Chemistry 1011 Introductory Chemistry II http www mi



- Slides: 17

Chemistry 1011 Introductory Chemistry II http: //www. mi. mun. ca/~pfisher/chemistry. html Chemistry 1011 Slot 5 1

Chemistry 1011 TOPIC Rate of Reaction TEXT REFERENCE Masterton and Hurley Chapter 11 Chemistry 1011 Slot 5 2

11. 1 Meaning of Reaction Rate YOU ARE EXPECTED TO BE ABLE TO: • Define the average rate and instantaneous rate of a chemical reaction in terms of reactant and/or product concentration. • Sketch a concentration vs time curve for a reactant of product in a reaction given experimental data. • Calculate average rate or instantaneous rate of reaction from concentration vs time data. • State the qualitative effects of changes in concentration, temperature, surface area of solid reactants and the presence of a catalyst on rate of reaction. Chemistry 1011 Slot 5 3

Rate of Reaction – What is it? • Some exothermic reactions proceed very quickly at room temperature P 4(s) + 5 O 2(g) P 4 O 10(s) • Other exothermic reactions proceed very slowly at room temperature C 6 H 12 O 6(s) + 5 O 2(g) 6 CO 2(g) + 6 H 2 O(l) • Other reactions, such as the burning of a match after striking, require an initial input of energy Chemistry 1011 Slot 5 4

Rate of Reaction – What is it? • The rate of a reaction is a measure of how fast a reaction proceeds • The rate of a reaction is a measure of how fast a reactant is used up or how fast a product is formed • The rate is a measure of the change in concentration of reactant or product with time Chemistry 1011 Slot 5 5

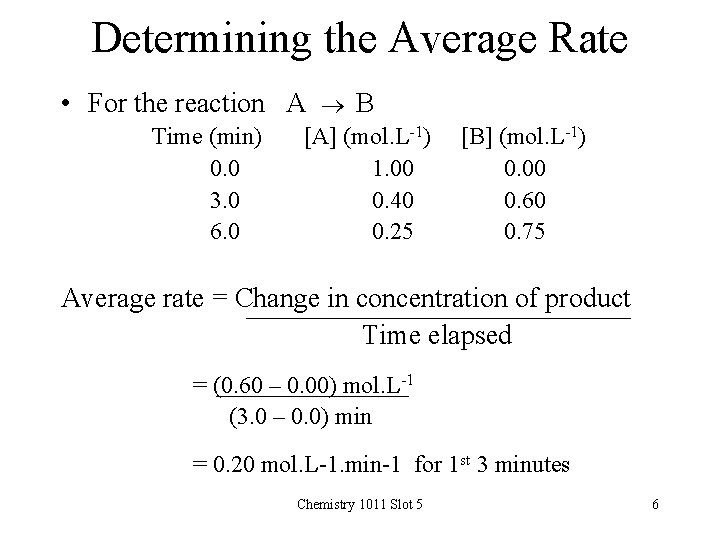
Determining the Average Rate • For the reaction A B Time (min) 0. 0 3. 0 6. 0 [A] (mol. L-1) 1. 00 0. 40 0. 25 [B] (mol. L-1) 0. 00 0. 60 0. 75 Average rate = Change in concentration of product Time elapsed = (0. 60 – 0. 00) mol. L-1 (3. 0 – 0. 0) min = 0. 20 mol. L-1. min-1 for 1 st 3 minutes Chemistry 1011 Slot 5 6

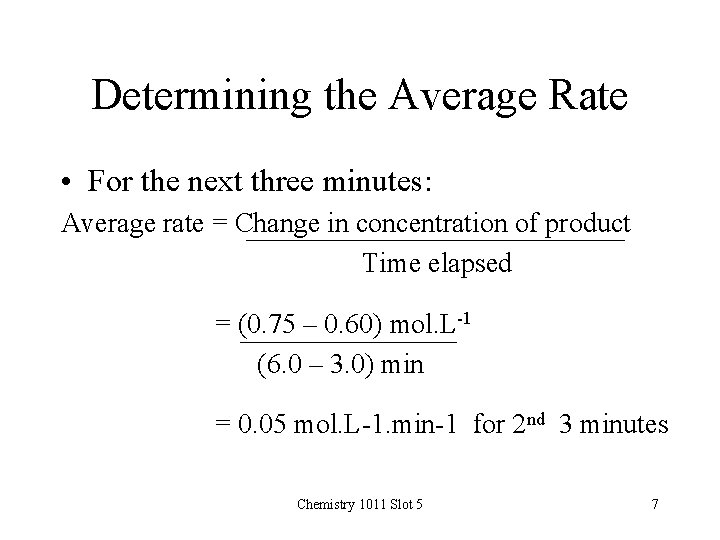
Determining the Average Rate • For the next three minutes: Average rate = Change in concentration of product Time elapsed = (0. 75 – 0. 60) mol. L-1 (6. 0 – 3. 0) min = 0. 05 mol. L-1. min-1 for 2 nd 3 minutes Chemistry 1011 Slot 5 7

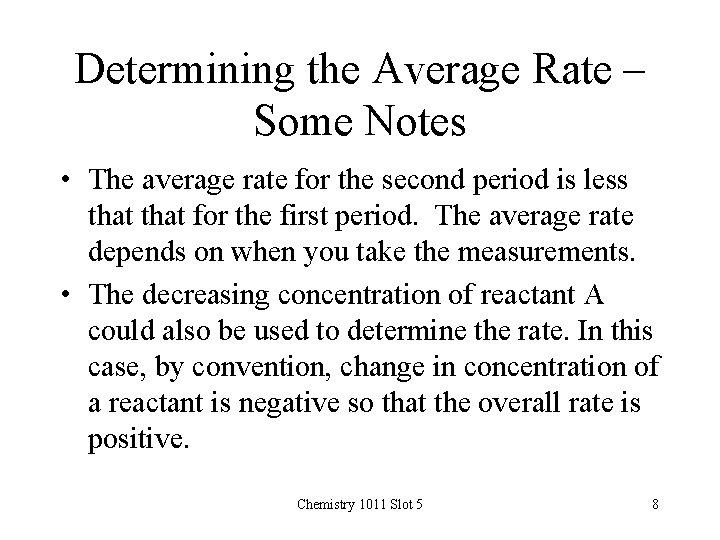
Determining the Average Rate – Some Notes • The average rate for the second period is less that for the first period. The average rate depends on when you take the measurements. • The decreasing concentration of reactant A could also be used to determine the rate. In this case, by convention, change in concentration of a reactant is negative so that the overall rate is positive. Chemistry 1011 Slot 5 8

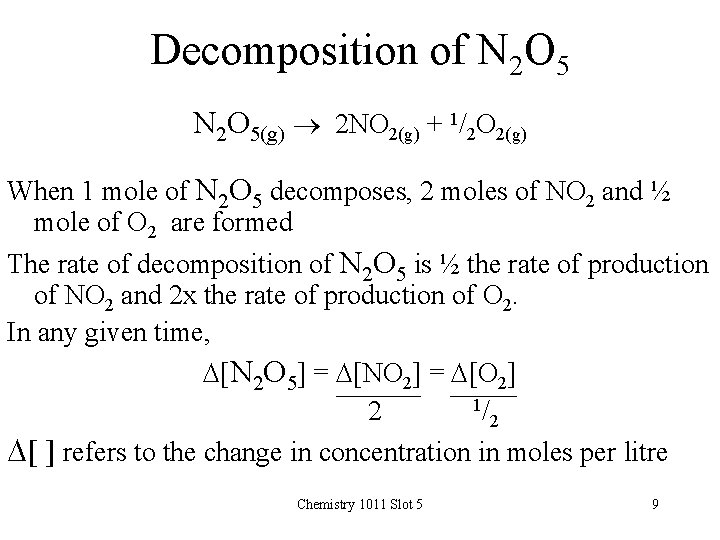
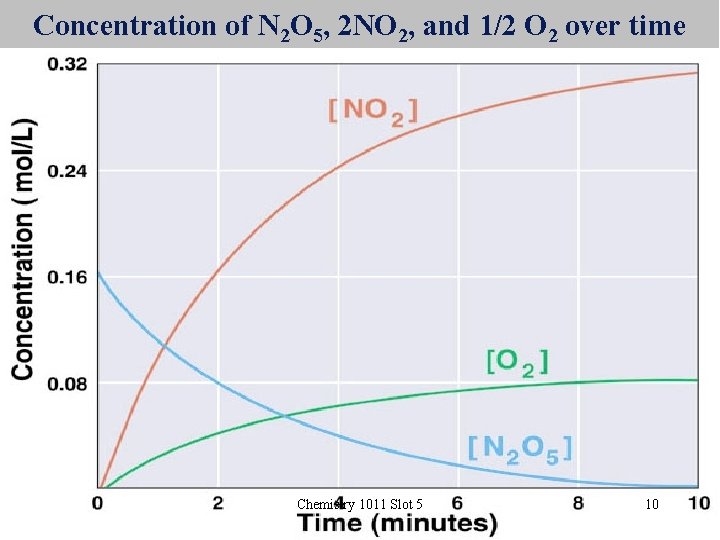
Decomposition of N 2 O 5(g) 2 NO 2(g) + 1/2 O 2(g) When 1 mole of N 2 O 5 decomposes, 2 moles of NO 2 and ½ mole of O 2 are formed The rate of decomposition of N 2 O 5 is ½ the rate of production of NO 2 and 2 x the rate of production of O 2. In any given time, D[N 2 O 5] = D[NO 2] = D[O 2] 1/ 2 2 D[ ] refers to the change in concentration in moles per litre Chemistry 1011 Slot 5 9

Concentration of N 2 O 5, 2 NO 2, and 1/2 O 2 over time Chemistry 1011 Slot 5 10

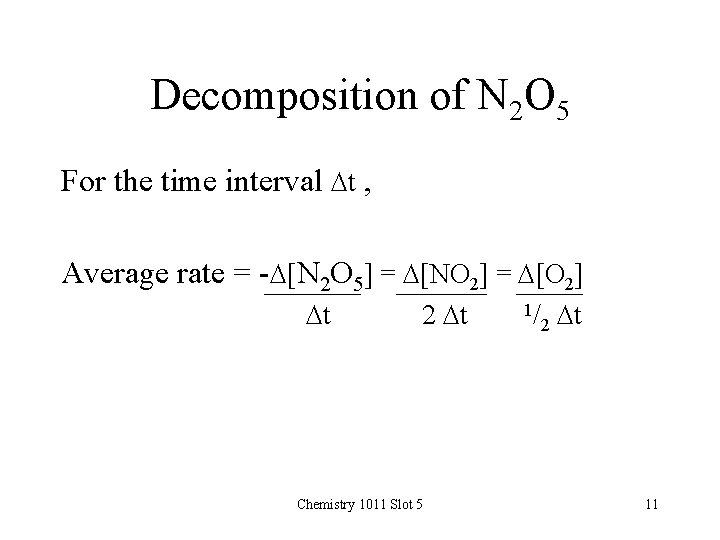
Decomposition of N 2 O 5 For the time interval Dt , Average rate = -D[N 2 O 5] = D[NO 2] = D[O 2] Dt 2 Dt Chemistry 1011 Slot 5 1/ 2 Dt 11

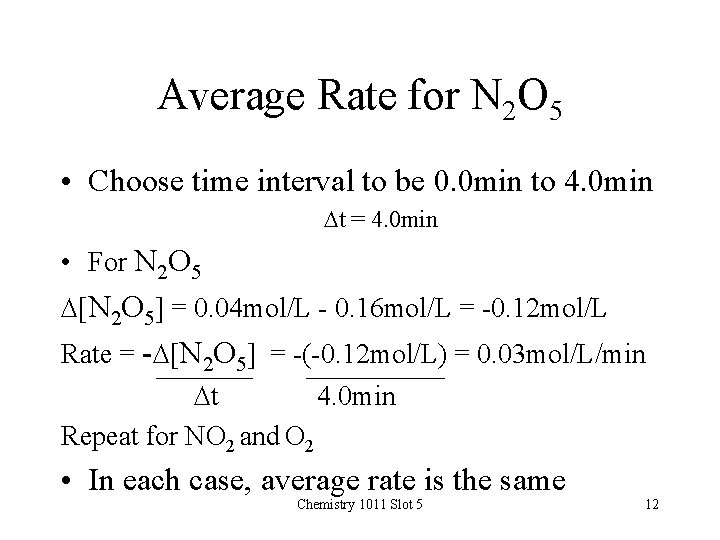
Average Rate for N 2 O 5 • Choose time interval to be 0. 0 min to 4. 0 min Dt = 4. 0 min • For N 2 O 5 D[N 2 O 5] = 0. 04 mol/L - 0. 16 mol/L = -0. 12 mol/L Rate = -D[N 2 O 5] = -(-0. 12 mol/L) = 0. 03 mol/L/min Dt 4. 0 min Repeat for NO 2 and O 2 • In each case, average rate is the same Chemistry 1011 Slot 5 12

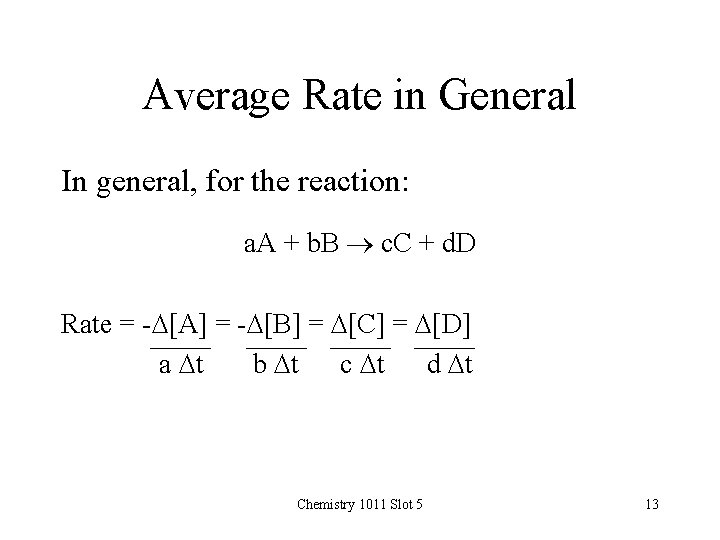
Average Rate in General In general, for the reaction: a. A + b. B c. C + d. D Rate = -D[A] = -D[B] = D[C] = D[D] a Dt b Dt c Dt d Dt Chemistry 1011 Slot 5 13

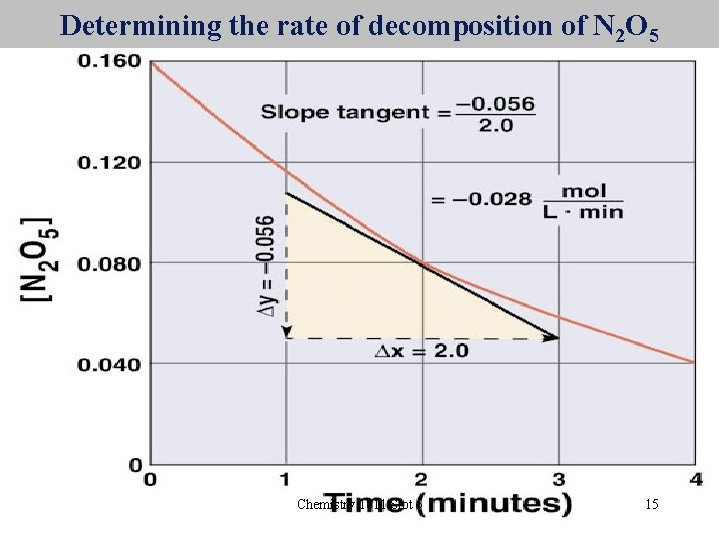
Instantaneous Rate • Instead of the average rate over a period of time, it is possible to determine the instantaneous rate. • This can be done by determining the slope of the tangent to the concentration vs time curve at the desired point • For N 2 O 5 at t = 2 minutes, the rate is 0. 28 mol/L/min Chemistry 1011 Slot 5 14

Determining the rate of decomposition of N 2 O 5 Chemistry 1011 Slot 5 15

Sketching a Concentration vs Time Curve • Textbook p 332 Q 7 • For A B + C Time (sec) [B] 0 2 4 6 8 10 0 0. 100 0. 130 0. 150 0. 165 0. 175 • Sketch the curve • Find instantaneous rate at t = 4 seconds Chemistry 1011 Slot 5 16

Factors Affecting the Rate • Concentration of reactants – Higher concentrations faster rates • Surface area of solid reactants – More surface area faster rates • Temperature – Higher temperatures faster rates • Catalysts – Speed up reactions without being consumed Chemistry 1011 Slot 5 17
 Introductory chemistry concepts and critical thinking
Introductory chemistry concepts and critical thinking Introductory chemistry 5th edition nivaldo j. tro
Introductory chemistry 5th edition nivaldo j. tro Introductory chemistry 5th edition answers
Introductory chemistry 5th edition answers Nivaldo j. tro introductory chemistry
Nivaldo j. tro introductory chemistry Introductory chemistry 4th edition
Introductory chemistry 4th edition 1001 1011
1001 1011 Các dạng biểu diễn số theo chuẩn ieee 754/85
Các dạng biểu diễn số theo chuẩn ieee 754/85 Decreto 1011 de 2006
Decreto 1011 de 2006 Types of positional number system
Types of positional number system B 1011
B 1011 Year 1011
Year 1011 Eecs 1011
Eecs 1011 Penjumlahan bilangan bcd
Penjumlahan bilangan bcd 1001 1011
1001 1011 Readone piece 1011
Readone piece 1011 0011 0011 1011 0001 0010 1000 0101
0011 0011 1011 0001 0010 1000 0101 En 1011
En 1011 Http //mbs.meb.gov.tr/ http //www.alantercihleri.com
Http //mbs.meb.gov.tr/ http //www.alantercihleri.com