Chemistry 1011 TOPIC Electrochemistry TEXT REFERENCE Masterton and



- Slides: 18

Chemistry 1011 TOPIC Electrochemistry TEXT REFERENCE Masterton and Hurley Chapter 18 Chemistry 1011 Slot 5 1

18. 5 Electrolytic Cells YOU ARE EXPECTED TO BE ABLE TO: • Construct a labelled diagram to show the structure of an electrolytic cell and describe its operation • Identify half reactions that take place at the anode and cathode of an electrolytic cell • Predict the most likely products of electrolysis of aqueous solutions and melts of ionic compounds • Calculate the minimum voltage necessary for the electrolysis of a solution under standard conditions • Carry out calculations related to the current passing through an electrolytic cell and the quantities of product formed Chemistry 1011 Slot 5 2

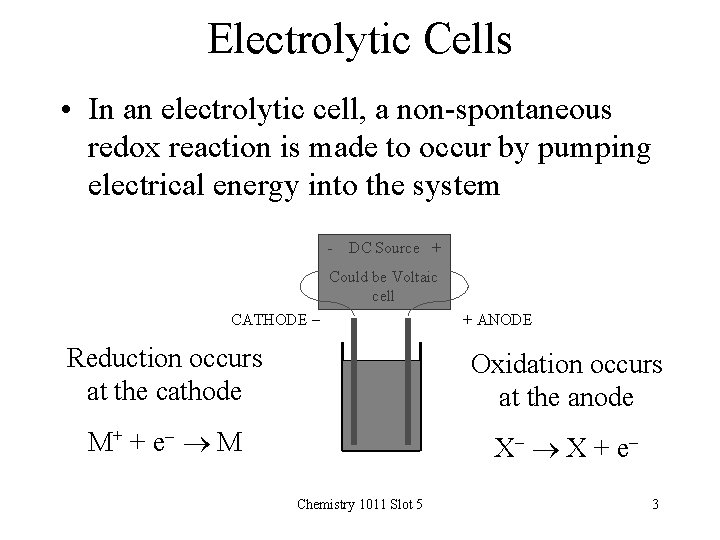
Electrolytic Cells • In an electrolytic cell, a non-spontaneous redox reaction is made to occur by pumping electrical energy into the system - DC Source + Could be Voltaic cell CATHODE - + ANODE Reduction occurs at the cathode Oxidation occurs at the anode M+ + e - M X- X + e Chemistry 1011 Slot 5 3

Electrolytic Cells • The simple electrolytic cell consists of – two electrodes, dipping into – a solution containing positive and negative ions • The external battery or DC source acts as a pump – It pushes electrons to the cathode and removes electrons from the anode • Metal ions in solution travel to the cathode and gain electrons (reduction) • Non-metal ions travel to the anode and give up electrons (oxidation) • The process is called electrolysis Chemistry 1011 Slot 5 4

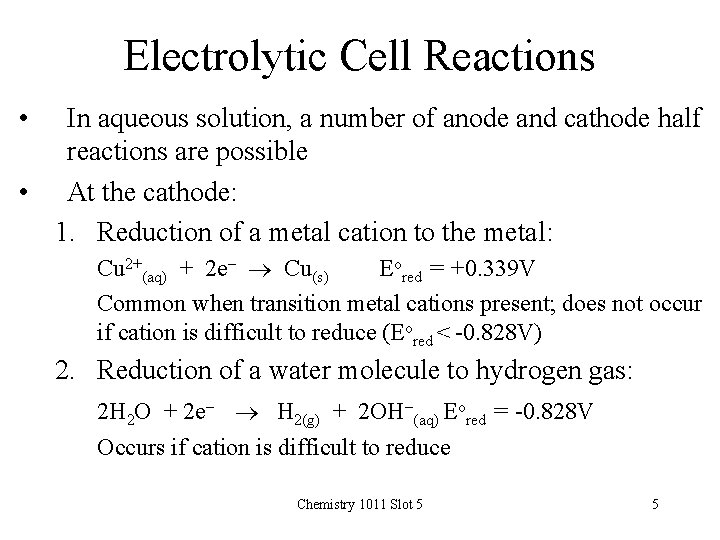
Electrolytic Cell Reactions • In aqueous solution, a number of anode and cathode half reactions are possible • At the cathode: 1. Reduction of a metal cation to the metal: Cu 2+(aq) + 2 e- Cu(s) Eored = +0. 339 V Common when transition metal cations present; does not occur if cation is difficult to reduce (Eored < -0. 828 V) 2. Reduction of a water molecule to hydrogen gas: 2 H 2 O + 2 e- H 2(g) + 2 OH-(aq) Eored = -0. 828 V Occurs if cation is difficult to reduce Chemistry 1011 Slot 5 5

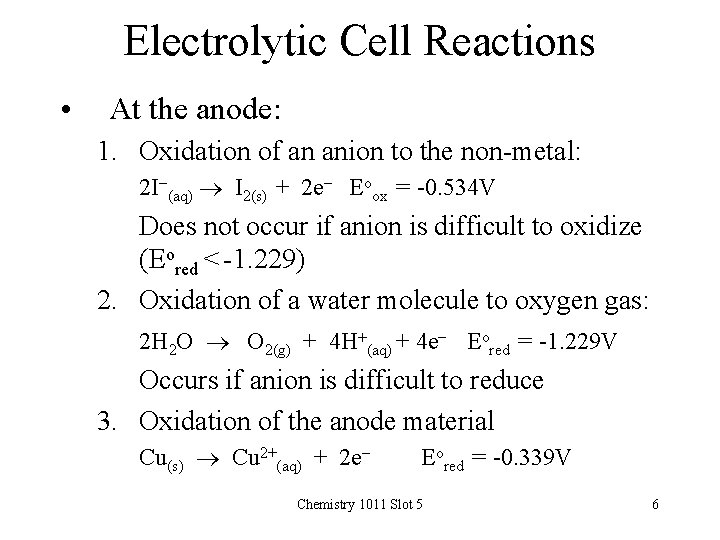
Electrolytic Cell Reactions • At the anode: 1. Oxidation of an anion to the non-metal: 2 I-(aq) I 2(s) + 2 e- Eoox = -0. 534 V Does not occur if anion is difficult to oxidize (Eored < -1. 229) 2. Oxidation of a water molecule to oxygen gas: 2 H 2 O O 2(g) + 4 H+(aq) + 4 e- Eored = -1. 229 V Occurs if anion is difficult to reduce 3. Oxidation of the anode material Cu(s) Cu 2+(aq) + 2 e- Eored = -0. 339 V Chemistry 1011 Slot 5 6

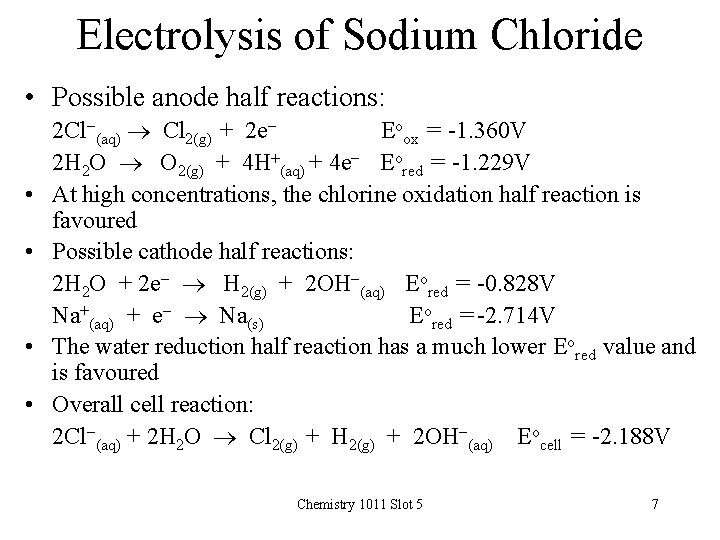
Electrolysis of Sodium Chloride • Possible anode half reactions: • • 2 Cl-(aq) Cl 2(g) + 2 e. Eoox = -1. 360 V 2 H 2 O O 2(g) + 4 H+(aq) + 4 e- Eored = -1. 229 V At high concentrations, the chlorine oxidation half reaction is favoured Possible cathode half reactions: 2 H 2 O + 2 e- H 2(g) + 2 OH-(aq) Eored = -0. 828 V Na+(aq) + e- Na(s) Eored = -2. 714 V The water reduction half reaction has a much lower Eored value and is favoured Overall cell reaction: 2 Cl-(aq) + 2 H 2 O Cl 2(g) + H 2(g) + 2 OH-(aq) Eocell = -2. 188 V Chemistry 1011 Slot 5 7

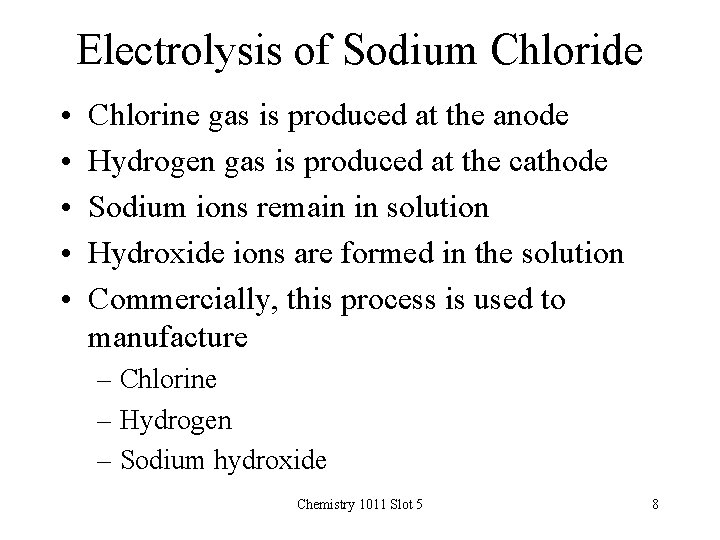
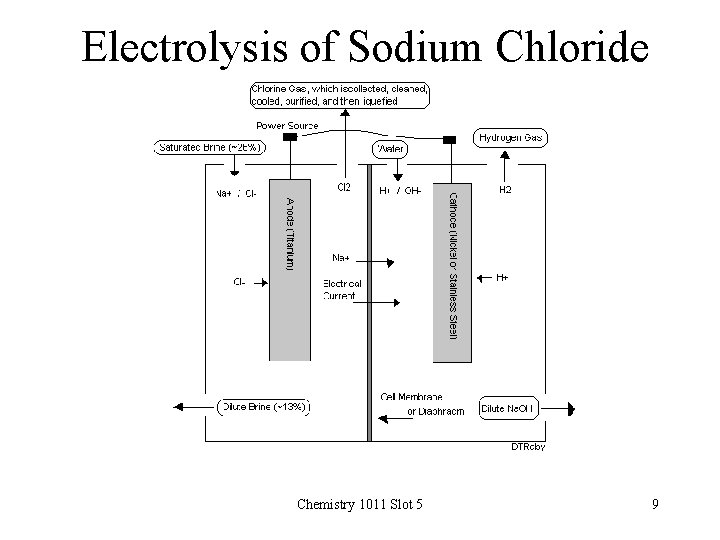
Electrolysis of Sodium Chloride • • • Chlorine gas is produced at the anode Hydrogen gas is produced at the cathode Sodium ions remain in solution Hydroxide ions are formed in the solution Commercially, this process is used to manufacture – Chlorine – Hydrogen – Sodium hydroxide Chemistry 1011 Slot 5 8

Electrolysis of Sodium Chloride Chemistry 1011 Slot 5 9

Electroplating • In many electrolytic cells, metal is deposited at the cathode • An object can be electroplated by making it the cathode • Copper, chromium and silver are often plated onto brass, nickel, or other metals by this method • The process is also used to purify copper, as the last step in the manufacture of copper from copper ore Chemistry 1011 Slot 5 10

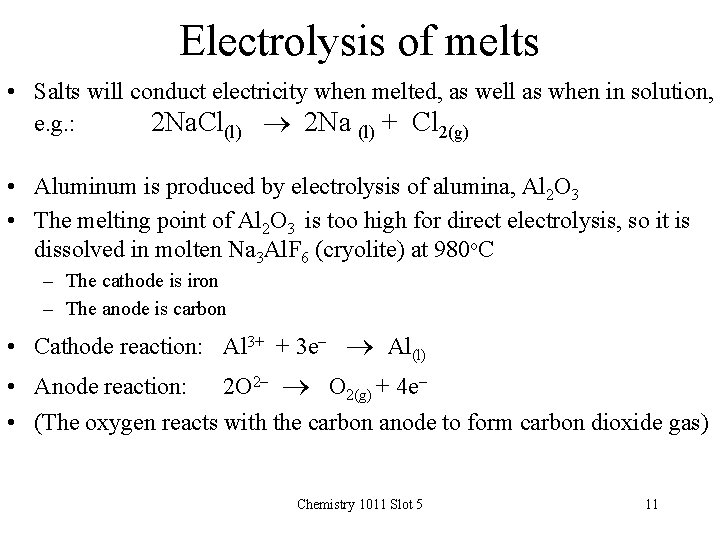
Electrolysis of melts • Salts will conduct electricity when melted, as well as when in solution, e. g. : 2 Na. Cl(l) 2 Na (l) + Cl 2(g) • Aluminum is produced by electrolysis of alumina, Al 2 O 3 • The melting point of Al 2 O 3 is too high for direct electrolysis, so it is dissolved in molten Na 3 Al. F 6 (cryolite) at 980 o. C – The cathode is iron – The anode is carbon • Cathode reaction: Al 3+ + 3 e- Al(l) • Anode reaction: 2 O 2 - O 2(g) + 4 e • (The oxygen reacts with the carbon anode to form carbon dioxide gas) Chemistry 1011 Slot 5 11

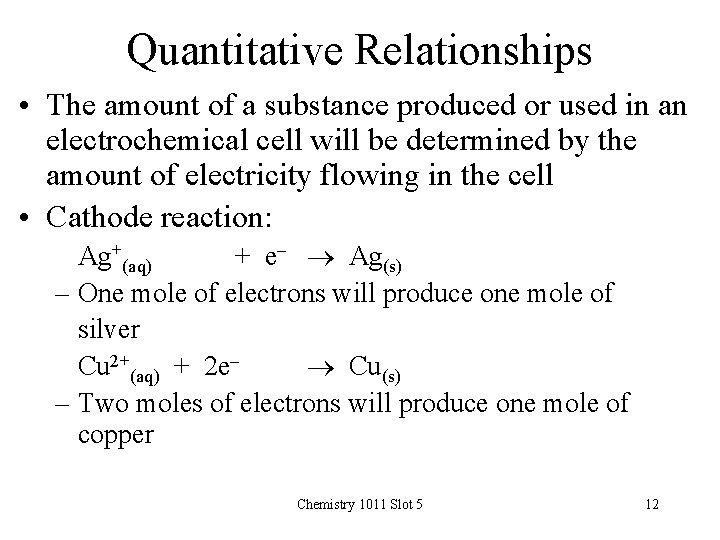
Quantitative Relationships • The amount of a substance produced or used in an electrochemical cell will be determined by the amount of electricity flowing in the cell • Cathode reaction: Ag+(aq) + e- Ag(s) – One mole of electrons will produce one mole of silver Cu 2+(aq) + 2 e Cu(s) – Two moles of electrons will produce one mole of copper Chemistry 1011 Slot 5 12

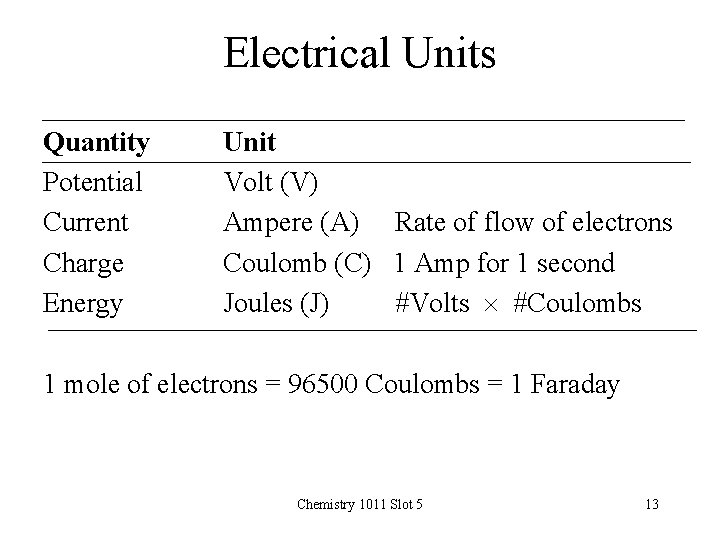
Electrical Units Quantity Potential Current Charge Energy Unit Volt (V) Ampere (A) Rate of flow of electrons Coulomb (C) 1 Amp for 1 second Joules (J) #Volts #Coulombs 1 mole of electrons = 96500 Coulombs = 1 Faraday Chemistry 1011 Slot 5 13

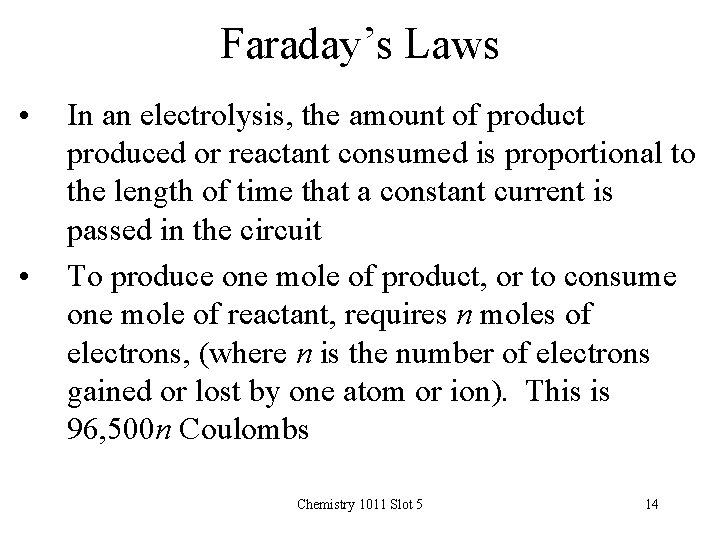
Faraday’s Laws • • In an electrolysis, the amount of product produced or reactant consumed is proportional to the length of time that a constant current is passed in the circuit To produce one mole of product, or to consume one mole of reactant, requires n moles of electrons, (where n is the number of electrons gained or lost by one atom or ion). This is 96, 500 n Coulombs Chemistry 1011 Slot 5 14

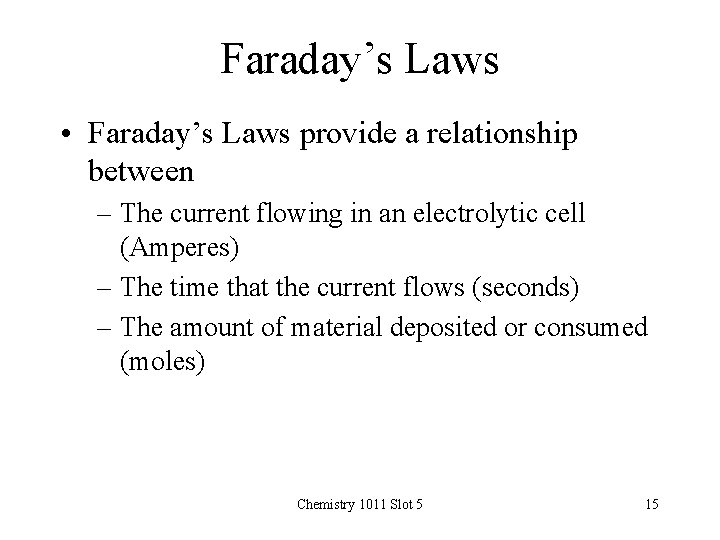
Faraday’s Laws • Faraday’s Laws provide a relationship between – The current flowing in an electrolytic cell (Amperes) – The time that the current flows (seconds) – The amount of material deposited or consumed (moles) Chemistry 1011 Slot 5 15

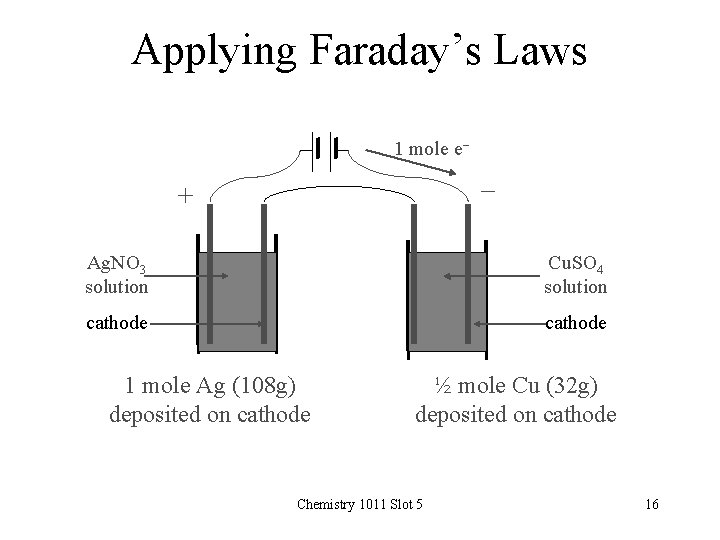
Applying Faraday’s Laws 1 mole e- - + Ag. NO 3 solution Cu. SO 4 solution cathode 1 mole Ag (108 g) deposited on cathode ½ mole Cu (32 g) deposited on cathode Chemistry 1011 Slot 5 16

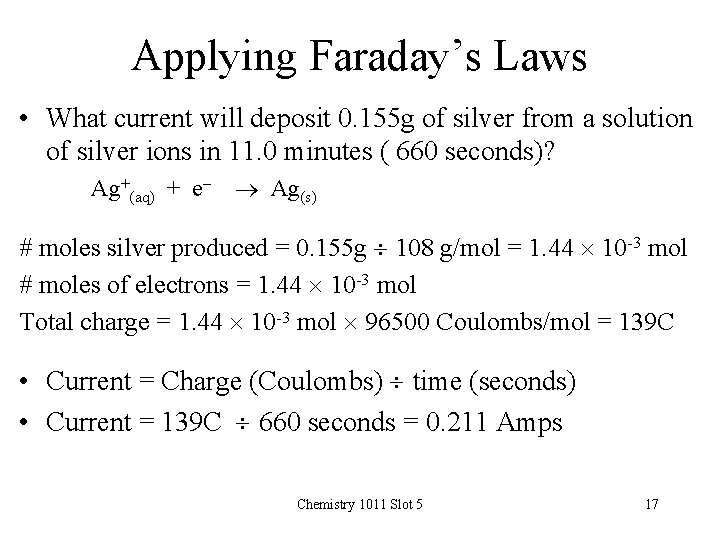
Applying Faraday’s Laws • What current will deposit 0. 155 g of silver from a solution of silver ions in 11. 0 minutes ( 660 seconds)? Ag+(aq) + e- Ag(s) # moles silver produced = 0. 155 g 108 g/mol = 1. 44 10 -3 mol # moles of electrons = 1. 44 10 -3 mol Total charge = 1. 44 10 -3 mol 96500 Coulombs/mol = 139 C • Current = Charge (Coulombs) time (seconds) • Current = 139 C 660 seconds = 0. 211 Amps Chemistry 1011 Slot 5 17

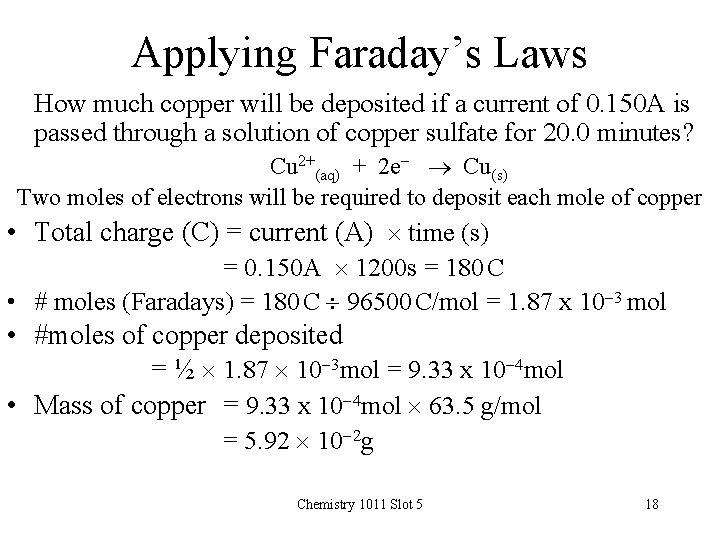
Applying Faraday’s Laws How much copper will be deposited if a current of 0. 150 A is passed through a solution of copper sulfate for 20. 0 minutes? Cu 2+(aq) + 2 e- Cu(s) Two moles of electrons will be required to deposit each mole of copper • Total charge (C) = current (A) time (s) = 0. 150 A 1200 s = 180 C • # moles (Faradays) = 180 C 96500 C/mol = 1. 87 x 10 -3 mol • #moles of copper deposited = ½ 1. 87 10 -3 mol = 9. 33 x 10 -4 mol • Mass of copper = 9. 33 x 10 -4 mol 63. 5 g/mol = 5. 92 10 -2 g Chemistry 1011 Slot 5 18