Chemistry 1011 Introductory Chemistry II http www mi










- Slides: 24

Chemistry 1011 Introductory Chemistry II http: //www. mi. mun. ca/~pfisher/chemistry. html Password for final exams Midgley Chemistry 1011 Slot 5 1

Chemistry 1011 TOPIC Electrochemistry TEXT REFERENCE Masterton and Hurley Chapter 18 Chemistry 1011 Slot 5 2

18. 2 Standard Voltages YOU ARE EXPECTED TO BE ABLE TO: • Define the standard electrode potential of a half cell • Order species according to their ease of oxidation or reduction based on a table of standard reduction potentials • Calculate the net cell voltage, Eo, of a combination of half cells from standard electrode potential data • Determine whether a given redox reaction will be spontaneous or non-spontaneous Chemistry 1011 Slot 5 3

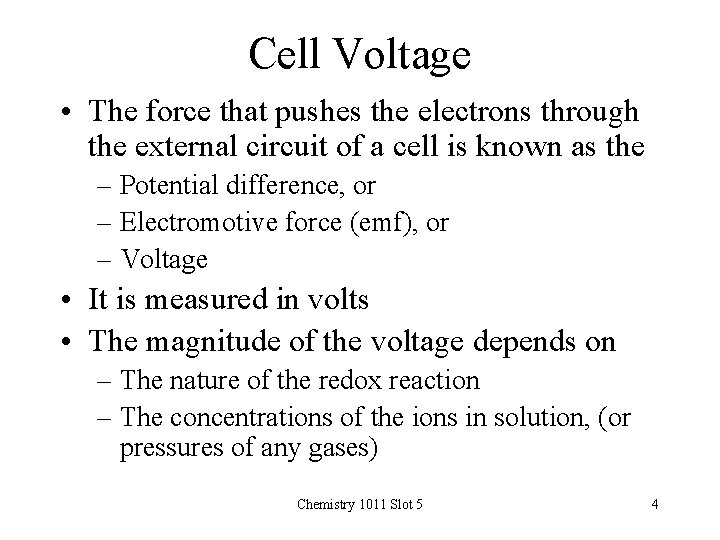
Cell Voltage • The force that pushes the electrons through the external circuit of a cell is known as the – Potential difference, or – Electromotive force (emf), or – Voltage • It is measured in volts • The magnitude of the voltage depends on – The nature of the redox reaction – The concentrations of the ions in solution, (or pressures of any gases) Chemistry 1011 Slot 5 4

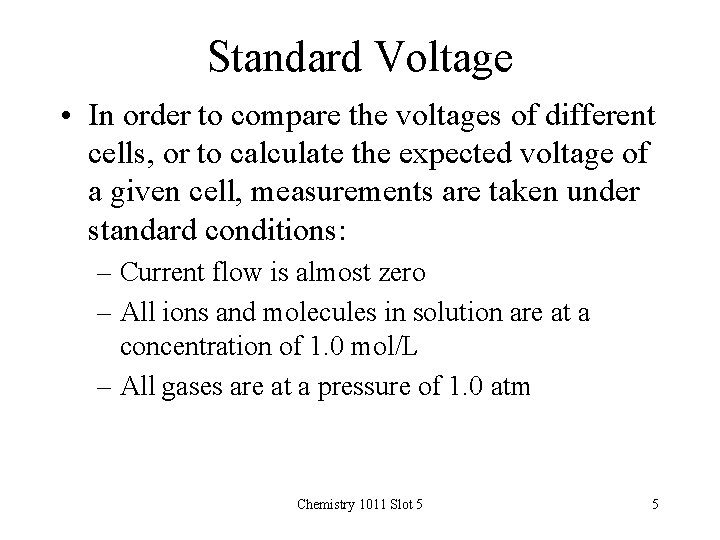
Standard Voltage • In order to compare the voltages of different cells, or to calculate the expected voltage of a given cell, measurements are taken under standard conditions: – Current flow is almost zero – All ions and molecules in solution are at a concentration of 1. 0 mol/L – All gases are at a pressure of 1. 0 atm Chemistry 1011 Slot 5 5

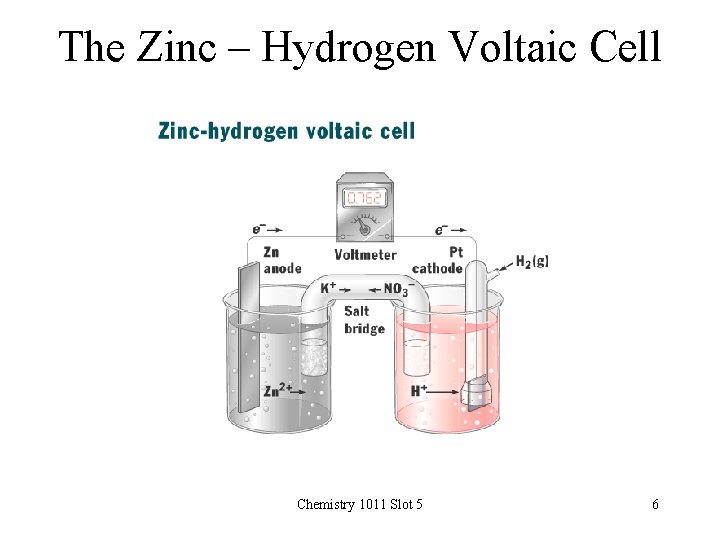
The Zinc – Hydrogen Voltaic Cell Chemistry 1011 Slot 5 6

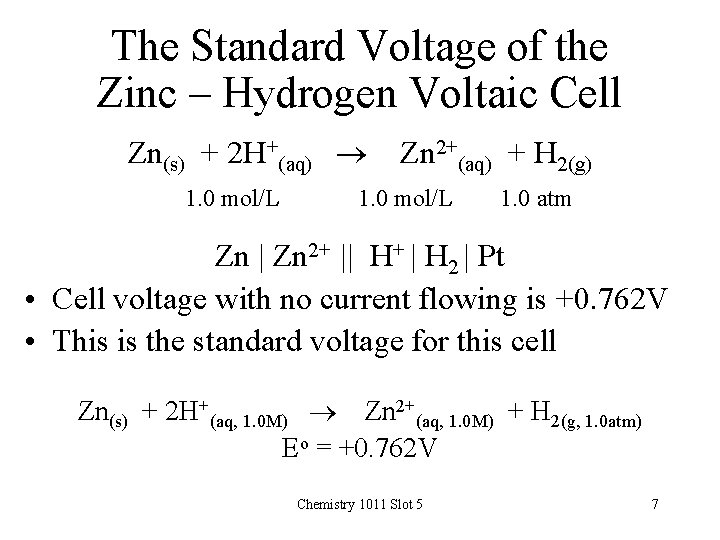
The Standard Voltage of the Zinc – Hydrogen Voltaic Cell Zn(s) + 2 H+(aq) 1. 0 mol/L Zn 2+(aq) + H 2(g) 1. 0 mol/L 1. 0 atm Zn | Zn 2+ || H+ | H 2 | Pt • Cell voltage with no current flowing is +0. 762 V • This is the standard voltage for this cell Zn(s) + 2 H+(aq, 1. 0 M) Zn 2+(aq, 1. 0 M) + H 2(g, 1. 0 atm) Eo = +0. 762 V Chemistry 1011 Slot 5 7

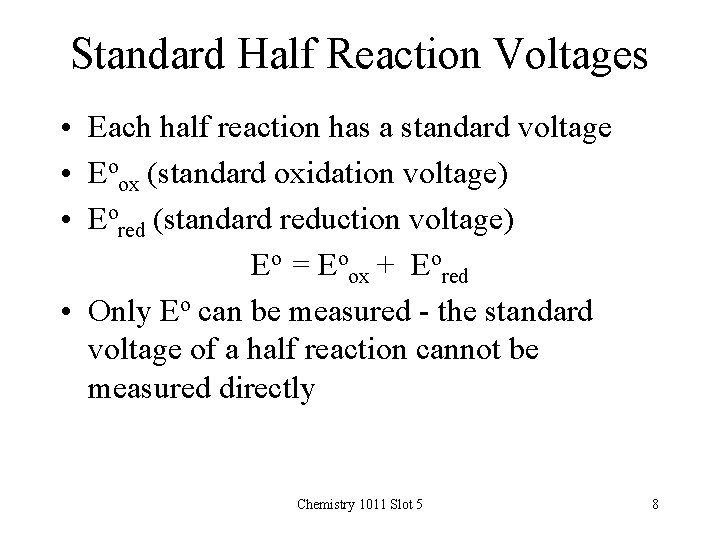
Standard Half Reaction Voltages • Each half reaction has a standard voltage • Eoox (standard oxidation voltage) • Eored (standard reduction voltage) Eo = Eoox + Eored • Only Eo can be measured - the standard voltage of a half reaction cannot be measured directly Chemistry 1011 Slot 5 8

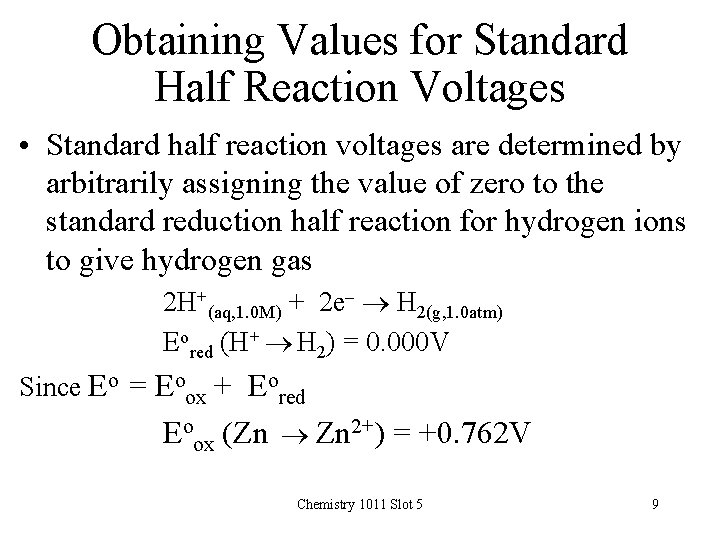
Obtaining Values for Standard Half Reaction Voltages • Standard half reaction voltages are determined by arbitrarily assigning the value of zero to the standard reduction half reaction for hydrogen ions to give hydrogen gas 2 H+(aq, 1. 0 M) + 2 e- H 2(g, 1. 0 atm) Eored (H+ H 2) = 0. 000 V Since Eo = Eoox + Eored Eoox (Zn Zn 2+) = +0. 762 V Chemistry 1011 Slot 5 9

Obtaining Values for Standard Half Reaction Voltages • Once one half reaction standard voltage is established, others can be deduced: • For: Zn(s) + Cu 2+(aq) Zn 2+(aq) + Cu(s) standard cell voltage is +1. 101 V Zn(s) Zn 2+(aq, 1. 0 M) + 2 e. Cu 2+(aq, 1. 0 M) + 2 e- Cu(s) the Eoox (Zn Zn 2+) = +0. 762 V Eored (Cu 2+ Cu) = ? ? V Since Eo = Eoox + Eored +1. 101 V = +0. 762 V + Eored = +0. 339 V Chemistry 1011 Slot 5 10

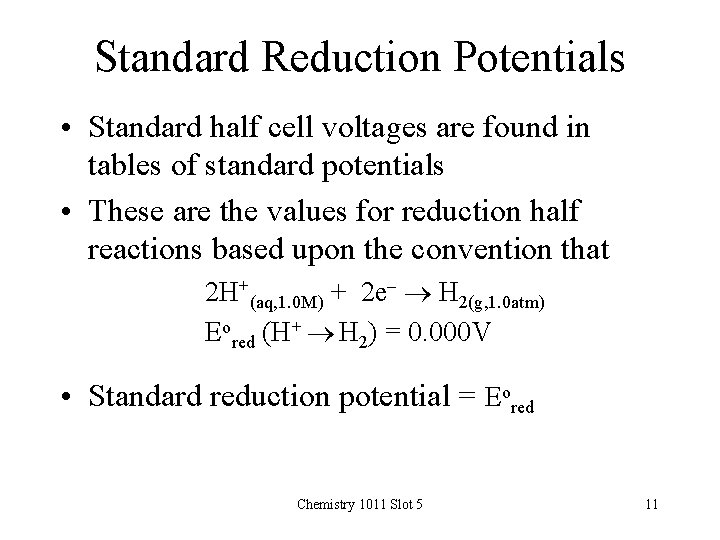
Standard Reduction Potentials • Standard half cell voltages are found in tables of standard potentials • These are the values for reduction half reactions based upon the convention that 2 H+(aq, 1. 0 M) + 2 e- H 2(g, 1. 0 atm) Eored (H+ H 2) = 0. 000 V • Standard reduction potential = Eored Chemistry 1011 Slot 5 11

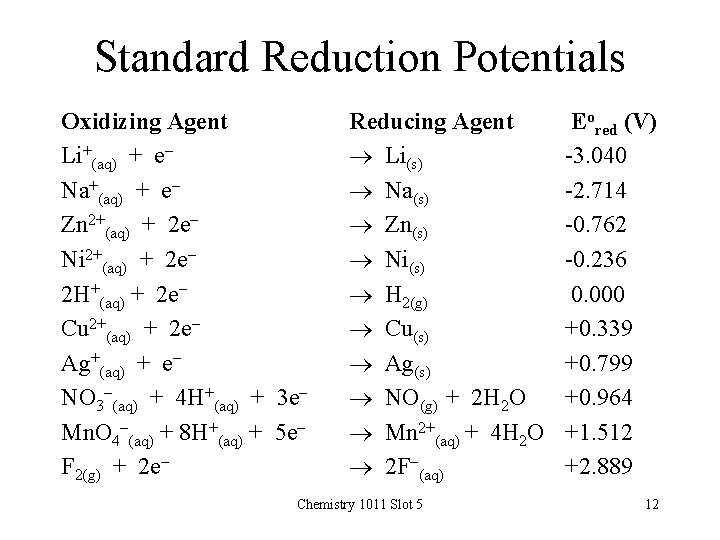
Standard Reduction Potentials Oxidizing Agent Li+(aq) + e. Na+(aq) + e. Zn 2+(aq) + 2 e. Ni 2+(aq) + 2 e 2 H+(aq) + 2 e. Cu 2+(aq) + 2 e. Ag+(aq) + e. NO 3 -(aq) + 4 H+(aq) + 3 e. Mn. O 4 -(aq) + 8 H+(aq) + 5 e. F 2(g) + 2 e- Reducing Agent Li(s) Na(s) Zn(s) Ni(s) H 2(g) Cu(s) Ag(s) NO(g) + 2 H 2 O Mn 2+(aq) + 4 H 2 O 2 F-(aq) Chemistry 1011 Slot 5 Eored (V) -3. 040 -2. 714 -0. 762 -0. 236 0. 000 +0. 339 +0. 799 +0. 964 +1. 512 +2. 889 12

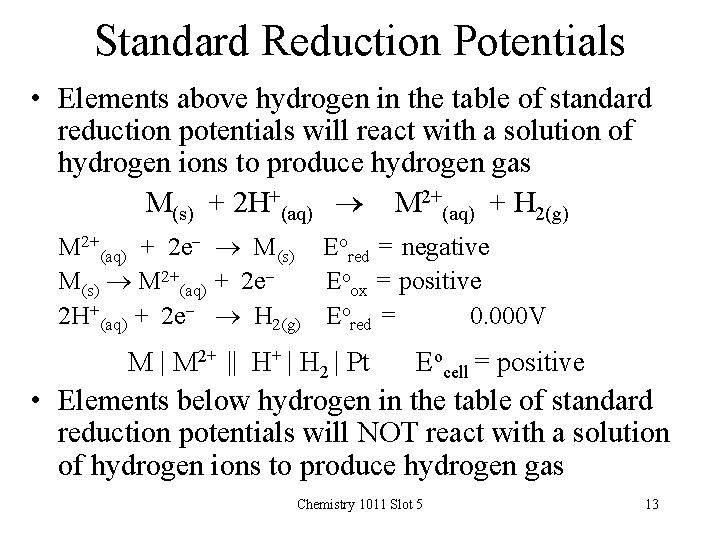
Standard Reduction Potentials • Elements above hydrogen in the table of standard reduction potentials will react with a solution of hydrogen ions to produce hydrogen gas M(s) + 2 H+(aq) M 2+(aq) + H 2(g) M 2+(aq) + 2 e- M(s) Eored = negative M(s) M 2+(aq) + 2 e. Eoox = positive 2 H+(aq) + 2 e- H 2(g) Eored = 0. 000 V M | M 2+ || H+ | H 2 | Pt Eocell = positive • Elements below hydrogen in the table of standard reduction potentials will NOT react with a solution of hydrogen ions to produce hydrogen gas Chemistry 1011 Slot 5 13

Standard Voltages for Voltaic Cells • The table of standard reduction potentials gives standard voltages for reduction half reactions • Standard voltages for oxidation half reactions are obtained by reversing these reactions and changing the sign of the Eored value • If: Zn 2+(aq) + 2 e Zn(s) Eored = -0. 762 • Then: Zn(s) Zn 2+(aq) + 2 e. Eoox = +0. 762 Chemistry 1011 Slot 5 14

Computing Standard Cell Potential • The standard voltage of a cell is the sum of the standard potentials for the two half reactions • For the cell: Zn | Zn 2+ || Cu 2+ | Cu Zn(s) Zn 2+(aq) + 2 e. Eoox = +0. 762 V Cu 2+(aq) + 2 e- Cu(s) Eored = +0. 339 V Zn(s) + Cu 2+(aq) Zn 2+(aq) + Cu(s) • Eocell = Eoox + Eored = + 0. 762 + 0. 339 = 1. 101 V Chemistry 1011 Slot 5 15

Oxidizing Agents • An oxidizing agent is a species that can gain electrons – The strongest oxidizing agents are the species that gain electrons most readily – They have the largest positive Eored values – Oxidizing strength increases moving down the left column of the table of standard reduction potentials – Oxidizing agents in the table of standard reduction potentials can oxidize any species above Chemistry 1011 Slot 5 16

Reducing Agents • A reducing agent is a species that readily loses electrons – The strongest reducing agents are the species that lose electrons most readily – They have the largest negative Eored values (The largest positive Eoox values) – Reducing strength increases moving up the right column of the table of standard reduction potentials – Reducing agents in the table of standard reduction potentials can reduce any species below Chemistry 1011 Slot 5 17

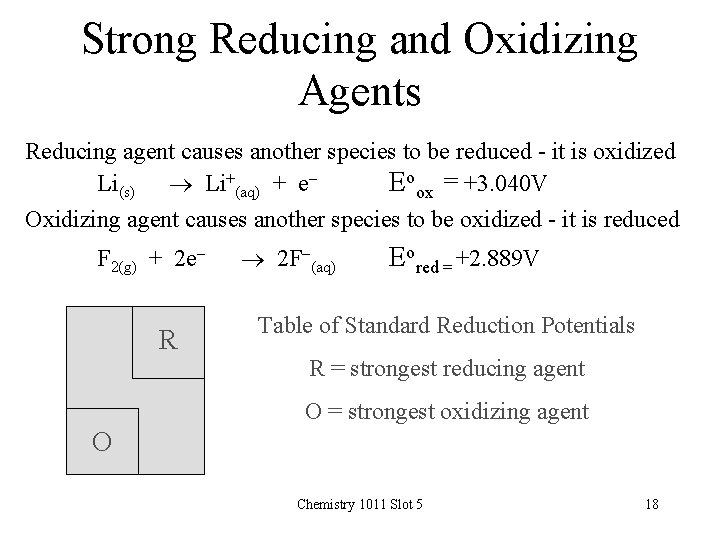
Strong Reducing and Oxidizing Agents Reducing agent causes another species to be reduced - it is oxidized Li(s) Li+(aq) + e. Eoox = +3. 040 V Oxidizing agent causes another species to be oxidized - it is reduced F 2(g) + 2 e- R 2 F-(aq) Eored = +2. 889 V Table of Standard Reduction Potentials R = strongest reducing agent O = strongest oxidizing agent O Chemistry 1011 Slot 5 18

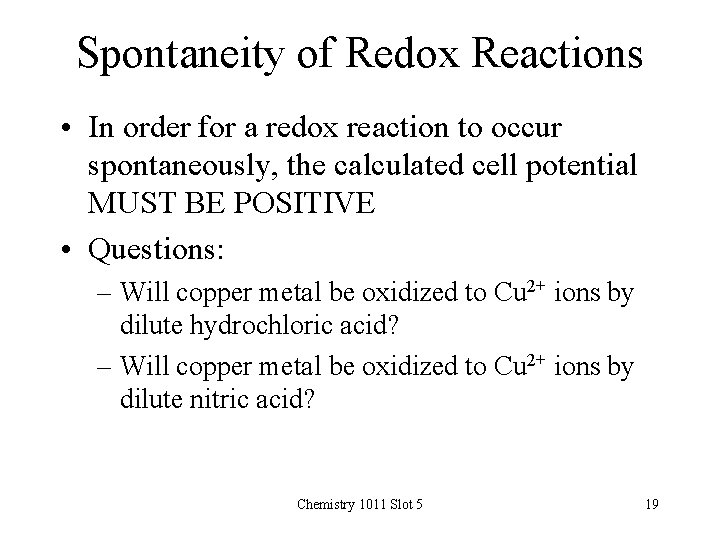
Spontaneity of Redox Reactions • In order for a redox reaction to occur spontaneously, the calculated cell potential MUST BE POSITIVE • Questions: – Will copper metal be oxidized to Cu 2+ ions by dilute hydrochloric acid? – Will copper metal be oxidized to Cu 2+ ions by dilute nitric acid? Chemistry 1011 Slot 5 19

Reaction of Copper with Dilute Hydrochloric Acid? ? • Possible oxidation half reaction: Cu(s) Cu 2+(aq) + 2 e. Eoox = -0. 339 V • Possible reduction half reaction (H+ and Cl- ions are present - Cl- ions cannot be reduced): 2 H+(aq) + 2 e- H 2(g) Eored = 0. 000 • Net possible reaction: Cu(s) + 2 H+(aq) Cu 2+(aq) + H 2(g) • Net calculated cell voltage Eocell = Eoox + Eored = - 0. 339 + 0. 000 = - 0. 339 V • Reaction will not be spontaneous i. e no reaction Chemistry 1011 Slot 5 20

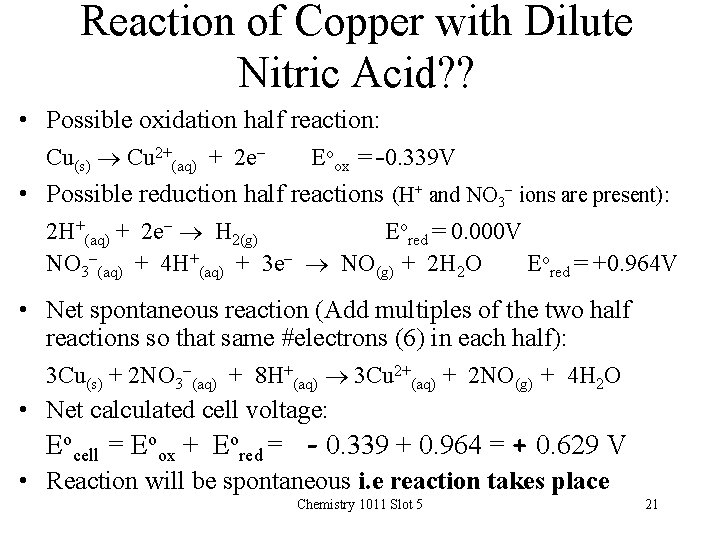
Reaction of Copper with Dilute Nitric Acid? ? • Possible oxidation half reaction: Cu(s) Cu 2+(aq) + 2 e- Eoox = -0. 339 V • Possible reduction half reactions (H+ and NO 3 - ions are present): 2 H+(aq) + 2 e- H 2(g) Eored = 0. 000 V NO 3 -(aq) + 4 H+(aq) + 3 e- NO(g) + 2 H 2 O Eored = +0. 964 V • Net spontaneous reaction (Add multiples of the two half reactions so that same #electrons (6) in each half): 3 Cu(s) + 2 NO 3 -(aq) + 8 H+(aq) 3 Cu 2+(aq) + 2 NO(g) + 4 H 2 O • Net calculated cell voltage: Eocell = Eoox + Eored = - 0. 339 + 0. 964 = + 0. 629 V • Reaction will be spontaneous i. e reaction takes place Chemistry 1011 Slot 5 21

Voltaic Cells with Inert Electrodes • Half cells will frequently be constructed with inert electrodes (often carbon or platinum) • The Hydrogen half cell is one example: H+ | H 2 | Pt • A cell with two inert electrodes might be: Pt | Fe 2+(aq) | Fe 3+(aq) || Cl -(aq) | Cl 2(g) | Pt Chemistry 1011 Slot 5 22

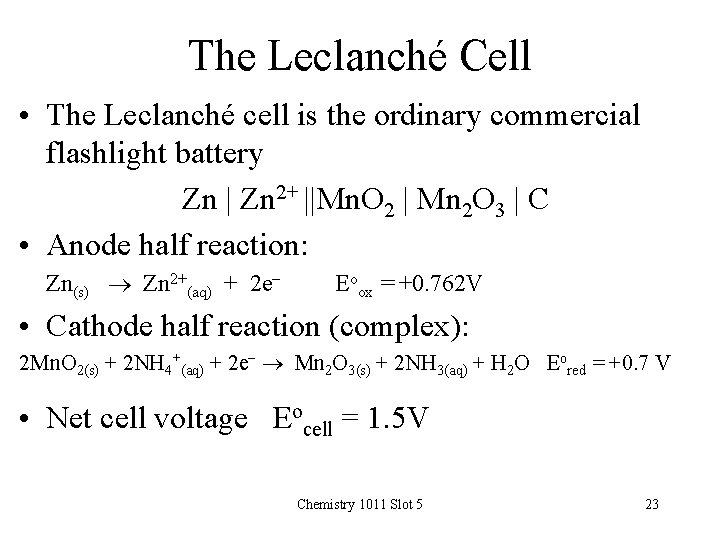
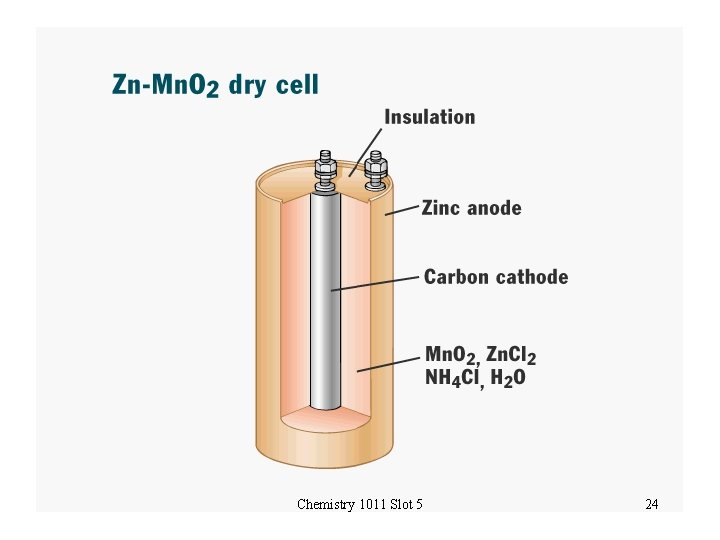
The Leclanché Cell • The Leclanché cell is the ordinary commercial flashlight battery Zn | Zn 2+ ||Mn. O 2 | Mn 2 O 3 | C • Anode half reaction: Zn(s) Zn 2+(aq) + 2 e- Eoox = +0. 762 V • Cathode half reaction (complex): 2 Mn. O 2(s) + 2 NH 4+(aq) + 2 e- Mn 2 O 3(s) + 2 NH 3(aq) + H 2 O Eored = +0. 7 V • Net cell voltage Eocell = 1. 5 V Chemistry 1011 Slot 5 23

Chemistry 1011 Slot 5 24
 Nivaldo j. tro introductory chemistry
Nivaldo j. tro introductory chemistry Introductory chemistry 4th edition
Introductory chemistry 4th edition Introductory chemistry concepts and critical thinking
Introductory chemistry concepts and critical thinking Prefix multipliers
Prefix multipliers Introductory chemistry 5th edition nivaldo j. tro
Introductory chemistry 5th edition nivaldo j. tro Pengurangan bcd
Pengurangan bcd Hasse diagram
Hasse diagram Readone piece 1011
Readone piece 1011 0101 0010 0000 0110 1000
0101 0010 0000 0110 1000 En 1011-1
En 1011-1 1001 1011
1001 1011 Biểu diễn số chấm động
Biểu diễn số chấm động Decreto 1011 de 2006
Decreto 1011 de 2006 Hexadecimal number system in computer
Hexadecimal number system in computer B 1011
B 1011 Year 1011
Year 1011 Eecs 1011
Eecs 1011 Http //mbs.meb.gov.tr/ http //www.alantercihleri.com
Http //mbs.meb.gov.tr/ http //www.alantercihleri.com Siat.ung.ac.id krs
Siat.ung.ac.id krs Introductory paragraph
Introductory paragraph Main clause
Main clause Introductory prepositional phrase
Introductory prepositional phrase Intro body conclusion
Intro body conclusion Introductory paragraph for persuasive essay
Introductory paragraph for persuasive essay General stament
General stament