Introductory Chemistry Chapter 1 Chemistry and You Tuesday






























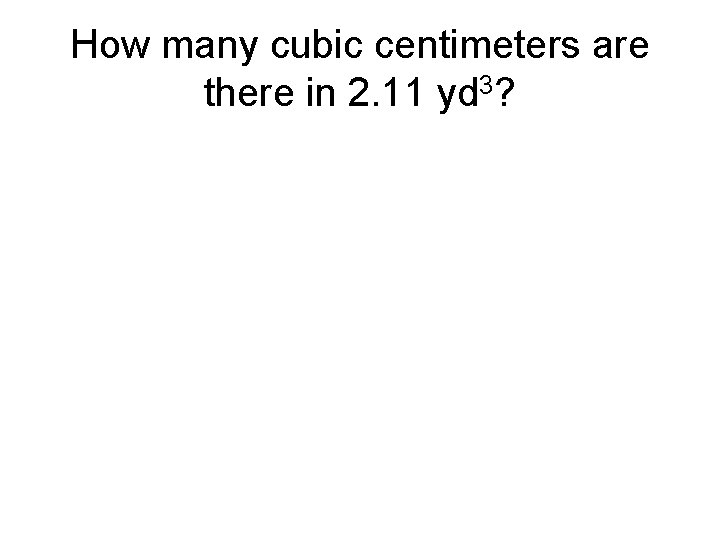




- Slides: 70

Introductory Chemistry: Chapter 1 Chemistry and You

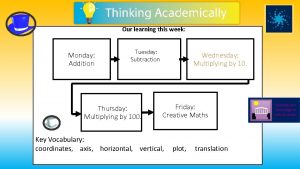
Tuesday, 9/9/14 • Learning Target: Students must be able to explain why chemistry is central to many human endeavors. Chapter 1 2

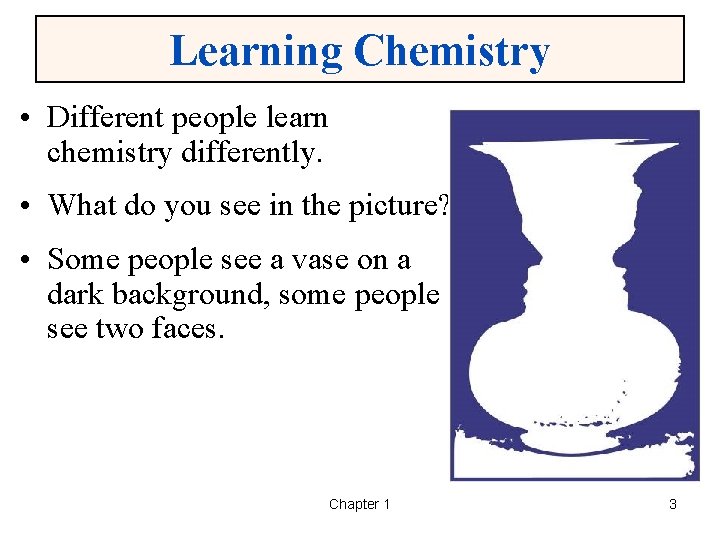
Learning Chemistry • Different people learn chemistry differently. • What do you see in the picture? • Some people see a vase on a dark background, some people see two faces. Chapter 1 3

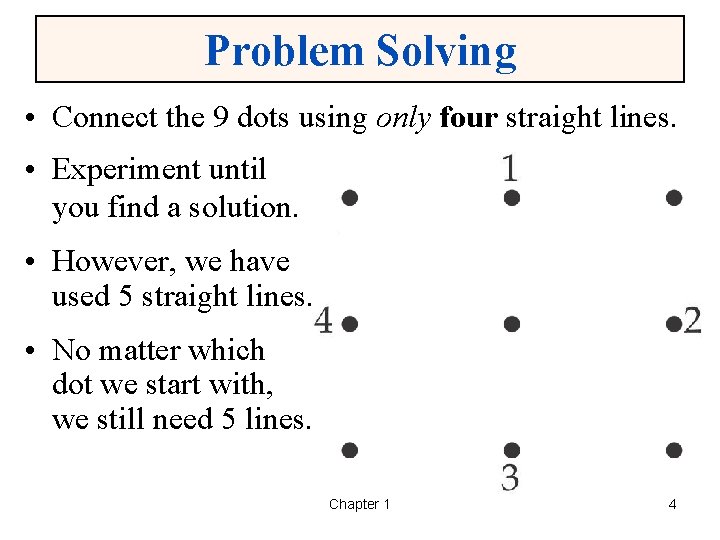
Problem Solving • Connect the 9 dots using only four straight lines. • Experiment until you find a solution. • However, we have used 5 straight lines. • No matter which dot we start with, we still need 5 lines. Chapter 1 4

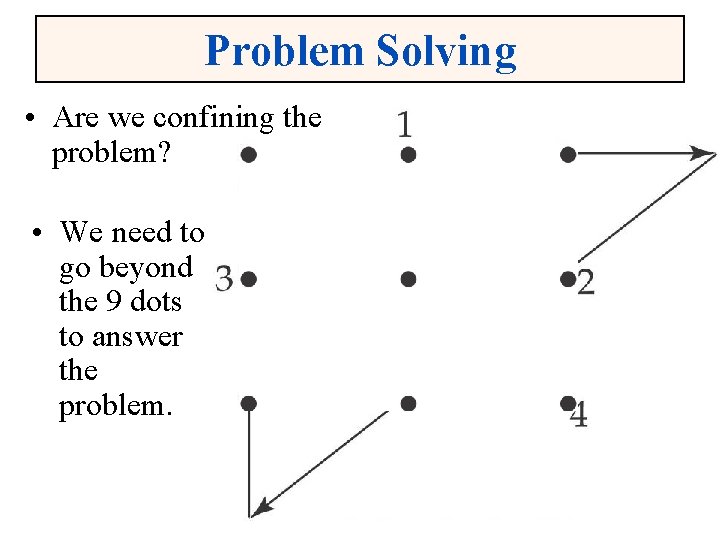
Problem Solving • Are we confining the problem? • We need to go beyond the 9 dots to answer the problem. Chapter 1 5

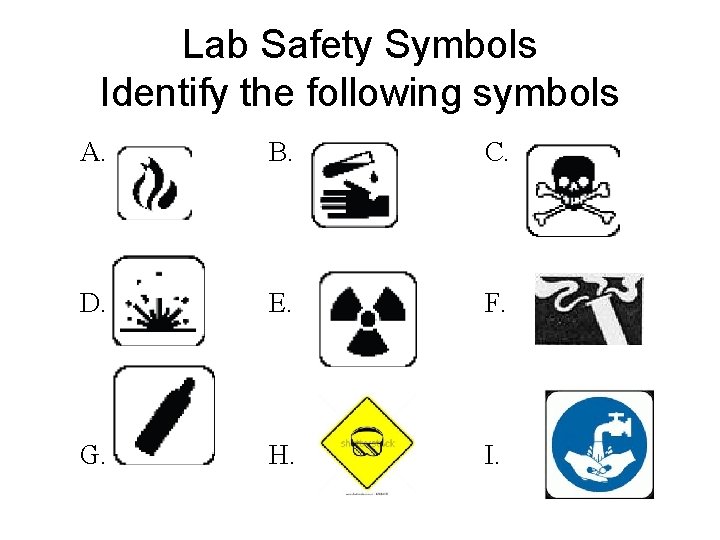
Lab Safety Symbols Identify the following symbols A. B. C. D. E. F. G. H. I.

• What is the definition of chemistry? – The science that studies the composition of matter and its properties.

Chemistry: The Central Science • Why? ? • Most other sciences demand an understanding of basic chemical principles, and Chemistry is often referred to as the Central Science Chapter 1 8

Modern Chemistry • Chemistry is a science that studies the composition of matter and its properties. • Chemistry is divided into several branches: – Organic chemistry is the study of substances containing carbon – Inorganic chemistry is the study of all other substances – Biochemistry is the study of substances derived from plants and animals – Analytical is the study of matter and ways to study the properties of matter. – Physical is the physics of chemistry. Thermodynamics and quantum mechanics. Chapter 1 9

Wednesday, 9/10/14 Learning Target: Students must know the metric system, SI units and derived units. Learning Outcome: Measurement Pre-Lab Chapter 1 10

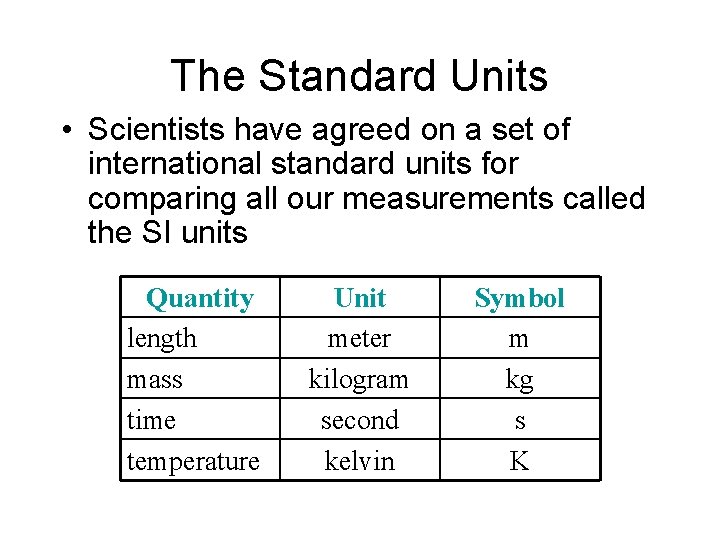
The Standard Units • Scientists have agreed on a set of international standard units for comparing all our measurements called the SI units Quantity length mass time temperature Unit meter kilogram second kelvin Symbol m kg s K

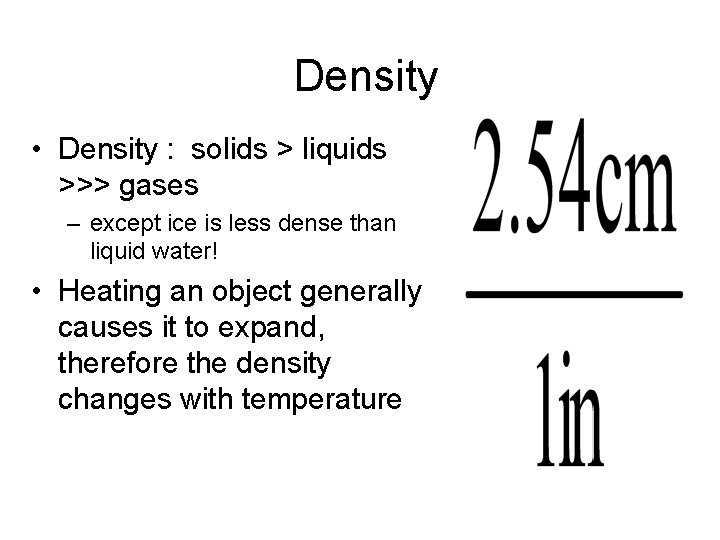
Length • SI unit = meter – About a yard • Commonly use centimeters (cm) – 1 m = 100 cm – 1 cm = 0. 01 m = 10 mm – 1 inch = 2. 54 cm

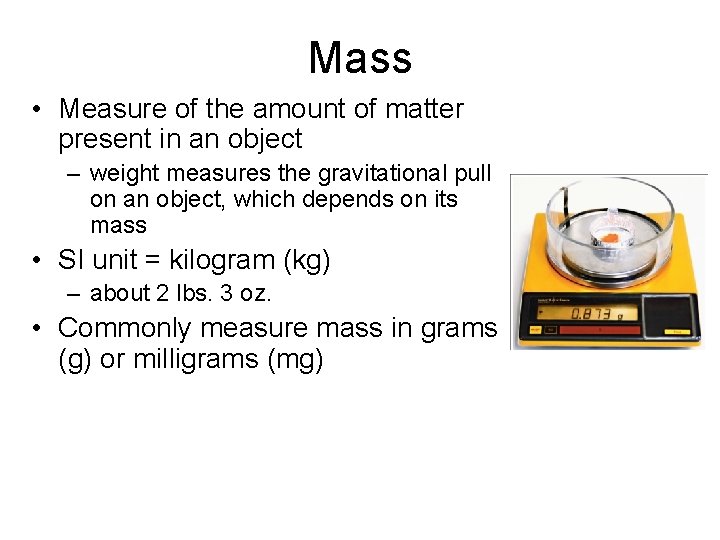
Mass • Measure of the amount of matter present in an object – weight measures the gravitational pull on an object, which depends on its mass • SI unit = kilogram (kg) – about 2 lbs. 3 oz. • Commonly measure mass in grams (g) or milligrams (mg)

Time • measure of the duration of an event • SI units = second (s)

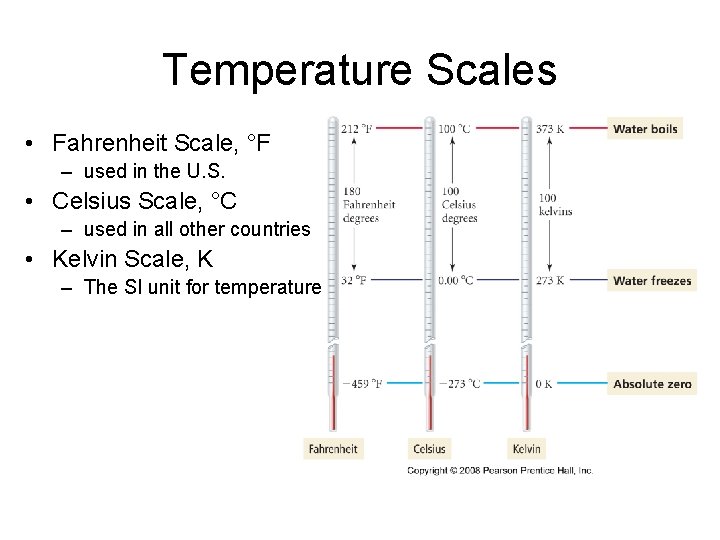
Temperature Scales • Fahrenheit Scale, °F – used in the U. S. • Celsius Scale, °C – used in all other countries • Kelvin Scale, K – The SI unit for temperature

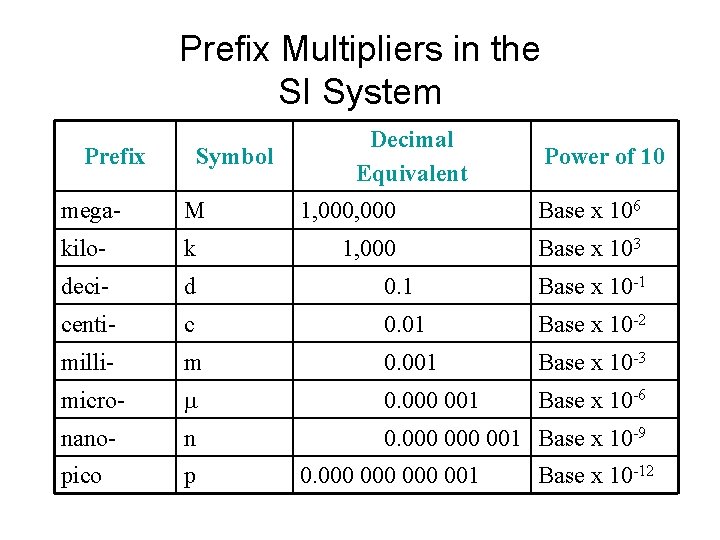
Prefix Multipliers in the SI System Prefix Symbol Decimal Equivalent Power of 10 mega- M 1, 000 Base x 106 kilo- k 1, 000 Base x 103 deci- d 0. 1 Base x 10 -1 centi- c 0. 01 Base x 10 -2 milli- m 0. 001 Base x 10 -3 micro- m 0. 000 001 Base x 10 -6 nano- n 0. 000 001 Base x 10 -9 pico p 0. 000 000 001 Base x 10 -12

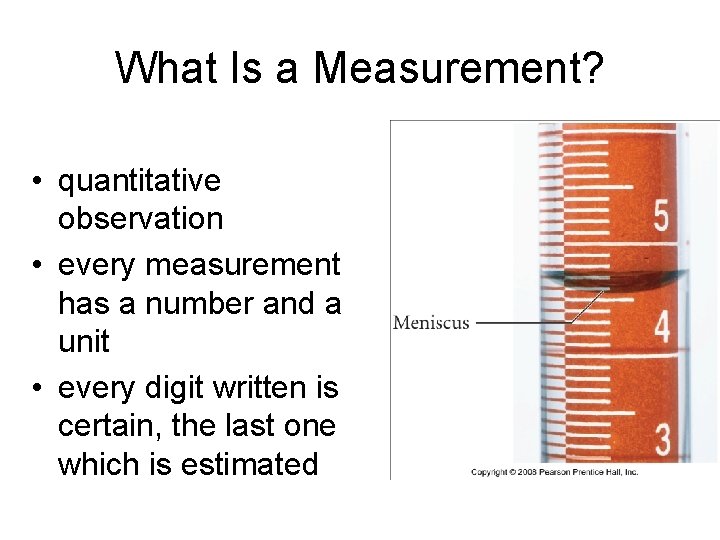
What Is a Measurement? • quantitative observation • every measurement has a number and a unit • every digit written is certain, the last one which is estimated


Estimation in Weighing • What is the uncertainty in this reading?

Thursday, 9/11/14 Learning Target: Students must be able to compare and contrast accuracy and precision in measurement. Learning Outcome: Complete “Measurement Lab” Chapter 1 20

Uncertainty in Measured Numbers uncertainty comes from: • limitations of the instruments used for comparison, • the experimental design, • the experimenter, • nature’s random behavior

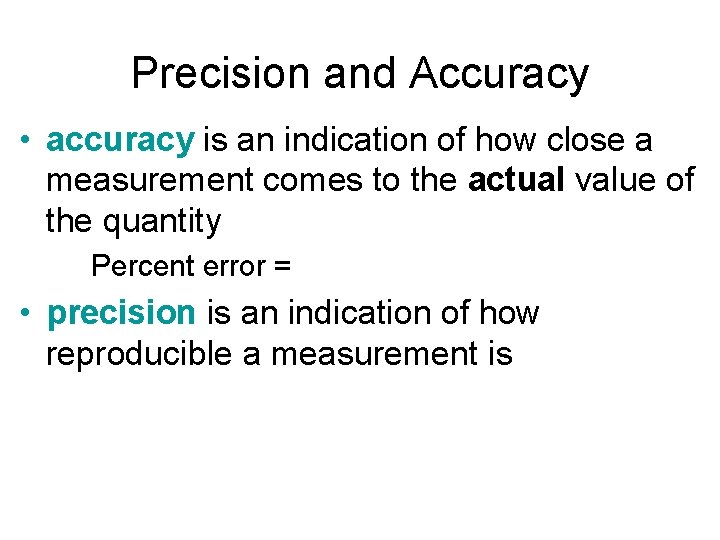
Precision and Accuracy • accuracy is an indication of how close a measurement comes to the actual value of the quantity Percent error = • precision is an indication of how reproducible a measurement is

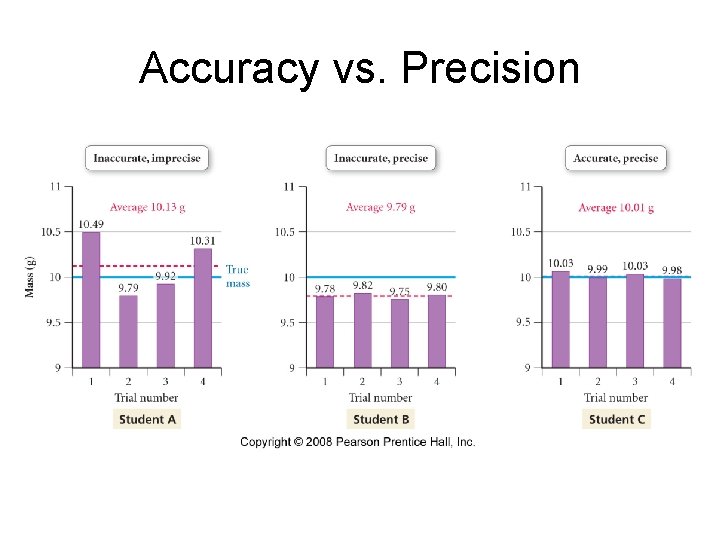
Accuracy vs. Precision

Precision • imprecision in measurements is caused by random errors – errors that result from random fluctuations • we determine the precision of a set of measurements by evaluating how far they are from the actual value and each other called standard deviation. • Do multiple trials to lesson error and improve precision.

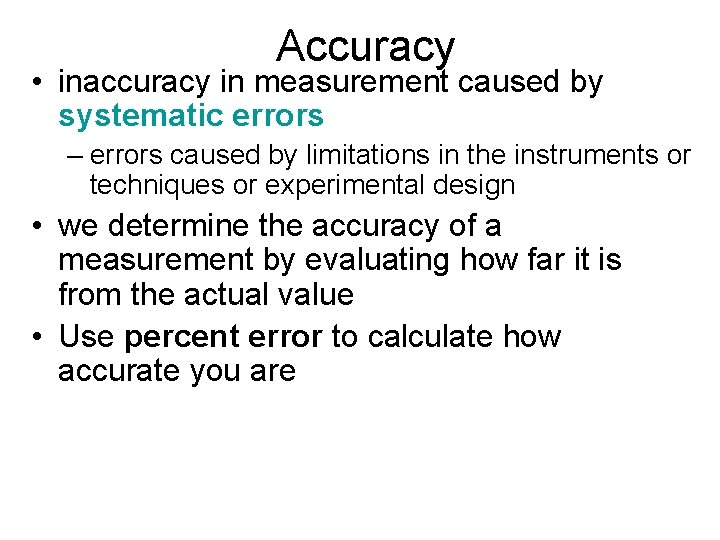
Accuracy • inaccuracy in measurement caused by systematic errors – errors caused by limitations in the instruments or techniques or experimental design • we determine the accuracy of a measurement by evaluating how far it is from the actual value • Use percent error to calculate how accurate you are

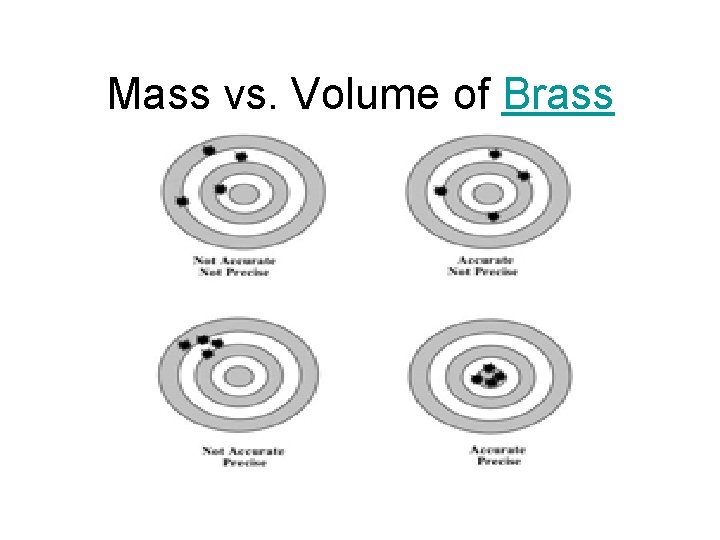
Mass & Volume • mass and volume are extensive properties – the value depends on the quantity of matter – extensive properties cannot be used to identify what type of matter something is • if you are given a large glass containing 100 g of a clear, colorless liquid and a small glass containing 25 g of a clear, colorless liquid - are both liquids the same stuff?

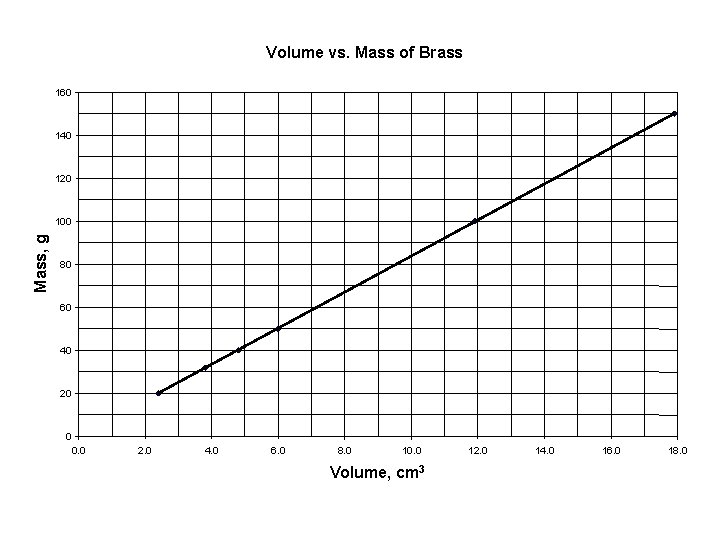
Mass vs. Volume of Brass

Monday 9/15/14 Learning Target: Know how to use significant figures in labs and in problems. Learning Outcome: Complete significant figures problems. Chapter 1 28

Accuracy versus Precision Chapter 1 29

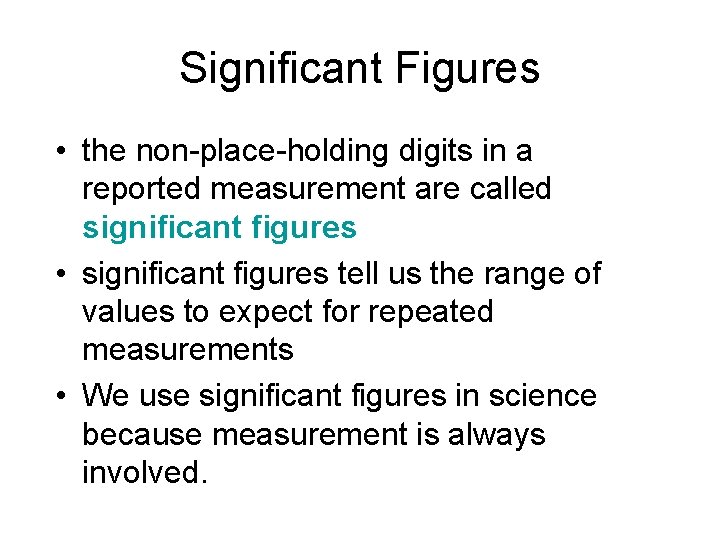
Significant Figures • the non-place-holding digits in a reported measurement are called significant figures • significant figures tell us the range of values to expect for repeated measurements • We use significant figures in science because measurement is always involved.

Counting Significant Figures 1) All non-zero digits are significant – 1. 5 has 2 sig. figs. 2) Interior zeros are significant – 1. 05 has 3 sig. figs. 3) Leading zeros are NOT significant – 0. 001050 has 4 sig. figs.

Counting Significant Figures 4) Trailing zeros may or may not be significant 1) If a decimal is present, trailing zeros are significant • 1. 050 has 4 sig. figs. 2) If a decimal is NOT present, trailing zeros are NOT significant. • if 150 has 2 sig. figs. then 1. 5 x 102 • but if 150. has 3 sig. figs. then 1. 50 x 102 **These are considered ambiguous and should be avoided by using scientific notation

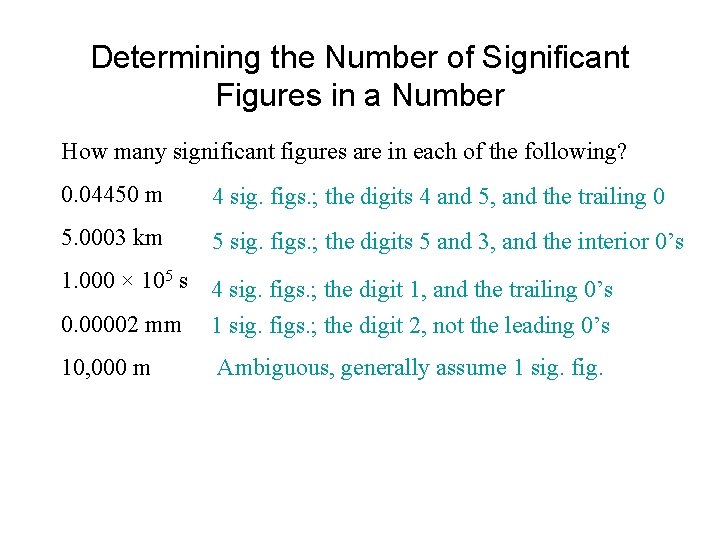
Determining the Number of Significant Figures in a Number How many significant figures are in each of the following? 0. 04450 m 4 sig. figs. ; the digits 4 and 5, and the trailing 0 5. 0003 km 5 sig. figs. ; the digits 5 and 3, and the interior 0’s 1. 000 × 105 s 4 sig. figs. ; the digit 1, and the trailing 0’s 0. 00002 mm 1 sig. figs. ; the digit 2, not the leading 0’s 10, 000 m Ambiguous, generally assume 1 sig. fig.

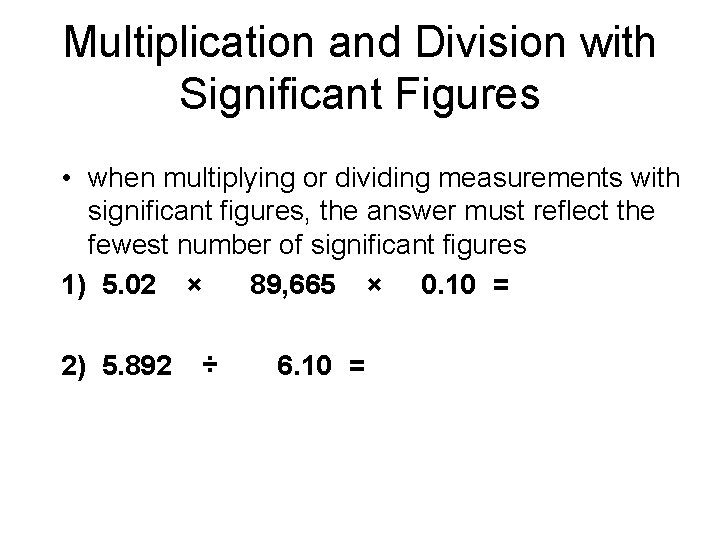
Multiplication and Division with Significant Figures • when multiplying or dividing measurements with significant figures, the answer must reflect the fewest number of significant figures 1) 5. 02 × 89, 665 × 0. 10 = 2) 5. 892 ÷ 6. 10 =

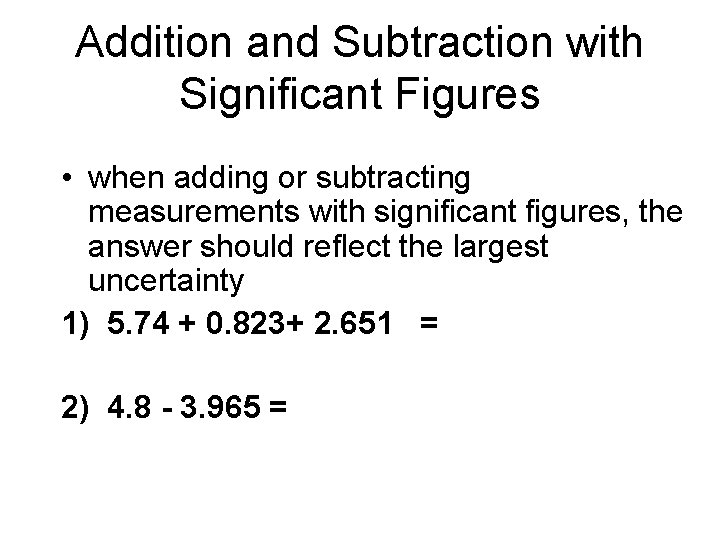
Addition and Subtraction with Significant Figures • when adding or subtracting measurements with significant figures, the answer should reflect the largest uncertainty 1) 5. 74 + 0. 823+ 2. 651 = 2) 4. 8 - 3. 965 =

Rounding if the number after the place of the last significant figure is: 0 to 4, round down – drop all digits after the last sig. fig. and leave the last sig. fig. alone 5 to 9, round up – drop all digits after the last sig. fig. and increase the last sig. fig. by one To avoid accumulating extra error from rounding, round only at the end, keeping track of the last sig. for intermediate calculations

Rounding rounding to 2 significant figures • 2. 34 rounds to 2. 3 • 2. 37 rounds to 2. 4 • 2. 349865 rounds to 2. 3

Rounding rounding to 2 significant figures • 0. 0234 rounds to 0. 023 • 0. 0237 rounds to 0. 024 • 0. 02349865 rounds to 0. 023

Rounding rounding to 2 significant figures • 234 rounds to 230 • 237 rounds to 240 • 234. 9865 rounds to 230

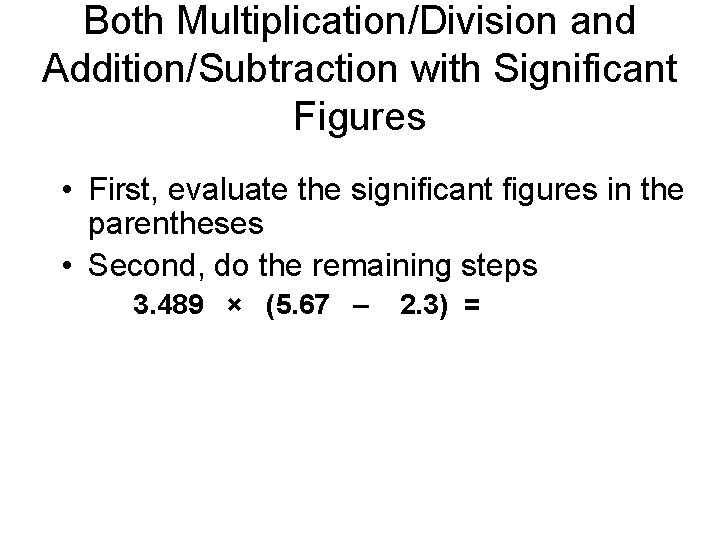
Both Multiplication/Division and Addition/Subtraction with Significant Figures • First, evaluate the significant figures in the parentheses • Second, do the remaining steps 3. 489 × (5. 67 – 2. 3) =

Perform the following calculations to the correct number of significant figures b)

Example 1. 6 Perform the following calculations to the correct number of significant figures b)

Tuesday 9/16/14 Learning Target: Know how to use and convert numbers into scientific notation. Learning Outcome: I will be able to use scientific notation in problems and convert standard notation into scientific notation. Chapter 1 43

• Why are significant figures not important in your math class? Chapter 1 44

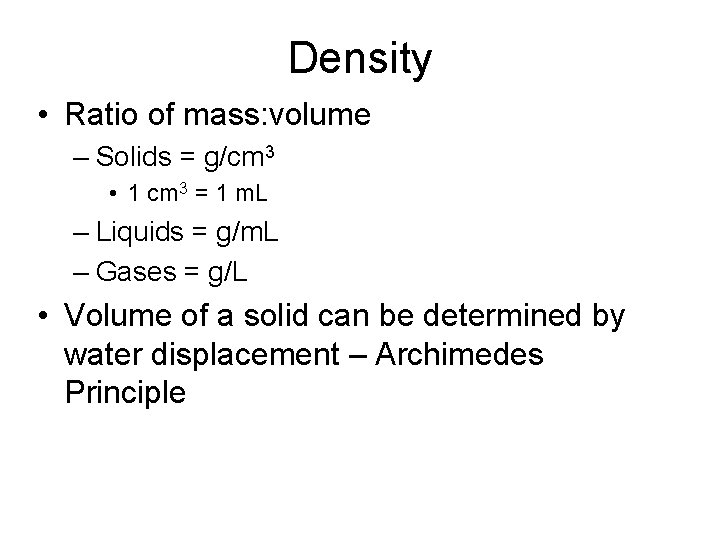
Density • Ratio of mass: volume – Solids = g/cm 3 • 1 cm 3 = 1 m. L – Liquids = g/m. L – Gases = g/L • Volume of a solid can be determined by water displacement – Archimedes Principle

Density • Density : solids > liquids >>> gases – except ice is less dense than liquid water! • Heating an object generally causes it to expand, therefore the density changes with temperature

Density • Iron has a density of 7. 86 g/cm 3. Could a block of metal with a mass of 18. 2 g and a volume of 2. 56 cm 3 be iron?

Density • What volume would a 0. 871 g sample of air occupy if the density of air is 1. 29 g/L?

Wednesday, 9/17/14 Learning Target: Be able to apply dimensional analysis to convert from one unit of measure to another. Learning Outcome: I will be able to complete single-step unit conversion problems. Chapter 1 49

Units • Always include units in your calculations – you can do the same kind of operations on units as you can with numbers • cm × cm = cm 2 • cm + cm = cm • cm ÷ cm = 1

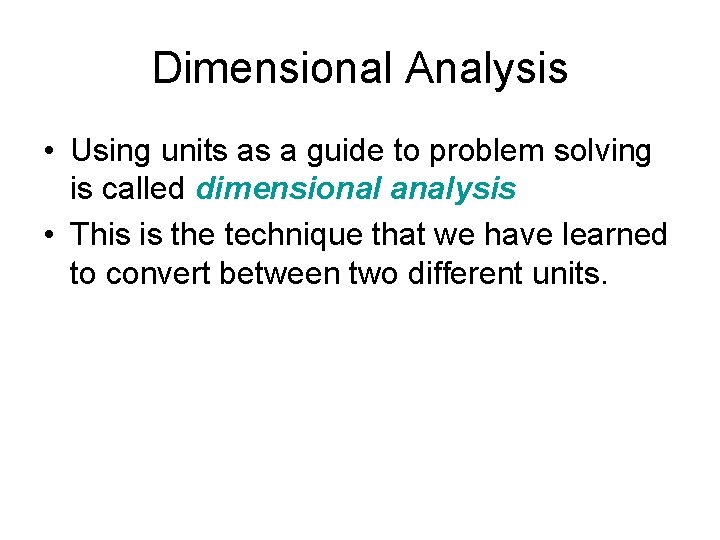
Dimensional Analysis • Using units as a guide to problem solving is called dimensional analysis • This is the technique that we have learned to convert between two different units.

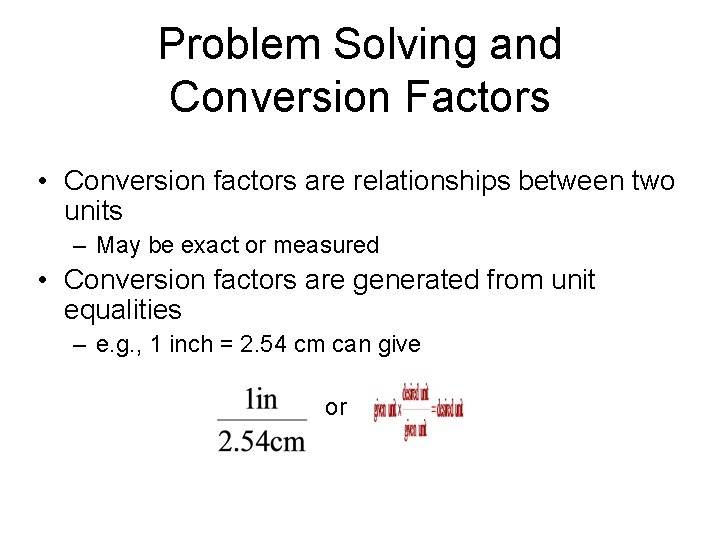
Problem Solving and Conversion Factors • Conversion factors are relationships between two units – May be exact or measured • Conversion factors are generated from unit equalities – e. g. , 1 inch = 2. 54 cm can give or

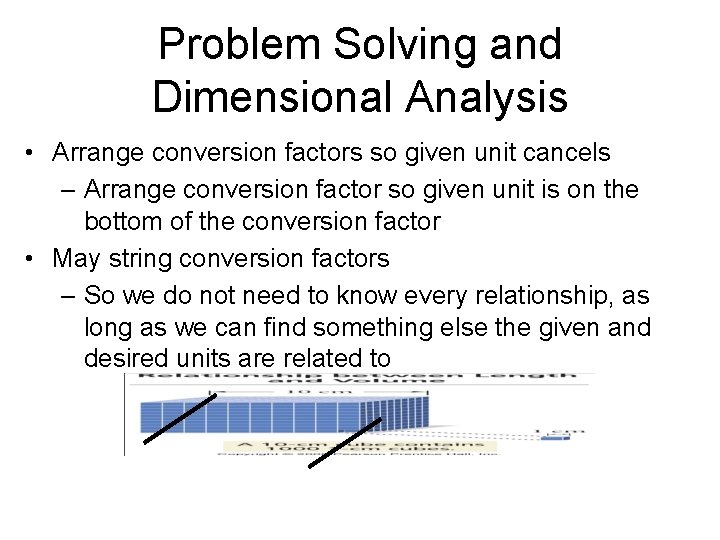
Problem Solving and Dimensional Analysis • Arrange conversion factors so given unit cancels – Arrange conversion factor so given unit is on the bottom of the conversion factor • May string conversion factors – So we do not need to know every relationship, as long as we can find something else the given and desired units are related to

• Using a ruler from the front counter, measure the length, width and height of a Chemistry textbook to the nearest 1 cm. • How many meters wide is it? • How many inches is the width of the textbook (2. 54 cm = 1 in)? • How many feet is your textbook? 54

Thursday, 9/18/14 Learning Target: Be able to apply dimensional analysis to convert from one unit of measure to another. Learning Outcome: I will be able to complete multi-step unit conversion problems. Chapter 1 55

Warm-Up Convert – 232. 1 k. Pa to Pa Chapter 1 56

Practice – Convert 154. 4 lbs to kg

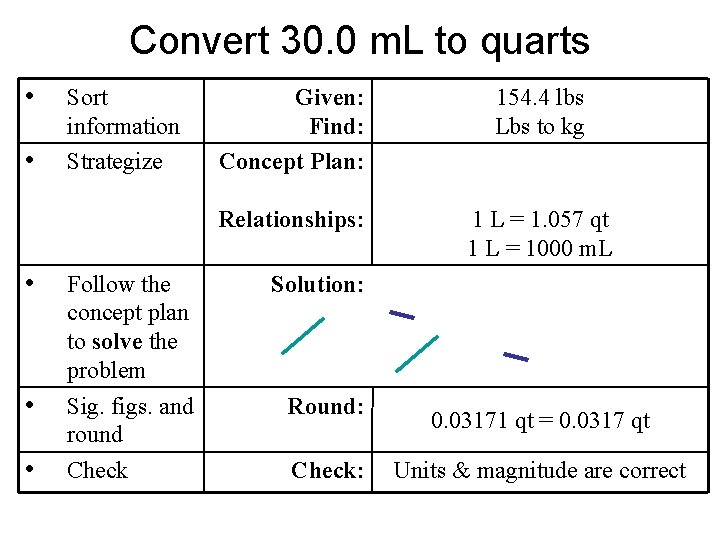
Practice – Convert 30. 0 m. L to quarts (1 L = 1. 057 qt)

Volume • Derived unit (width x length x height) – any length unit cubed • Measure of the amount of space occupied • SI unit = cubic meter (m 3) • Commonly measure solid volume in cubic centimeters (cm 3) – 1 m 3 = 106 cm 3 • Commonly measure liquid or gas volume in milliliters (m. L) – 1 L is slightly larger than 1 quart – 1 m. L = 1 cm 3
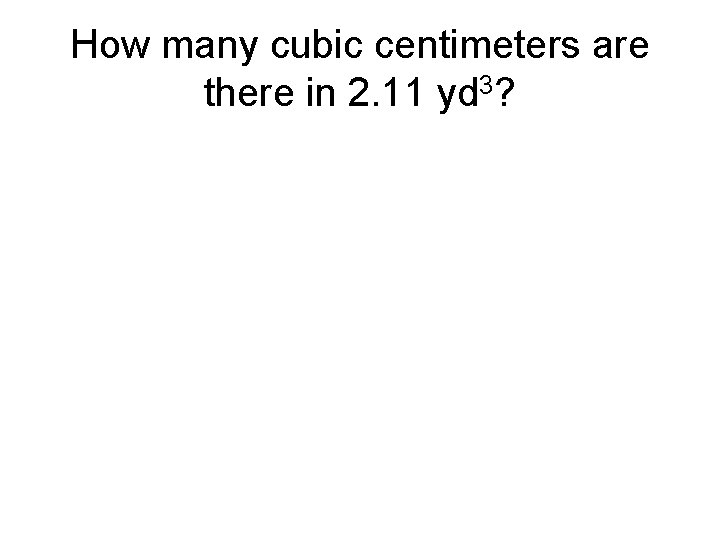
How many cubic centimeters are there in 2. 11 yd 3?

Impossible Conversions • Is it possible to find how many seconds in a kilogram? • In order to do unit conversions they must be able to correspond to the same quantity. – For example, kilograms and pounds are both units of mass.

Graphing in Science • All graphing that is done in science must include the following: 1. A descriptive title 2. X and Y axis labeled with units. 3. The X – axis is the manipulated variable and the Y- axis is the responding variable. 4. A trend line (or line of best fit) to show the trend in the data that has been plotted.

Volume vs. Mass of Brass 160 140 120 Mass, g 100 80 60 40 20 0 0. 0 2. 0 4. 0 6. 0 8. 0 10. 0 Volume, cm 3 12. 0 14. 0 16. 0 18. 0

Convert 30. 0 m. L to quarts • • • Sort information Strategize Follow the concept plan to solve the problem Sig. figs. and round Check Given: Find: Concept Plan: 154. 4 lbs Lbs to kg Relationships: 1 L = 1. 057 qt 1 L = 1000 m. L Solution: Round: Check: 0. 03171 qt = 0. 0317 qt Units & magnitude are correct

Scientific Investigations • Science is the methodical exploration of nature followed by a logical explanation of the observations. • Scientific investigation entails: – planning an investigation – carefully recording observations – gathering data – analyzing the results 65

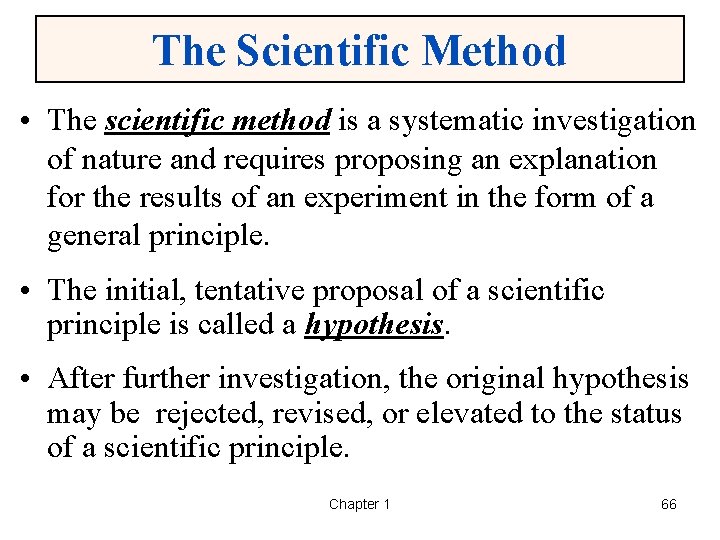
The Scientific Method • The scientific method is a systematic investigation of nature and requires proposing an explanation for the results of an experiment in the form of a general principle. • The initial, tentative proposal of a scientific principle is called a hypothesis. • After further investigation, the original hypothesis may be rejected, revised, or elevated to the status of a scientific principle. Chapter 1 66

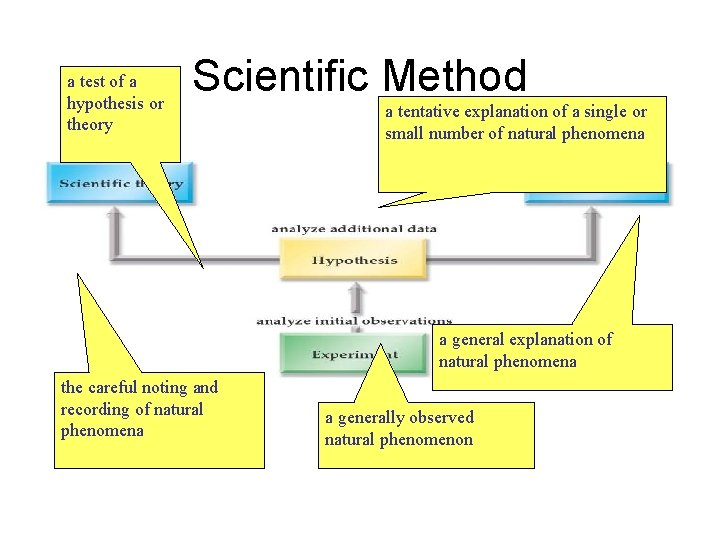
a test of a hypothesis or theory Scientific Method a tentative explanation of a single or small number of natural phenomena a general explanation of natural phenomena the careful noting and recording of natural phenomena a generally observed natural phenomenon

Conclusions Continued • After sufficient evidence, a hypothesis becomes a scientific theory. • A natural law is a measurable relationship. Chapter 1 68

Conclusions • Scientists use the scientific method to investigate the world around them. • Experiments lead to a hypothesis, which may lead to a scientific theory or a natural law. • Chemistry is a central science with many branches. • The impact of chemistry is felt in many aspects of our daily lives. Chapter 1 69

QUIZE - CHAPTER -1 1. What is the difference between a hypothesis and theory 2. According to the ancient Greeks, which of the following are not basic elements found in nature: I. Air II. Coal III. Fire IV. Earth V. Gold VI. Water Chapter 1 70
 Introductory chemistry concepts and critical thinking
Introductory chemistry concepts and critical thinking Introductory chemistry 4th edition
Introductory chemistry 4th edition Prefix multipliers
Prefix multipliers Introductory chemistry 5th edition answers
Introductory chemistry 5th edition answers Nivaldo j. tro introductory chemistry
Nivaldo j. tro introductory chemistry Tuesday with morrie chapter 1
Tuesday with morrie chapter 1 Chapter 2 descriptive statistics answer key
Chapter 2 descriptive statistics answer key Tuesday morning prayer for family and friends
Tuesday morning prayer for family and friends On monday and tuesday
On monday and tuesday Lesson 1-1 variables and expressions answer key
Lesson 1-1 variables and expressions answer key Numerical
Numerical Intro conclusion
Intro conclusion Using therefore in middle of sentence
Using therefore in middle of sentence Commas after introductory phrases
Commas after introductory phrases Wonnacott and wonnacott introductory statistics pdf
Wonnacott and wonnacott introductory statistics pdf Signal phrasing
Signal phrasing Tell me what you eat and i shall tell you what you are
Tell me what you eat and i shall tell you what you are Monday tuesday wednesday thursday friday calendar
Monday tuesday wednesday thursday friday calendar Tuesday please
Tuesday please Saturday morning prayer
Saturday morning prayer Feathered friend by arthur c clarke
Feathered friend by arthur c clarke Tuesday morning prayer
Tuesday morning prayer Prayer for tuesday morning
Prayer for tuesday morning Tuesday night prayer images
Tuesday night prayer images Stock market crash cartoon 1929
Stock market crash cartoon 1929 Black tuesday political cartoon
Black tuesday political cartoon John gave a bar of chocolate to jill.
John gave a bar of chocolate to jill. Pancake tuesday 2012
Pancake tuesday 2012 Dua for tuesday
Dua for tuesday Sunday monday tuesday thursday friday saturday
Sunday monday tuesday thursday friday saturday Monday tuesday wednesday thursday friday saturday sunday
Monday tuesday wednesday thursday friday saturday sunday Week 10 dgp
Week 10 dgp On a tuesday
On a tuesday Aphorisms in tuesdays with morrie
Aphorisms in tuesdays with morrie Tuesday friends
Tuesday friends Wednesday bellwork
Wednesday bellwork Monday tuesday
Monday tuesday Marvelous monday terrific tuesday wonderful wednesday
Marvelous monday terrific tuesday wonderful wednesday Tuesday homework
Tuesday homework Thrilling tuesday
Thrilling tuesday Enum day sunday=1 monday tuesday=5
Enum day sunday=1 monday tuesday=5 Tuesday bellringer
Tuesday bellringer Tuesday warm up questions
Tuesday warm up questions What did you do last weekend conversation
What did you do last weekend conversation Adjectives for tuesday
Adjectives for tuesday Shrove tuesday quiz
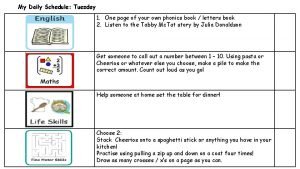
Shrove tuesday quiz My tuesday schedule
My tuesday schedule Sentences from before
Sentences from before Wire delay
Wire delay Is today tuesday
Is today tuesday Good morning tuesday stay safe images
Good morning tuesday stay safe images Tuesday evening prayer
Tuesday evening prayer Flashback tuesday
Flashback tuesday Evening prayer
Evening prayer Tuesday the 6th
Tuesday the 6th The last week of jesus life
The last week of jesus life Great depression 1929
Great depression 1929 Tuesday evening prayer
Tuesday evening prayer Lobsang rampa prophecy
Lobsang rampa prophecy Working tuesday
Working tuesday Wednesday or thursday
Wednesday or thursday 1
1 Monday=621 tuesday=732 wednesday=933
Monday=621 tuesday=732 wednesday=933 Sunday, tuesday, january, saturday
Sunday, tuesday, january, saturday Due tuesday
Due tuesday Overview tuesday
Overview tuesday Tuesday supper
Tuesday supper Breakfast tuesday
Breakfast tuesday Good morning miss in german
Good morning miss in german Ela lab
Ela lab Tuesday bell ringer
Tuesday bell ringer