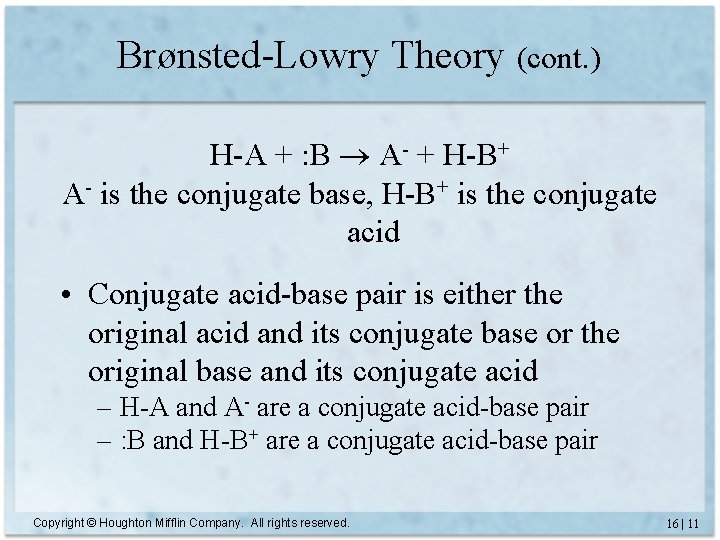
Brønsted-Lowry Theory (cont. ) H-A + : B A- + H-B+ A- is the conjugate base, H-B+ is the conjugate acid • Conjugate acid-base pair is either the original acid and its conjugate base or the original base and its conjugate acid – H-A and A- are a conjugate acid-base pair – : B and H-B+ are a conjugate acid-base pair Copyright © Houghton Mifflin Company. All rights reserved. 16 | 11

Example #1: Write the conjugate base for the acid H 3 PO 4 • Determine what species you will get if you remove 1 H+1 from the acid. – Conjugate base will have one more negative charge than the original acid H 3 PO 4 H+ + H 2 PO 4 - Copyright © Houghton Mifflin Company. All rights reserved. 16 | 12

Self- check p 490 • Which of the following represent conjugate acid base pairs? • A. H 2 O, H 3 O+ • B. OH-, HNO 3 • C. H 2 SO 4, SO 42 • D. HC 2 H 3 O 2, C 2 H 3 O 2 - Copyright © Houghton Mifflin Company. All rights reserved. 16 | 13

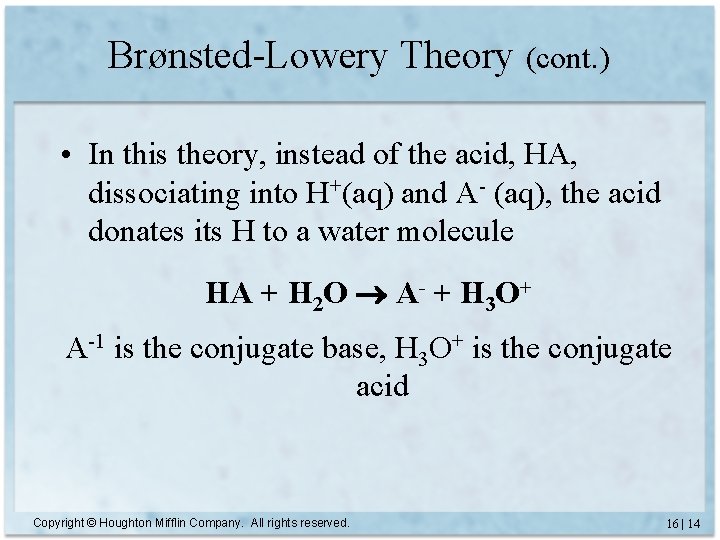
Brønsted-Lowery Theory (cont. ) • In this theory, instead of the acid, HA, dissociating into H+(aq) and A- (aq), the acid donates its H to a water molecule HA + H 2 O A- + H 3 O+ A-1 is the conjugate base, H 3 O+ is the conjugate acid Copyright © Houghton Mifflin Company. All rights reserved. 16 | 14

Brønsted-Lowry Theory (cont. ) • H 3 O+ is called the hydronium ion • In this theory, substances that do not have OH- ions can act as a base if they can accept a H+1 from water. H 2 O + : B OH- + H-B+ : B is acting here as a base. Copyright © Houghton Mifflin Company. All rights reserved. 16 | 15

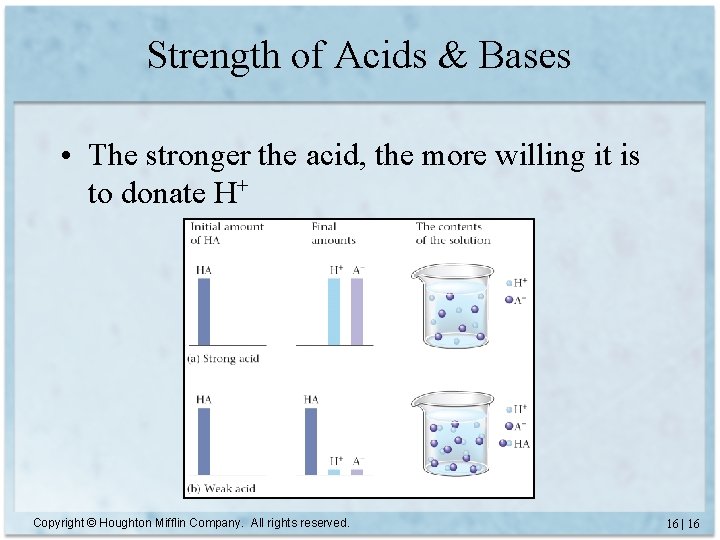
Strength of Acids & Bases • The stronger the acid, the more willing it is to donate H+ Copyright © Houghton Mifflin Company. All rights reserved. 16 | 16

Strength of Acids & Bases (cont. ) • Strong bases will react completely with water to form hydroxide: CO 3 -2 + H 2 O HCO 3 - + OH • Only small fraction of weak base molecules pull H+ off water: HCO 3 - + H 2 O H 2 CO 3 + OH- Copyright © Houghton Mifflin Company. All rights reserved. 16 | 17

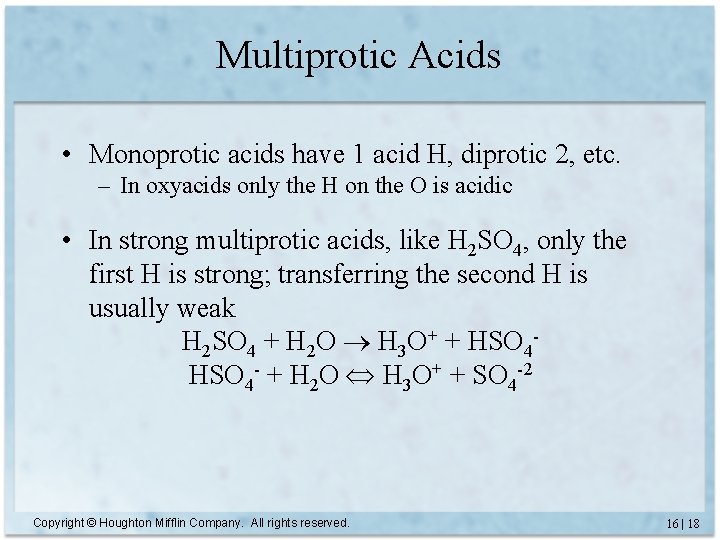
Multiprotic Acids • Monoprotic acids have 1 acid H, diprotic 2, etc. – In oxyacids only the H on the O is acidic • In strong multiprotic acids, like H 2 SO 4, only the first H is strong; transferring the second H is usually weak H 2 SO 4 + H 2 O H 3 O+ + HSO 4 - + H 2 O H 3 O+ + SO 4 -2 Copyright © Houghton Mifflin Company. All rights reserved. 16 | 18

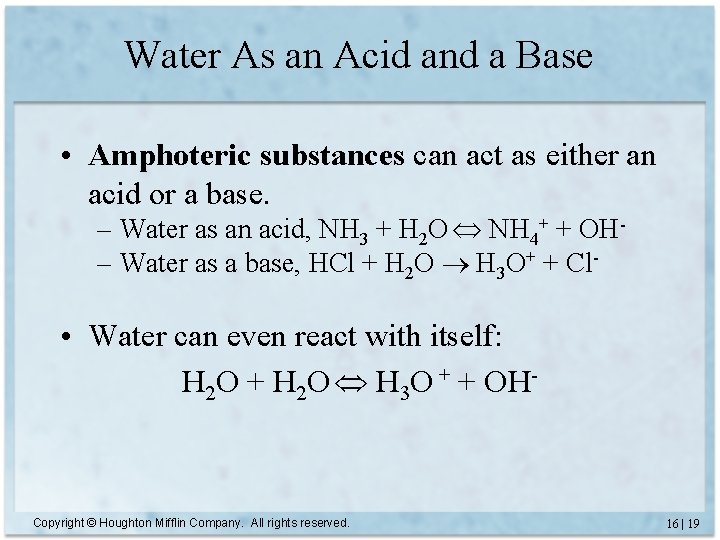
Water As an Acid and a Base • Amphoteric substances can act as either an acid or a base. – Water as an acid, NH 3 + H 2 O NH 4+ + OH– Water as a base, HCl + H 2 O H 3 O+ + Cl- • Water can even react with itself: H 2 O + H 2 O H 3 O + + OH- Copyright © Houghton Mifflin Company. All rights reserved. 16 | 19

Autoionization of Water • Water is an extremely weak electrolyte. – Therefore there must be a few ions present H 2 O + H 2 O H 3 O+ + OH- Copyright © Houghton Mifflin Company. All rights reserved. 16 | 20

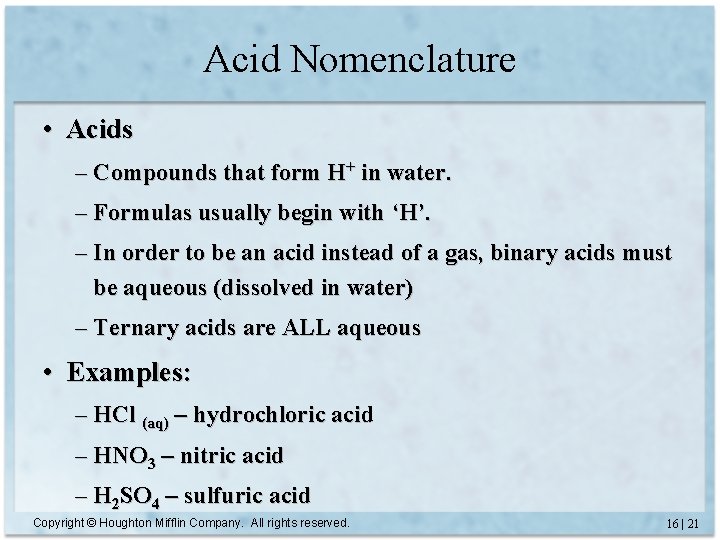
Acid Nomenclature • Acids – Compounds that form H+ in water. – Formulas usually begin with ‘H’. – In order to be an acid instead of a gas, binary acids must be aqueous (dissolved in water) – Ternary acids are ALL aqueous • Examples: – HCl (aq) – hydrochloric acid – HNO 3 – nitric acid – H 2 SO 4 – sulfuric acid Copyright © Houghton Mifflin Company. All rights reserved. 16 | 21

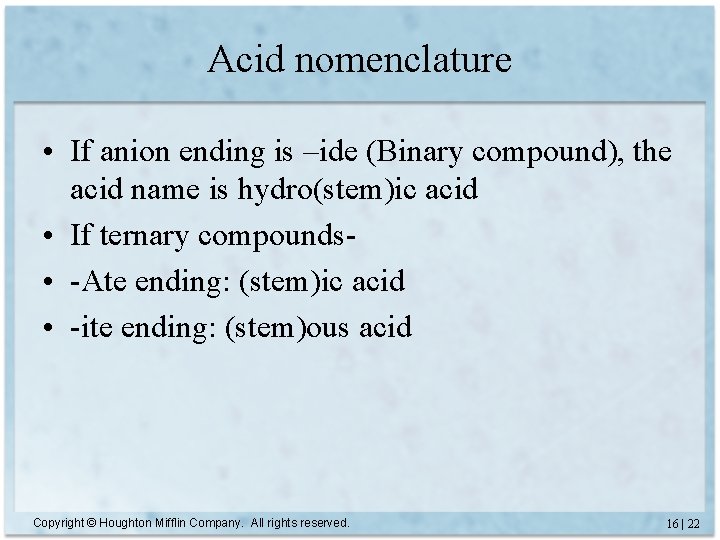
Acid nomenclature • If anion ending is –ide (Binary compound), the acid name is hydro(stem)ic acid • If ternary compounds • -Ate ending: (stem)ic acid • -ite ending: (stem)ous acid Copyright © Houghton Mifflin Company. All rights reserved. 16 | 22

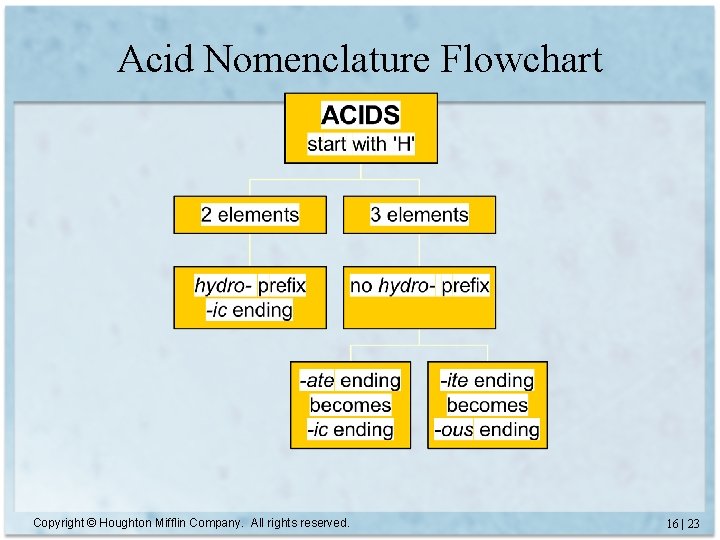
Acid Nomenclature Flowchart Copyright © Houghton Mifflin Company. All rights reserved. 16 | 23

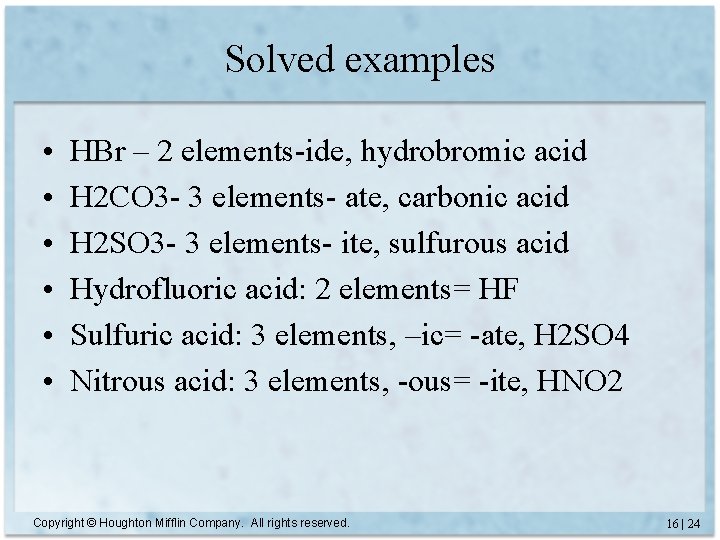
Solved examples • • • HBr – 2 elements-ide, hydrobromic acid H 2 CO 3 - 3 elements- ate, carbonic acid H 2 SO 3 - 3 elements- ite, sulfurous acid Hydrofluoric acid: 2 elements= HF Sulfuric acid: 3 elements, –ic= -ate, H 2 SO 4 Nitrous acid: 3 elements, -ous= -ite, HNO 2 Copyright © Houghton Mifflin Company. All rights reserved. 16 | 24

Now your turn! • HI (aq) • HCl • H 2 SO 3 • HNO 3 • HIO 4 Copyright © Houghton Mifflin Company. All rights reserved. 16 | 25

• Hydrobromic acid • Nitrous acid • Carbonic acid • Phosphoric acid Copyright © Houghton Mifflin Company. All rights reserved. 16 | 26
![Acidic and Basic Solutions Acidic solutions have a larger H than OH Acidic and Basic Solutions • Acidic solutions have a larger [H+] than [OH-] •](https://slidetodoc.com/presentation_image_h2/74c22ddbcb8538e6bd29e947a2c22fe5/image-27.jpg)
Acidic and Basic Solutions • Acidic solutions have a larger [H+] than [OH-] • Basic solutions have a larger [OH-] than [H+] • Neutral solutions have [H+]=[OH-]= 1 x 10 -7 M [H+] = 1 x 10 -14 [OH-] Copyright © Houghton Mifflin Company. All rights reserved. [OH-] -14 1 x 10 = [H+] 16 | 27

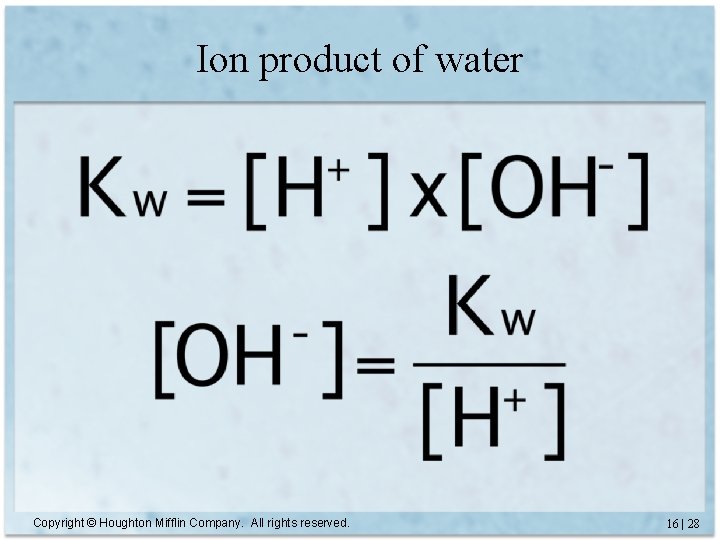
Ion product of water Copyright © Houghton Mifflin Company. All rights reserved. 16 | 28
![Example 2 Determine the H and OH in a 10 0 M H solution Example #2 Determine the [H+] and [OH-] in a 10. 0 M H+ solution](https://slidetodoc.com/presentation_image_h2/74c22ddbcb8538e6bd29e947a2c22fe5/image-29.jpg)
Example #2 Determine the [H+] and [OH-] in a 10. 0 M H+ solution Copyright © Houghton Mifflin Company. All rights reserved. 16 | 29

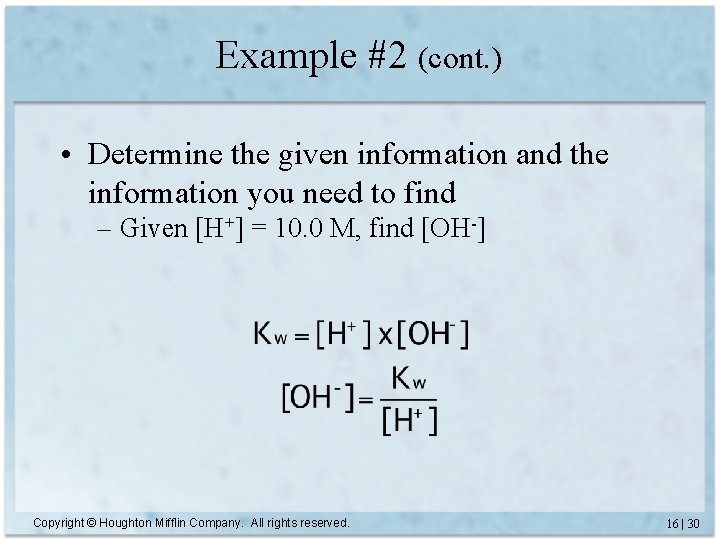
Example #2 (cont. ) • Determine the given information and the information you need to find – Given [H+] = 10. 0 M, find [OH-] Copyright © Houghton Mifflin Company. All rights reserved. 16 | 30
![Example 2 cont Given H 10 0 M 1 00 x Example #2 (cont. ) Given [H+] = 10. 0 M = 1. 00 x](https://slidetodoc.com/presentation_image_h2/74c22ddbcb8538e6bd29e947a2c22fe5/image-31.jpg)
Example #2 (cont. ) Given [H+] = 10. 0 M = 1. 00 x 101 M Kw = 1. 0 x 10 -14 Copyright © Houghton Mifflin Company. All rights reserved. 16 | 31
![Self check p 497 Calculate H in a solution in which OH Self check p 497 • Calculate [H+] in a solution in which [OH-] =](https://slidetodoc.com/presentation_image_h2/74c22ddbcb8538e6bd29e947a2c22fe5/image-32.jpg)
Self check p 497 • Calculate [H+] in a solution in which [OH-] = • 2. 0 X 10 -2 M. Is this solution acidic, neutral or basic? Copyright © Houghton Mifflin Company. All rights reserved. 16 | 32

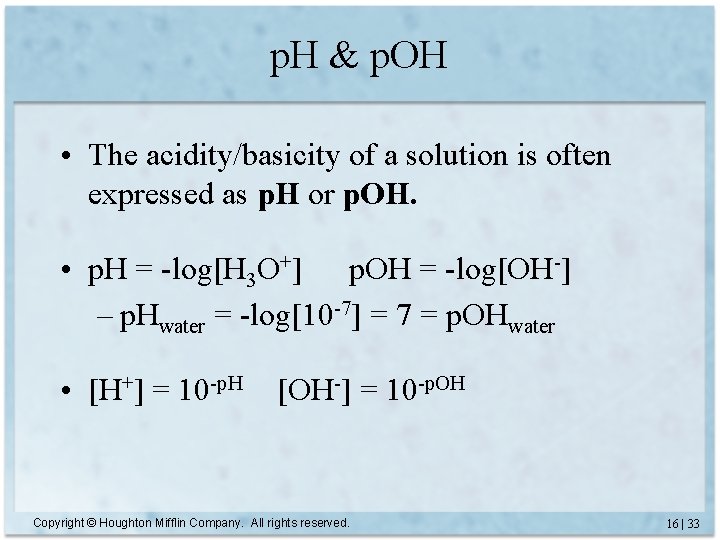
p. H & p. OH • The acidity/basicity of a solution is often expressed as p. H or p. OH. • p. H = -log[H 3 O+] p. OH = -log[OH-] – p. Hwater = -log[10 -7] = 7 = p. OHwater • [H+] = 10 -p. H [OH-] = 10 -p. OH Copyright © Houghton Mifflin Company. All rights reserved. 16 | 33

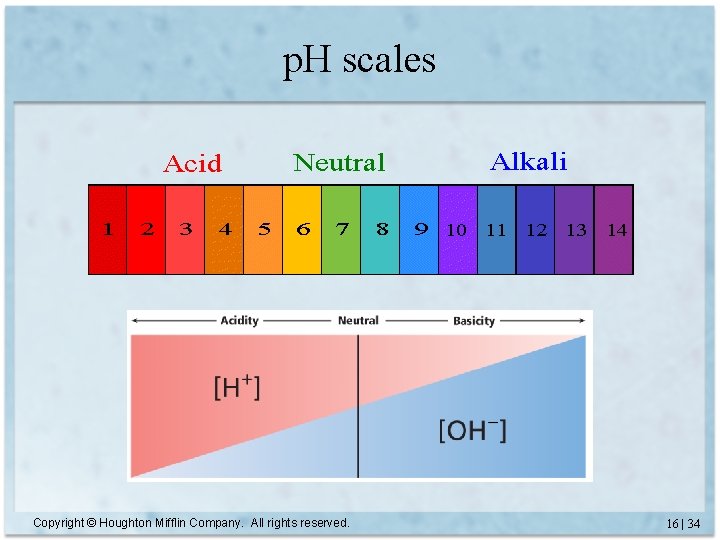
p. H scales Copyright © Houghton Mifflin Company. All rights reserved. 16 | 34

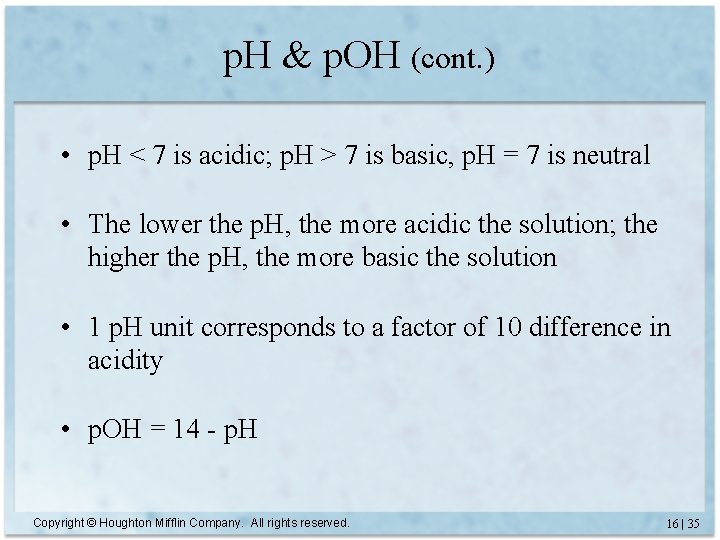
p. H & p. OH (cont. ) • p. H < 7 is acidic; p. H > 7 is basic, p. H = 7 is neutral • The lower the p. H, the more acidic the solution; the higher the p. H, the more basic the solution • 1 p. H unit corresponds to a factor of 10 difference in acidity • p. OH = 14 - p. H Copyright © Houghton Mifflin Company. All rights reserved. 16 | 35

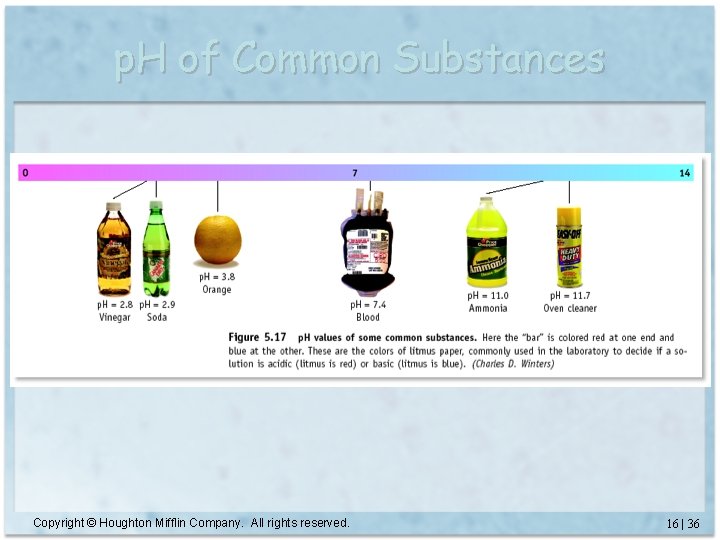
p. H of Common Substances Copyright © Houghton Mifflin Company. All rights reserved. 16 | 36
![Example 3 Calculate the p H of a solution with a OH 1 Example #3 Calculate the p. H of a solution with a [OH-] = 1.](https://slidetodoc.com/presentation_image_h2/74c22ddbcb8538e6bd29e947a2c22fe5/image-37.jpg)
Example #3 Calculate the p. H of a solution with a [OH-] = 1. 0 x 10 -6 M Copyright © Houghton Mifflin Company. All rights reserved. 16 | 37
![Example 3 cont Find the concentration of H Copyright Houghton Mifflin Example #3 (cont. ) • Find the concentration of [H+] Copyright © Houghton Mifflin](https://slidetodoc.com/presentation_image_h2/74c22ddbcb8538e6bd29e947a2c22fe5/image-38.jpg)
Example #3 (cont. ) • Find the concentration of [H+] Copyright © Houghton Mifflin Company. All rights reserved. 16 | 38
![Example 3 cont Enter the H concentration into your calculator and press Example #3 (cont. ) • Enter the [H+] concentration into your calculator and press](https://slidetodoc.com/presentation_image_h2/74c22ddbcb8538e6bd29e947a2c22fe5/image-39.jpg)
Example #3 (cont. ) • Enter the [H+] concentration into your calculator and press the log key – log(1. 0 x 10 -8) = -8. 0 • Change the sign to get the p. H – p. H = -(-8. 0) = 8. 0 Copyright © Houghton Mifflin Company. All rights reserved. 16 | 39

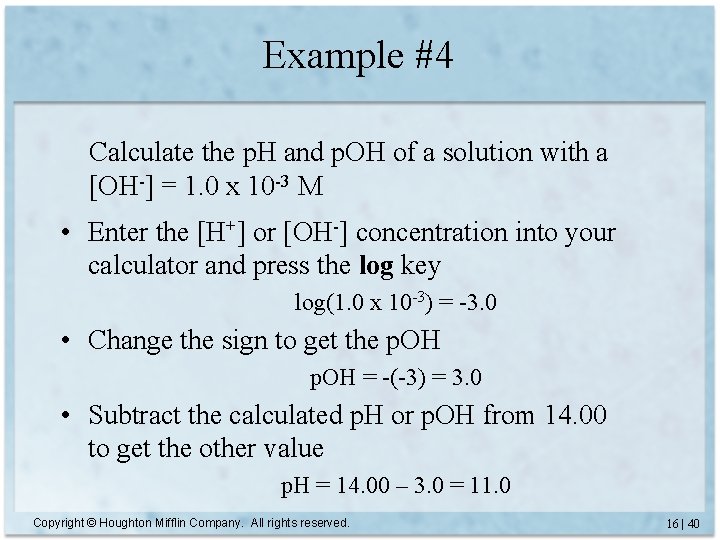
Example #4 Calculate the p. H and p. OH of a solution with a [OH-] = 1. 0 x 10 -3 M • Enter the [H+] or [OH-] concentration into your calculator and press the log key log(1. 0 x 10 -3) = -3. 0 • Change the sign to get the p. OH = -(-3) = 3. 0 • Subtract the calculated p. H or p. OH from 14. 00 to get the other value p. H = 14. 00 – 3. 0 = 11. 0 Copyright © Houghton Mifflin Company. All rights reserved. 16 | 40
![Solving concentration from p H or p OH Calculate the OH of a solution Solving concentration from p. H or p. OH Calculate the [OH-] of a solution](https://slidetodoc.com/presentation_image_h2/74c22ddbcb8538e6bd29e947a2c22fe5/image-41.jpg)
Solving concentration from p. H or p. OH Calculate the [OH-] of a solution with a p. H of 7. 41 • If you want to calculate [OH-] use p. OH; if you want [H+] use p. H. It may be necessary to convert one to the other using 14 = [H+] + [OH-] p. OH = 14. 00 – 7. 41 = 6. 59 Copyright © Houghton Mifflin Company. All rights reserved. 16 | 41

Example #5 (cont. ) • Enter the p. H or p. OH concentration into your calculator • Change the sign of the p. H or p. OH -p. OH = -(6. 59) • Press the button(s) on you calculator to take the inverse log or 10 x [OH-] = 10 -6. 59 = 2. 6 x 10 -7 M Copyright © Houghton Mifflin Company. All rights reserved. 16 | 42

Self check p 499 • Calculate the p. H value for each of the following solutions at 25°C. • A. a solution in which [H+] =1. 0 X 10 -9 M. • B. a solution in which [OH-] =1. 0 X 10 -6 M. • P 501 - A sample of rain in an area with severe air pollution has a p. H of 3. 5. What is the p. OH of this rainwater? Copyright © Houghton Mifflin Company. All rights reserved. 16 | 43

Calculating the p. H of a Strong, Monoprotic Acid • A strong acid will dissociate 100% HA H+ + A • Therefore the molarity of H+ ions will be the same as the molarity of the acid • Once the H+ molarity is determined, the p. H can be determined p. H = -log[H+] Copyright © Houghton Mifflin Company. All rights reserved. 16 | 44

Example #6 Calculate the p. H of a 0. 10 M HNO 3 solution. Copyright © Houghton Mifflin Company. All rights reserved. 16 | 45
![Example 6 cont Determine the H from the acid concentration HNO 3 Example #6 (cont. ) • Determine the [H+] from the acid concentration HNO 3](https://slidetodoc.com/presentation_image_h2/74c22ddbcb8538e6bd29e947a2c22fe5/image-46.jpg)
Example #6 (cont. ) • Determine the [H+] from the acid concentration HNO 3 H+ + NO 30. 10 M HNO 3 = 0. 10 M H+ • Enter the [H+] concentration into your calculator and press the log key log(0. 10) = -1. 00 • Change the sign to get the p. H = -(-1. 00) = 1. 00 Copyright © Houghton Mifflin Company. All rights reserved. 16 | 46

Self check p 503 • The p. H of rainwater in a polluted area was measured to be 3. 5. What is the [H+] in this rainwater? • The p. OH of a liquid drain cleaner was found to be 10. 50. What is the [OH-] for this cleaner? • P 505 - Calculate the p. H of a solution of 5. 0 X 10 -3 M HCl. Copyright © Houghton Mifflin Company. All rights reserved. 16 | 47

Buffered Solutions • Buffered solutions resist change in p. H when an acid or base is added to it. • Used when need to maintain a certain p. H in the system – Blood Copyright © Houghton Mifflin Company. All rights reserved. 16 | 48

Buffered Solutions (cont. ) • A buffer solution contains a weak acid and its conjugate base. • Buffers work by reacting with added H+ or OHions so they do not accumulate and change the p. H. • Buffers will only work as long as there are sufficient weak acid and conjugate base molecules present. Copyright © Houghton Mifflin Company. All rights reserved. 16 | 49

Buffered Solutions (cont. ) Copyright © Houghton Mifflin Company. All rights reserved. 16 | 50