Introductory Chemistry A Foundation 6 th Ed Introductory





























- Slides: 49

Introductory Chemistry: A Foundation, 6 th Ed. Introductory Chemistry, 6 th Ed. Basic Chemistry, 6 th Ed. by Steven S. Zumdahl & Donald J. De. Coste University of Illinois

Chapter 13 Gases

Properties of Gases • Expand to completely fill their container • Take the shape of their container • Low density – Much less than solid or liquid state • Compressible • Mixtures of gases are always homogeneous • Fluid Copyright © Houghton Mifflin Company. All rights reserved. 13 | 3

Gas Pressure • Pressure = total force applied to a certain area – Larger force = larger pressure – Smaller area = larger pressure • Gas pressure caused by gas molecules colliding with container or surface • More forceful or more frequent collisions mean higher gas pressure Copyright © Houghton Mifflin Company. All rights reserved. 13 | 4

Air Pressure • Constantly present when air present • Decreases with altitude – Less air = less pressure • Varies with weather conditions Copyright © Houghton Mifflin Company. All rights reserved. 13 | 5

Air Pressure (cont. ) • Measured using a barometer – Column of mercury supported by air pressure – Longer mercury column supported = higher pressure – Force of the air on the surface of the mercury balanced by the pull of gravity on the column of mercury Copyright © Houghton Mifflin Company. All rights reserved. 13 | 6

Measuring Pressure of a Trapped Gas Copyright © Houghton Mifflin Company. All rights reserved. 13 | 7

Measuring Pressure of a Trapped Gas (cont. ) Copyright © Houghton Mifflin Company. All rights reserved. 13 | 8

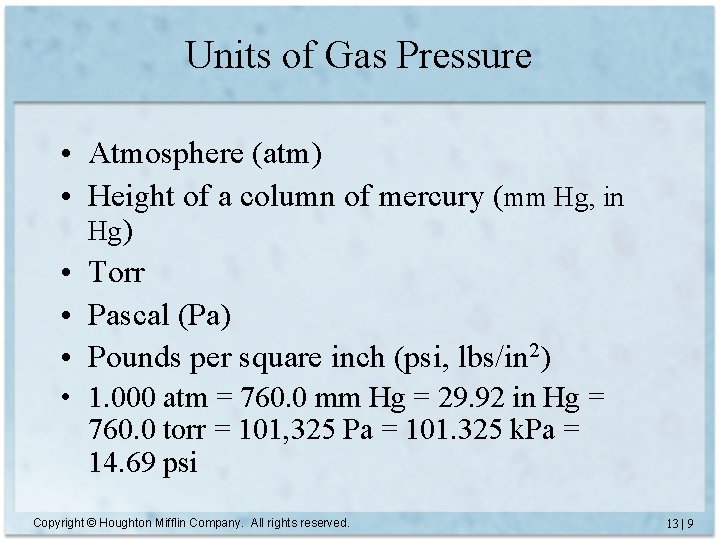
Units of Gas Pressure • Atmosphere (atm) • Height of a column of mercury (mm Hg, in Hg) • Torr • Pascal (Pa) • Pounds per square inch (psi, lbs/in 2) • 1. 000 atm = 760. 0 mm Hg = 29. 92 in Hg = 760. 0 torr = 101, 325 Pa = 101. 325 k. Pa = 14. 69 psi Copyright © Houghton Mifflin Company. All rights reserved. 13 | 9

Self check p 390 • On a summer day in Colorado, the atmospheric pressure is 525 mm Hg. What is this air pressure in atmospheres? • P 418 • Q 7. Convert the following into atmospheres • 109. 2 k. Pa, 781 torr, 781 mm Hg, 15. 2 psi • Q 9. Convert the following into mm Hg • 822 torr, 121. 4 k. Pa, 1. 14 atm, 9. 75 psi Copyright © Houghton Mifflin Company. All rights reserved. 13 | 10

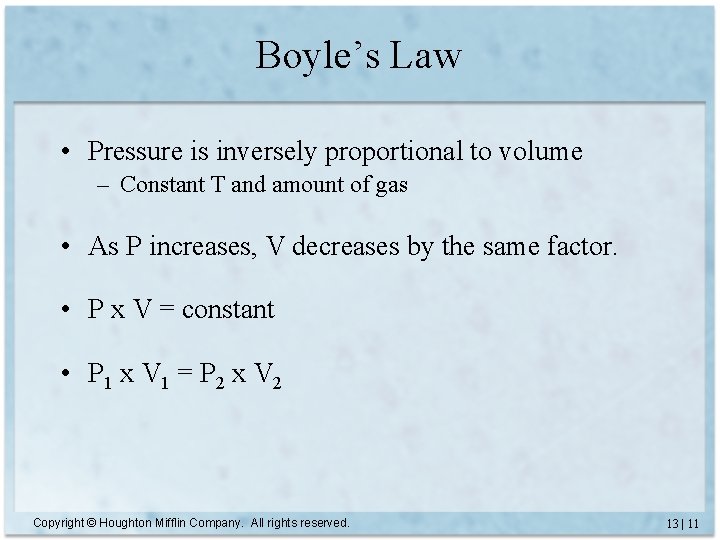
Boyle’s Law • Pressure is inversely proportional to volume – Constant T and amount of gas • As P increases, V decreases by the same factor. • P x V = constant • P 1 x V 1 = P 2 x V 2 Copyright © Houghton Mifflin Company. All rights reserved. 13 | 11

Boyle’s Law (cont. ) Copyright © Houghton Mifflin Company. All rights reserved. 13 | 12

Boyle’s Law (cont. ) Copyright © Houghton Mifflin Company. All rights reserved. 13 | 13

Example: What is the new volume if a 1. 5 L sample of Freon-12 at 56 torr is compressed to 150 torr? Copyright © Houghton Mifflin Company. All rights reserved. 13 | 14

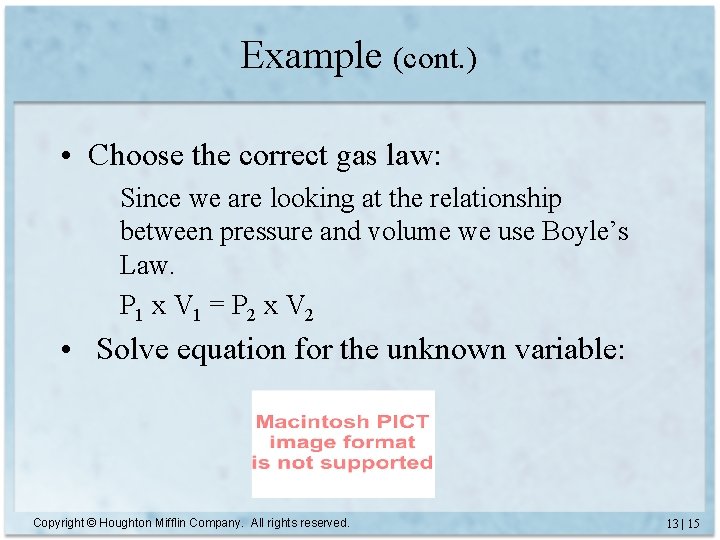
Example (cont. ) • Choose the correct gas law: Since we are looking at the relationship between pressure and volume we use Boyle’s Law. P 1 x V 1 = P 2 x V 2 • Solve equation for the unknown variable: Copyright © Houghton Mifflin Company. All rights reserved. 13 | 15

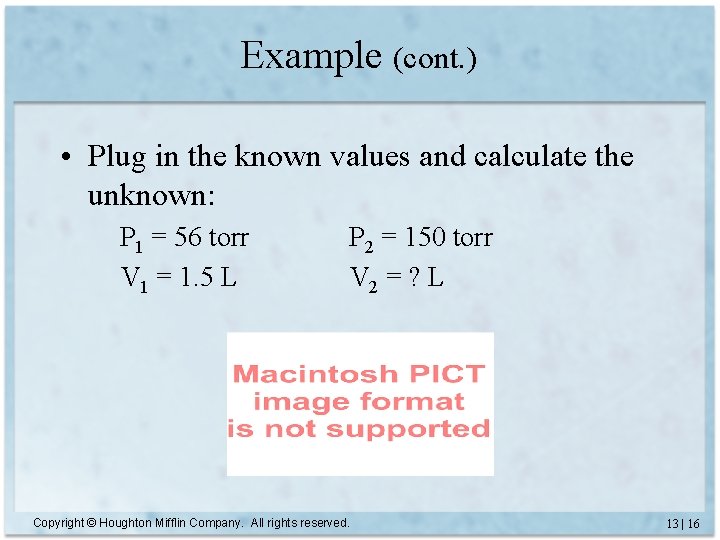
Example (cont. ) • Plug in the known values and calculate the unknown: P 1 = 56 torr V 1 = 1. 5 L P 2 = 150 torr V 2 = ? L Copyright © Houghton Mifflin Company. All rights reserved. 13 | 16

Self check p 394 • A sample of neon to be used in a sign has a volume of 1. 51 L at a pressure of 635 torr. Calculate the volume of a gas after it is pumped into the glass tubes of the signs where it shows a pressure of 785 torr. • P 418 q 17. Calculate new volume of the gas • V=249 m. L @764 mm. Hg; 654 mm. Hg, V=? L • V=1. 04 L @1. 21 atm; 0. 671 atm, V=? L • V=142 m. L @20. 9 atm; 760 mm. Hg, V=? Copyright © Houghton Mifflin Company. All rights reserved. 13 | 17

Absolute Zero • Theoretical temperature at which a gas would have zero volume and no pressure – Calculated by extrapolation • • 0 K = -273. 15 °C = -459 °F Kelvin T = Celsius T + 273. 15 Never attainable (though we’ve gotten really close) All gas law problems use Kelvin temperature scale. Copyright © Houghton Mifflin Company. All rights reserved. 13 | 18

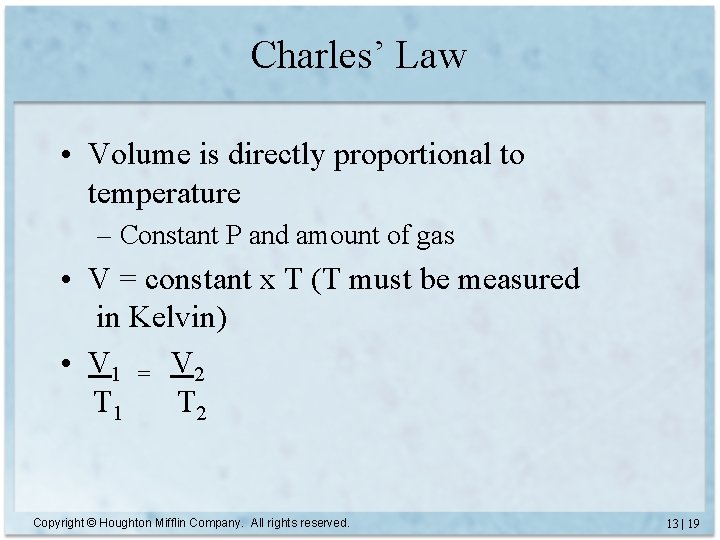
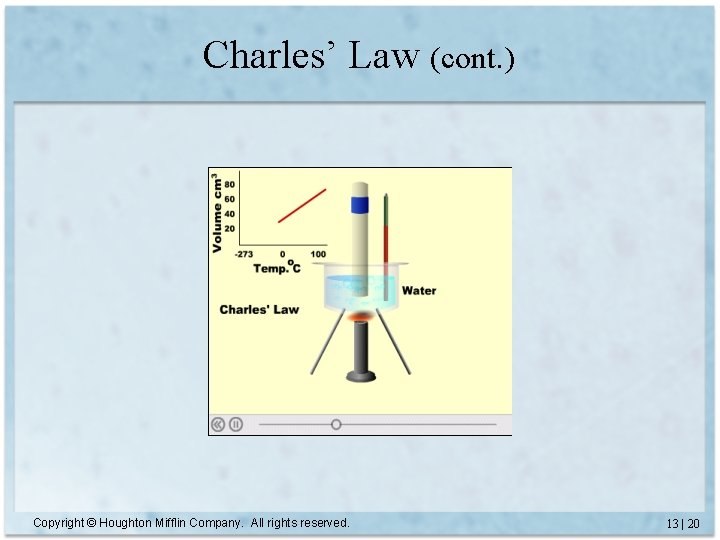
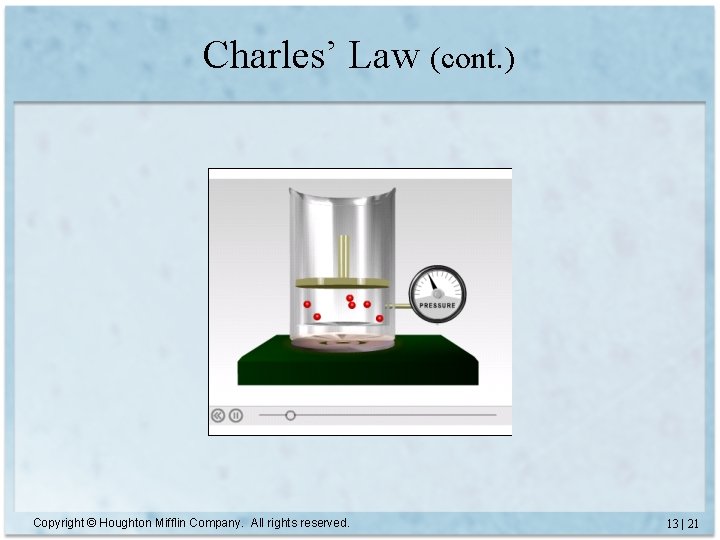
Charles’ Law • Volume is directly proportional to temperature – Constant P and amount of gas • V = constant x T (T must be measured in Kelvin) • V 1 = V 2 T 1 T 2 Copyright © Houghton Mifflin Company. All rights reserved. 13 | 19

Charles’ Law (cont. ) Copyright © Houghton Mifflin Company. All rights reserved. 13 | 20

Charles’ Law (cont. ) Copyright © Houghton Mifflin Company. All rights reserved. 13 | 21

Self check p 398 • A child blows a bubble that contains air at 8°C and has a volume of 23 cm 3 a atm. As the bubble rises, it encounters a pocket of cold air (temp 18 ° C). If there is no change in pressure, will the bubble get larger or smaller as the air inside cools to 18 ° C? Calculate the new volume of the gas. • P 419 q 31 Copyright © Houghton Mifflin Company. All rights reserved. 13 | 22

Avogadro’s Law • Volume directly proportional to the number of gas molecules – V = constant x n (moles) – Constant P and T – More gas molecules = larger volume Copyright © Houghton Mifflin Company. All rights reserved. 13 | 23

Avogadro’s Law (cont. ) • Count number of gas molecules by moles • One mole of any ideal gas occupies 22. 414 L at standard conditions = molar volume • Equal volumes of gases contain equal numbers of molecules. – It doesn’t matter what the gas is! Copyright © Houghton Mifflin Company. All rights reserved. 13 | 24

Ideal Gas Law • By combining the proportionality constants from the gas laws we can write a general equation. • R is called the gas constant. • The value of R depends on the units of P and V. – Generally use R = 0. 08206 when P in atm and V in L PV = n. RT Copyright © Houghton Mifflin Company. All rights reserved. 13 | 25

Ideal Gas Law (cont. ) • Use the ideal gas law when you have gas at one set of conditions • Most gases obey this law when pressure is low (at or below 1 atm) and temperature is high (above 0°C). Copyright © Houghton Mifflin Company. All rights reserved. 13 | 26

Ideal Gas Law (cont. ) Copyright © Houghton Mifflin Company. All rights reserved. 13 | 27

Combined Gas Law Copyright © Houghton Mifflin Company. All rights reserved. 13 | 28

Copyright © Houghton Mifflin Company. All rights reserved. 13 | 29

Self check p 405 • A sample of argon gas with a volume of 11 L at a temp of 13 C, and a pressure of 0. 747 atm is heated to 56°C and a pressure of 1. 18 atm. Calculate the final volume. • Avogadro’s law- p 420 q 41. If 0. 105 mol of helium gas occupies a vol of 2. 35 L at a certain temp and pressure, what volume would 0. 337 mol of helium occupy under the same conditions? • Q 43 - If 3. 25 mol of Ar gas occupies a vol of 100 L at a particular temp and pressure, what vol does 14. 15 mol of Ar occupy under the same conditions? Copyright © Houghton Mifflin Company. All rights reserved. 13 | 30

Self check p 403, 404 • A weather balloon contains 1. 1 X 105 mol of He and has a vol of 2. 7 X 106 L at 1 atm pressure. Calculate the temp of the helium in the balloon in kelvins and in �°C. • Ra can pose a hazard to humans by seeping into houses, and there is a concern about this problem in many areas. A 1. 5 mol sample of this gas has a vol of 21 L at 33°C. What is the pressure of this gas? • A sample of methane gas has a vol of 3. 8 L at 5 °C and is heated to 86 C at constant pressure. Calculate its new volume. Copyright © Houghton Mifflin Company. All rights reserved. 13 | 31

Dalton’s Law • The total pressure of a mixture of gases equals the sum of the pressures each gas would exert independently. – Partial pressure: the pressure a gas in a mixture would exert if it were alone in the container – Ptotal = Pgas A + Pgas B + … Copyright © Houghton Mifflin Company. All rights reserved. 13 | 32

Dalton’s Law (cont. ) • Particularly useful for determining the pressure a dry gas would have after it is collected over water – Pair = Pwet gas = Pdry gas + Pwater vapor – Pwater vapor depends on the temperature, look up in table Copyright © Houghton Mifflin Company. All rights reserved. 13 | 33

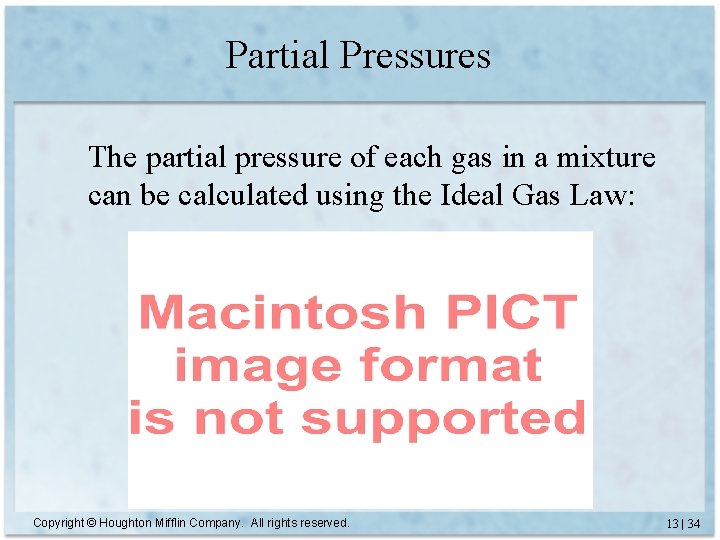
Partial Pressures The partial pressure of each gas in a mixture can be calculated using the Ideal Gas Law: Copyright © Houghton Mifflin Company. All rights reserved. 13 | 34

Self check p 409, 410 • A 2 L flask contains a mixture of nitrogen gas and oxygen gas at 25°C. The total pressure of the gaseous mixture is 0. 91 atm, and the mixture is known to contain 0. 050 mol of N 2. Calculate the partial pressure of oxygen and the moles of oxygen present. • Consider a sample of hydrogen gas collected over water at 25 C where the vapor pressure of water is 24 torr. The volume occupied by the gaseous mixture is 0. 5 L and the total pressure is 0. 95 atm. Calculate the partial pressure of H 2 and the number of moles of H 2 present. Copyright © Houghton Mifflin Company. All rights reserved. 13 | 35

Kinetic-Molecular Theory • The properties of solids, liquids, and gases can be explained based on the speed of the molecules and the attractive forces between molecules. • In solids, the molecules have no translational freedom. They are held in place by strong attractive forces. – May only vibrate Copyright © Houghton Mifflin Company. All rights reserved. 13 | 36

Kinetic-Molecular Theory (cont. ) • In liquids, the molecules have some translational freedom, but not enough to escape their attraction to neighboring molecules. – They can slide past one another and rotate, as well as vibrate. Copyright © Houghton Mifflin Company. All rights reserved. 13 | 37

Kinetic-Molecular Theory (cont. ) • In gases, the molecules have “complete” freedom from each other. They have enough energy to overcome “all” attractive forces. • Kinetic energy depends only on the temperature. Copyright © Houghton Mifflin Company. All rights reserved. 13 | 38

Describing a Gas • Gases are composed of tiny particles. • The particles are small compared to the average space between them. – Assume the molecules do not have volume – Ideal gas law assumes volume of molecules=zero! Copyright © Houghton Mifflin Company. All rights reserved. 13 | 39

Describing a Gas (cont. ) • Molecules constantly and rapidly move in a straight line until they bump into each other or the wall. – Average kinetic energy proportional to the temperature – Results in gas pressure • Assume that the gas molecules attraction for each other is negligible. • Ideal gas law assumes this attraction=zero! Copyright © Houghton Mifflin Company. All rights reserved. 13 | 40

Visual Copyright © Houghton Mifflin Company. All rights reserved. 13 | 41

The Meaning of Temperature • Temperature is a measure of the average kinetic energy of the molecules in a sample. – Not all molecules have same kinetic energy • Kinetic energy is directly proportional to the Kelvin temperature. – Average speed of molecules increases as the temperature increases Copyright © Houghton Mifflin Company. All rights reserved. 13 | 42

Gas Properties Explained • Gases have indefinite shape and volume because the freedom of the molecules allows them to move and fill the container they’re in. • Gases are compressible and have low density because of the large spaces between the molecules. Copyright © Houghton Mifflin Company. All rights reserved. 13 | 43

Pressure and Temperature • As the temperature of a gas increases, the average speed of the molecules increases. • The molecules hit the sides of the container with more force (on average) and more frequently, resulting in an increase in pressure. Copyright © Houghton Mifflin Company. All rights reserved. 13 | 44

Pressure and Temperature (cont. ) Copyright © Houghton Mifflin Company. All rights reserved. 13 | 45

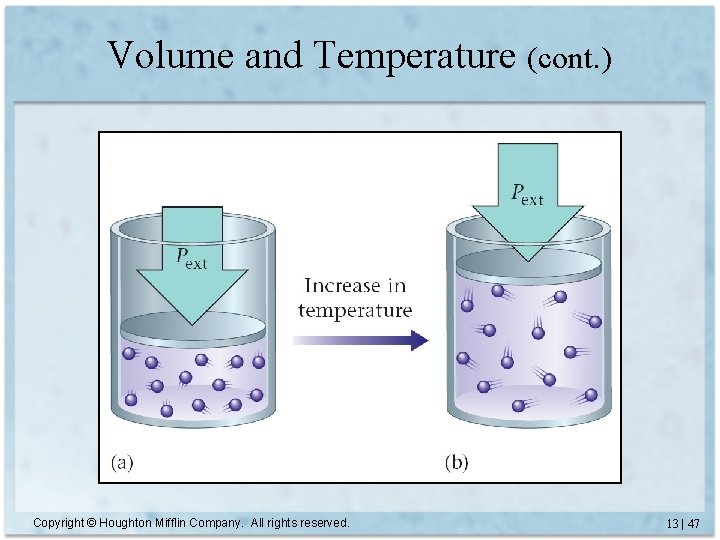
Volume and Temperature • In a rigid container, raising the temperature increases the pressure. • For a cylinder with a piston, the pressure outside and inside stays the same. Copyright © Houghton Mifflin Company. All rights reserved. 13 | 46

Volume and Temperature (cont. ) Copyright © Houghton Mifflin Company. All rights reserved. 13 | 47

Gas Stoichiometry • Use the general algorithms discussed previously to convert masses or solution amounts to moles. • Use gas laws to convert amounts of gas to moles. Copyright © Houghton Mifflin Company. All rights reserved. 13 | 48

Self check p 415 • Calculate the volume of hydrogen produced at 1. 5 atm and 19 C by the reaction of 26. 5 g of zinc with excess HCl according to the balanced equation • Zn (s)+ 2 HCl (aq)--> Zn. Cl 2(aq)+H 2(g) • Ammonia is commonly used as a fertilizer to provide a source of nitrogen for plants. A sample of NH 3 (g) occupies a volume of 5 L at 25°C and 15 atm. What volume will this sample occupy at STP? • Book Assignment- p 420 q 49, 51, 55, 57, 67, 73, 85, 87, 89, 95. Turn in on April 5 th. Late work will result in lowered credit. Copyright © Houghton Mifflin Company. All rights reserved. 13 | 49