HTA HITAP Yot Teerawattananon M D Ph D


























- Slides: 52

HTA & HITAP Yot Teerawattananon M. D. , Ph. D. Health Intervention and Technology Assessment (HITAP) Ministry of Public Health, Thailand 1

Which intervention is worth investing? • PSA screening for prostate cancer • Whole-body CT scans in routine check-up • Electronic fetal monitoring for normal labour • Hormone replacement therapy for postmenopausal women 2

3

4

5

6


PSA screening for prostate cancer • Screening for prostate cancer does not have a significant impact on either overall mortality or death from prostate cancer • Screening helps to diagnose prostate cancer at an earlier stage but at the risk of overtreatment and downstream adverse effects that currently cannot be precisely quantified http: //www. bmj. com/content/bmj/341/bmj. c 4543. full. pdf 8

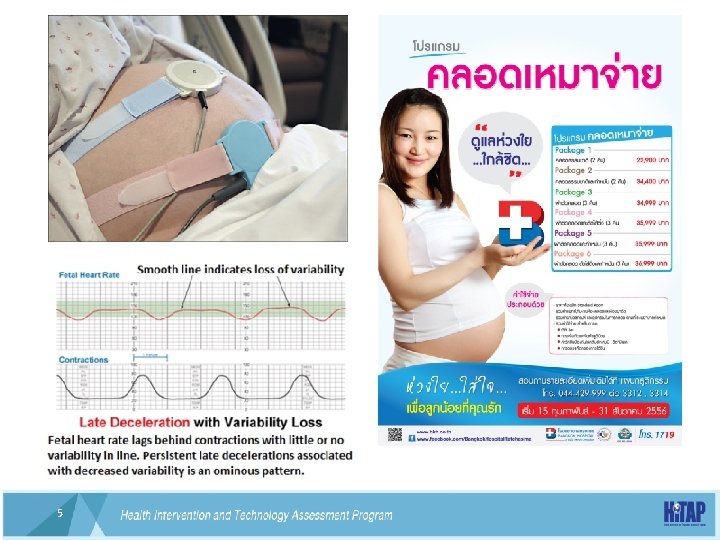
Electronic fetal monitoring • We found no evidence of benefit for the use of the admission CTG for low -riskwomen on admission in labour. Furthermore, the probability is that admission CTG increases the caesarean section rate by approximately 20%. The datalacked power to detect possible important differences in perinatal mortality. However, it is unlikely that any trial, or metaanalysis, will be adequately powered to detect such differences. The findings of this review support recommendations that the admission CTG not be used for women who are lowrisk on admission in labour. Women should be informed that admission CTG is likely associated with an increase in the incidence of caesarean section without evidence of benefit. http: //www. cochrane. org/CD 005122/PREG_comparing-electronicmonitoring-of-the-babys-heartbeat-on-a-womans-admission-in-labourusing-cardiotocography-ctg-with-intermittent-monitoring 9

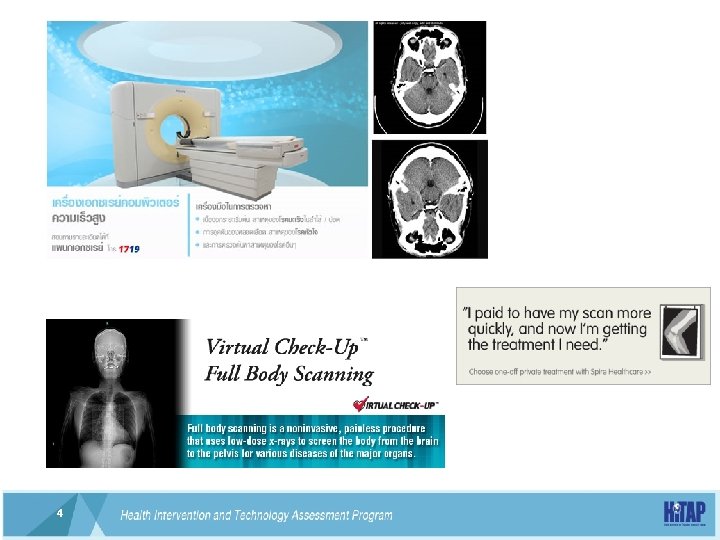
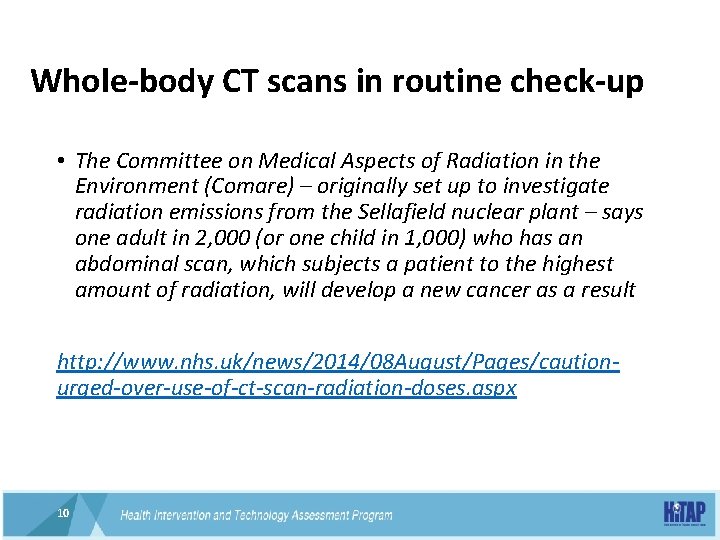
Whole-body CT scans in routine check-up • The Committee on Medical Aspects of Radiation in the Environment (Comare) – originally set up to investigate radiation emissions from the Sellafield nuclear plant – says one adult in 2, 000 (or one child in 1, 000) who has an abdominal scan, which subjects a patient to the highest amount of radiation, will develop a new cancer as a result http: //www. nhs. uk/news/2014/08 August/Pages/cautionurged-over-use-of-ct-scan-radiation-doses. aspx 10

Hormone replacement therapy for postmenopausal women • HT is not indicated for primary or secondary prevention of cardiovasculardisease or dementia, nor for preventing deterioration of cognitive function in postmenopausal women. Although HT is considered effective for the prevention of postmenopausal osteoporosis, it is generally recommended as an option only for women at significant risk, for whom non-oestrogen therapies are unsuitable. There are insufficient data to assess the risk of long term HT use in perimenopausal women or postmenopausal women younger than 50 years of age http: //www. cochrane. org/news/hormone-replacement-therapy-postmenopausalwomen-does-it-help-or-harm-your-heart http: //www. cochrane. org/CD 004143/MENSTR_long-term-hormone-therapy-forperimenopausal-and-postmenopausal-women 11

“Undertreatment improves but overtreatment does not” 12 12

The need for changes in medical education and practices • More in better than less • Early in better than late • More advance and higher cost of health technology is better than traditional and lower cost technology • FDA approved products are always safe • The need to address uncertainty • Placebo has no effect 13 13

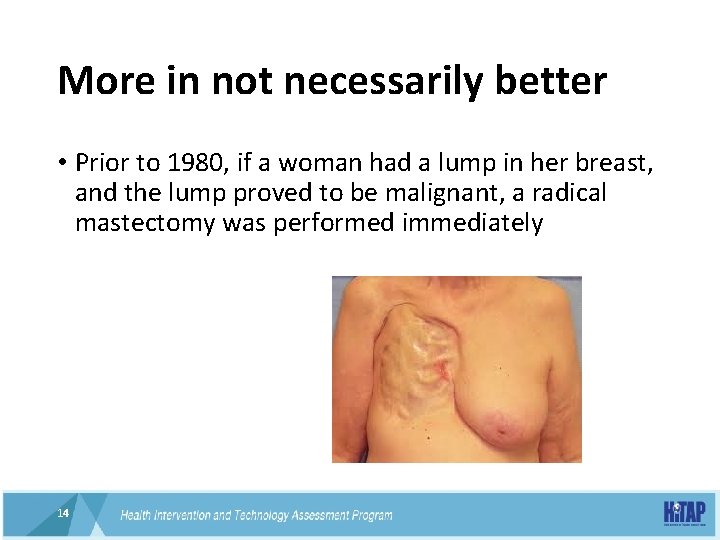
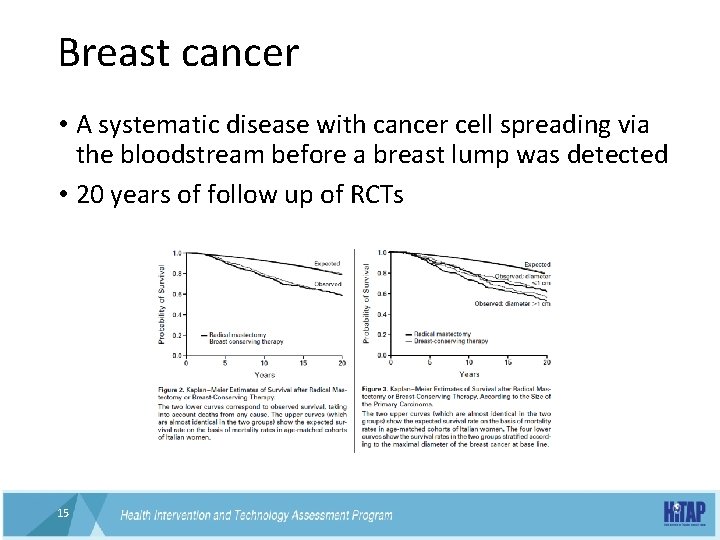
More in not necessarily better • Prior to 1980, if a woman had a lump in her breast, and the lump proved to be malignant, a radical mastectomy was performed immediately 14 14

Breast cancer • A systematic disease with cancer cell spreading via the bloodstream before a breast lump was detected • 20 years of follow up of RCTs 15 15

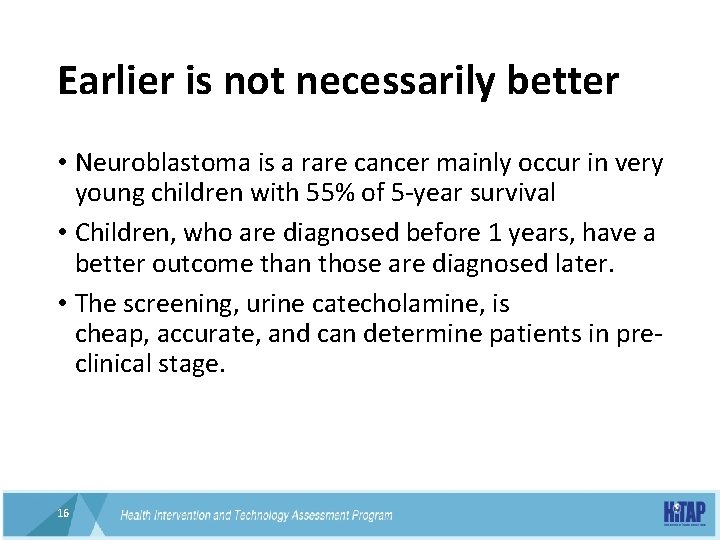
Earlier is not necessarily better • Neuroblastoma is a rare cancer mainly occur in very young children with 55% of 5 -year survival • Children, who are diagnosed before 1 years, have a better outcome than those are diagnosed later. • The screening, urine catecholamine, is cheap, accurate, and can determine patients in preclinical stage. 16 16

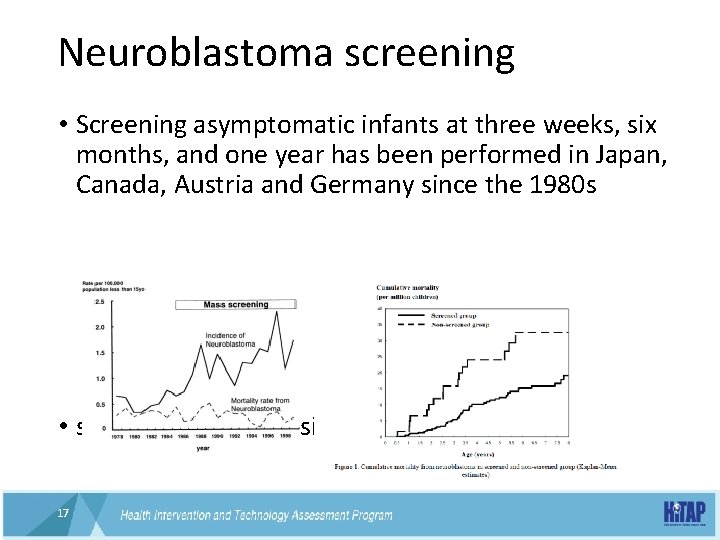
Neuroblastoma screening • Screening asymptomatic infants at three weeks, six months, and one year has been performed in Japan, Canada, Austria and Germany since the 1980 s • spontaneous regression 17 17

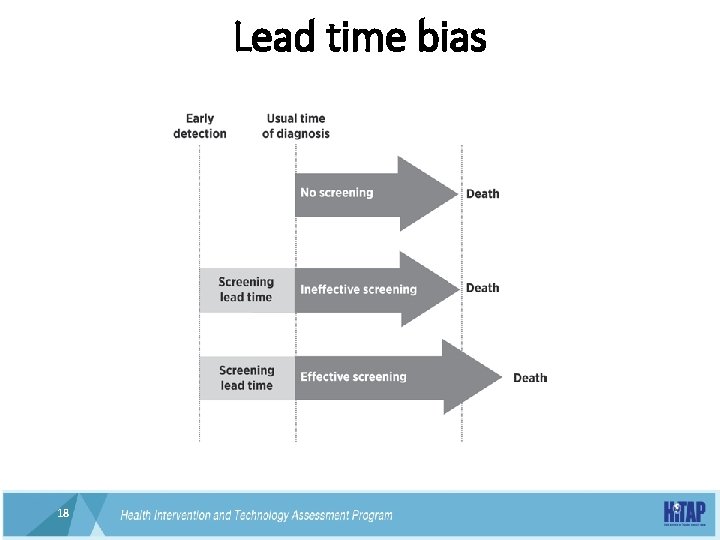
Lead time bias 18 18

FDA approved products is not always safe 19 19

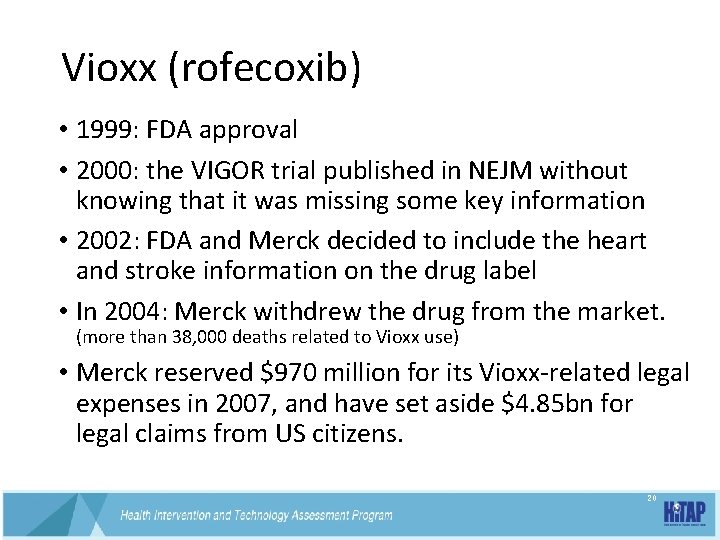
Vioxx (rofecoxib) • 1999: FDA approval • 2000: the VIGOR trial published in NEJM without knowing that it was missing some key information • 2002: FDA and Merck decided to include the heart and stroke information on the drug label • In 2004: Merck withdrew the drug from the market. (more than 38, 000 deaths related to Vioxx use) • Merck reserved $970 million for its Vioxx-related legal expenses in 2007, and have set aside $4. 85 bn for legal claims from US citizens. 20

21 21

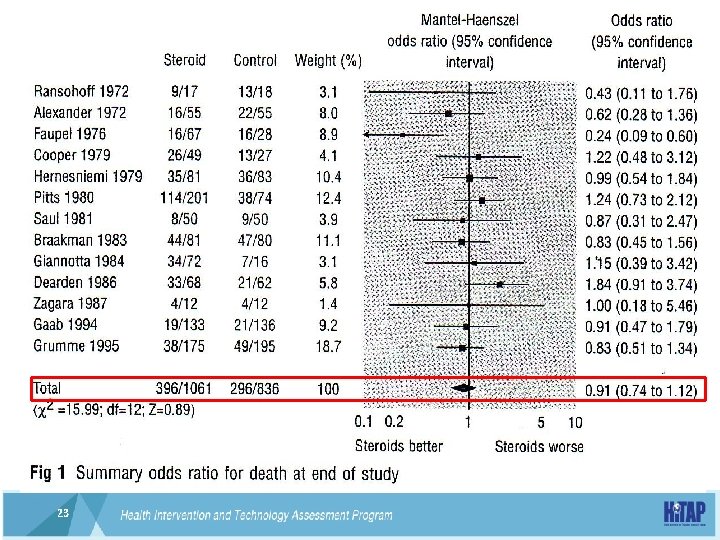
The need to address uncertainty • High-does intravenous corticosteroid for traumatic head injury 22 22

23

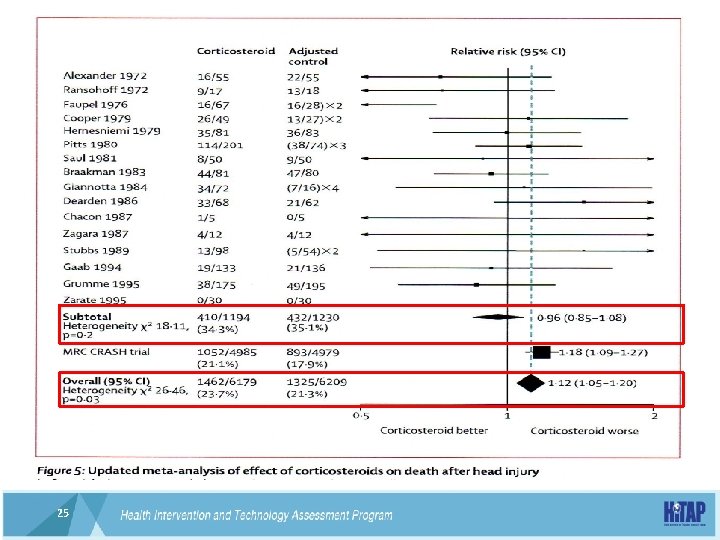
Lancet 2004; 364: 1321 -28 24 24

25 25

It’s so difficult to do ‘pragmatic’ research • Medical records: who’s own them? • Ethical approval • Confidentiality 26

Placebo effect 27 27

28 28

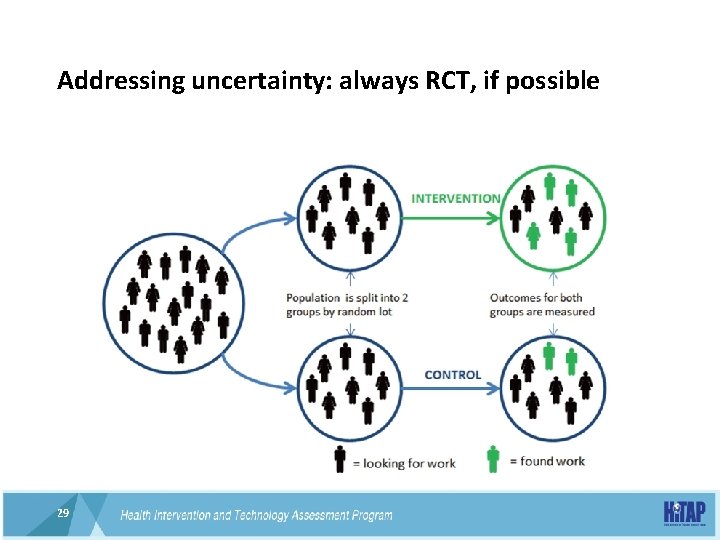
Addressing uncertainty: always RCT, if possible 29 29

Publication bias 30 30

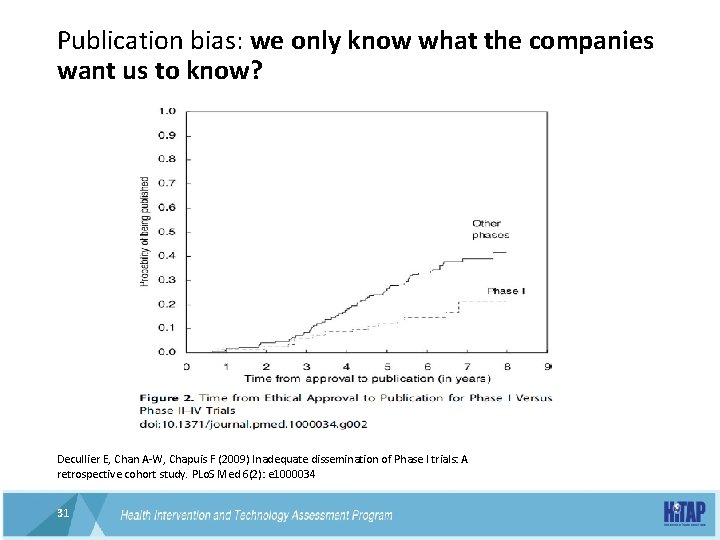
Publication bias: we only know what the companies want us to know? Decullier E, Chan A-W, Chapuis F (2009) Inadequate dissemination of Phase I trials: A retrospective cohort study. PLo. S Med 6(2): e 1000034 31 31

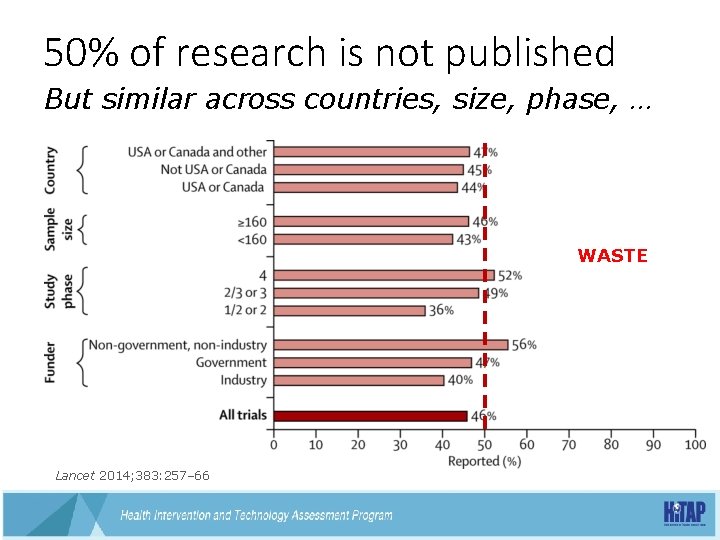
50% of research is not published But similar across countries, size, phase, … WASTE Lancet 2014; 383: 257– 66

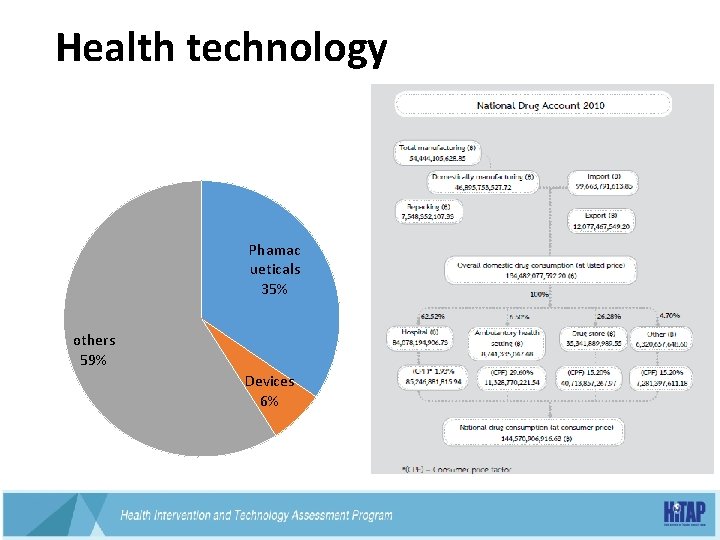
Health technology Phamac ueticals 35% others 59% Devices 6%

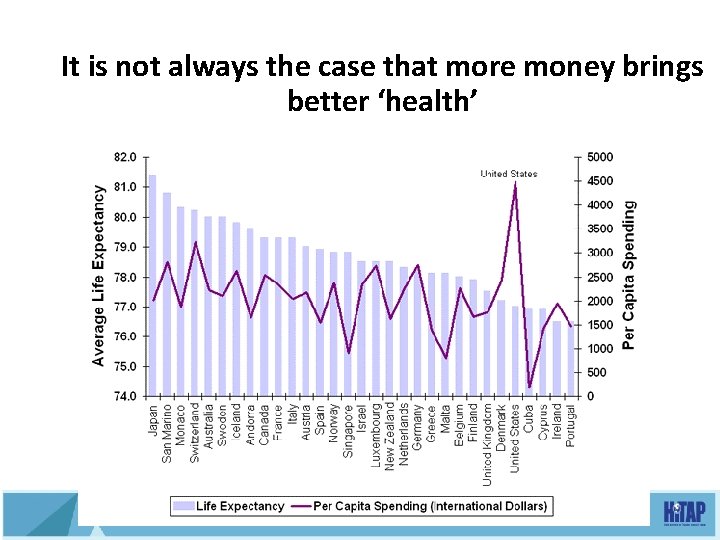
It is not always the case that more money brings better ‘health’ 34

Improving efficiency “ 20 -40% of all health spending is currently wasted through inefficiency use of resources. ” (WHR 2010) 35

HITAP at 9 years • 50 staff (8 Ph. D staff) • Revenue: 30 mil baht per year (60% from overseas) • 20 research projects per annual • Policy links • NLEM development • UC Benefit Package • Other areas • QOF • DFA Reform • Setting Priority Health Research 36

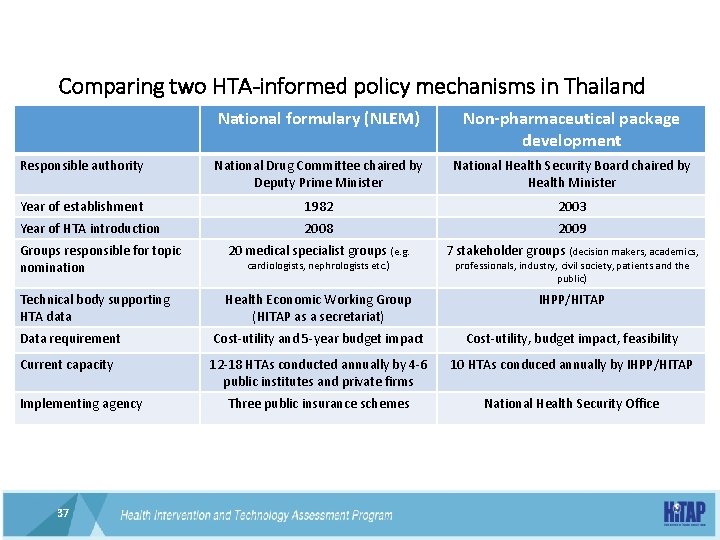
Comparing two HTA-informed policy mechanisms in Thailand National formulary (NLEM) Non-pharmaceutical package development Responsible authority National Drug Committee chaired by Deputy Prime Minister National Health Security Board chaired by Health Minister Year of establishment 1982 2003 Year of HTA introduction 2008 2009 Groups responsible for topic nomination 20 medical specialist groups (e. g. 7 stakeholder groups (decision makers, academics, Technical body supporting HTA data Health Economic Working Group (HITAP as a secretariat) IHPP/HITAP Data requirement Cost-utility and 5 -year budget impact Cost-utility, budget impact, feasibility Current capacity 12 -18 HTAs conducted annually by 4 -6 public institutes and private firms 10 HTAs conduced annually by IHPP/HITAP Three public insurance schemes National Health Security Office Implementing agency 37 cardiologists, nephrologists etc. ) professionals, industry, civil society, patients and the public)

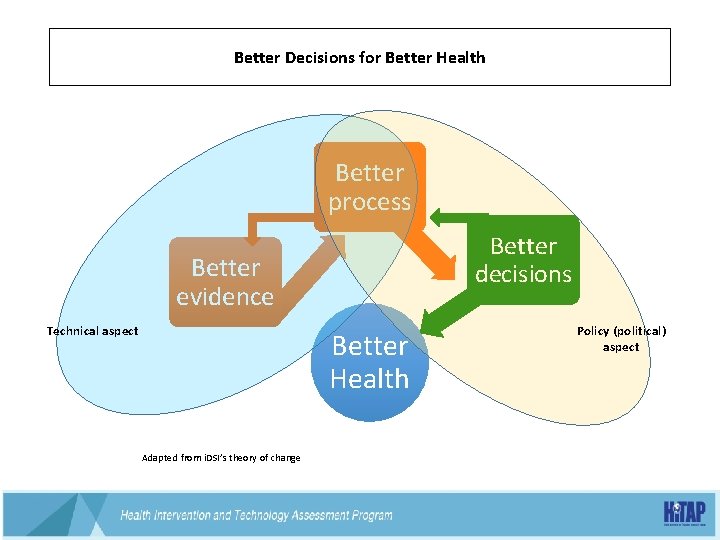
Better Decisions for Better Health Better process Better decisions Better evidence Technical aspect Better Health Policy (political) aspect 38 Adapted from i. DSI’s theory of change

Our approach • HTA research prioritization • Stakeholder’s involvement in HTA process • Research dissemination to wide rage of stakeholders (all are publicly available) • • 39 HTA process Research reports International journal publications (100+) Policy brief Social media Meeting/conference/teachning In-person communication

Future strategies for domestic works • HTA units in university hospitals • Mahidol University’s Center for Health Intervention & Technology Assessment • HTA units in universities • Social and Administrative Pharmacy, Silaprakorn University, Khon Kaen University, Chiang Mai University, Naresaun University, Phayao University • HTA capacity in public health authorities e. g. MOPH’s departments, NHSO, NLEM secretariat, etc. • HTA approach for other ministries e. g. education, social welfare, etc. 40

HITAP going global • Co-founder of HTAsia. Link (2010) http: //www. htasialink. org/ • Establishment of HITAP International Unit in 2013 • Joining NICE International-led i. DSI http: //www. idsihealth. org/ providing technical support to ministries of health in Asia • Supporting HITA resolution in WHO SEARO in 2013 • Supporting HITA resolution in WHA 2014 • Assisting PMAC 2016 41

42

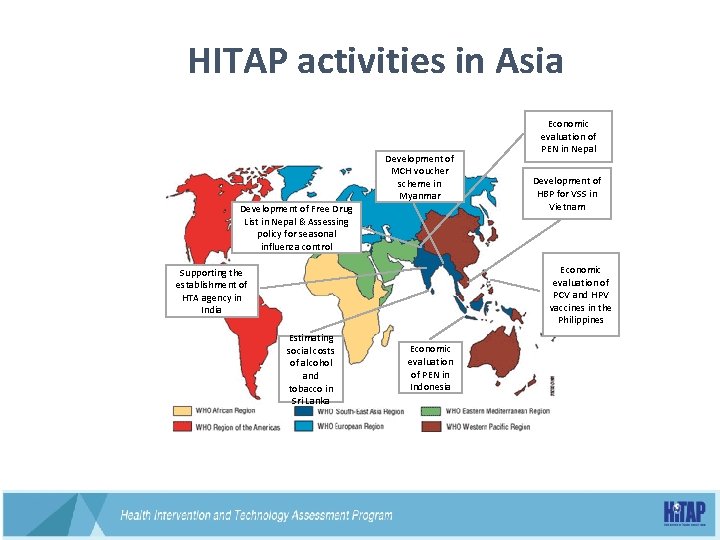
HITAP activities in Asia Development of Free Drug List in Nepal & Assessing policy for seasonal influenza control Development of MCH voucher scheme in Myanmar Economic evaluation of PEN in Nepal Development of HBP for VSS in Vietnam Economic evaluation of PCV and HPV vaccines in the Philippines Supporting the establishment of HTA agency in India Estimating social costs of alcohol and tobacco in Sri Lanka Economic evaluation of PEN in Indonesia 43

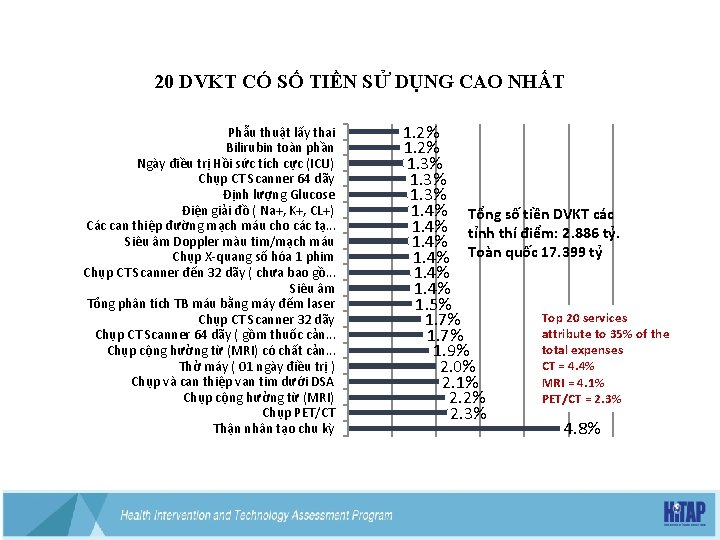
20 DVKT CÓ SỐ TIỀN SỬ DỤNG CAO NHẤT Phẫu thuật lấy thai Bilirubin toàn phần Ngày điều trị Hồi sức tích cực (ICU) Chụp CT Scanner 64 dãy Định lượng Glucose Điện giải đồ ( Na+, K+, CL+) Các can thiệp đường mạch máu cho các tạ. . . Siêu âm Doppler màu tim/mạch máu Chụp X-quang số hóa 1 phim Chụp CT Scanner đến 32 dãy ( chưa bao gồ. . . Siêu âm Tổng phân tích TB máu bằng máy đếm laser Chụp CT Scanner 32 dãy Chụp CT Scanner 64 dãy ( gồm thuốc cản. . . Chụp cộng hưởng từ (MRI) có chất cản. . . Thở máy ( 01 ngày điều trị ) Chụp và can thiệp van tim dưới DSA Chụp cộng hưởng từ (MRI) Chụp PET/CT Thận nhân tạo chu kỳ 1. 2% 1. 3% 1. 4% Tổng số tiền DVKT các 1. 4% tỉnh thí điểm: 2. 886 tỷ. 1. 4% Toàn quốc 17. 399 tỷ 1. 4% 1. 5% Top 20 services 1. 7% attribute to 35% of the 1. 7% total expenses 1. 9% CT = 4. 4% 2. 0% MRI = 4. 1% 2. 1% PET/CT = 2. 3% 2. 2% 2. 3% 4. 8%

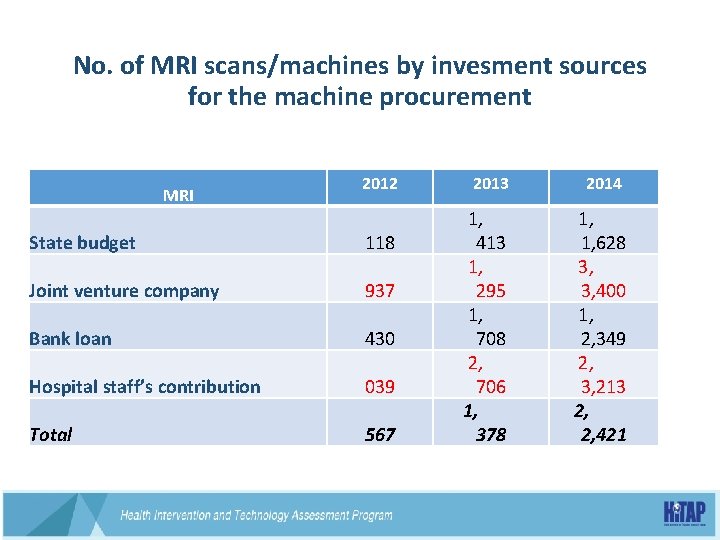
No. of MRI scans/machines by invesment sources for the machine procurement MRI State budget Joint venture company Bank loan Hospital staff’s contribution Total 2012 2013 2014 1, 118 413 1, 628 1, 3, 937 295 3, 400 1, 430 708 2, 349 2, 039 706 3, 213 1, 2, 567 378 2, 421

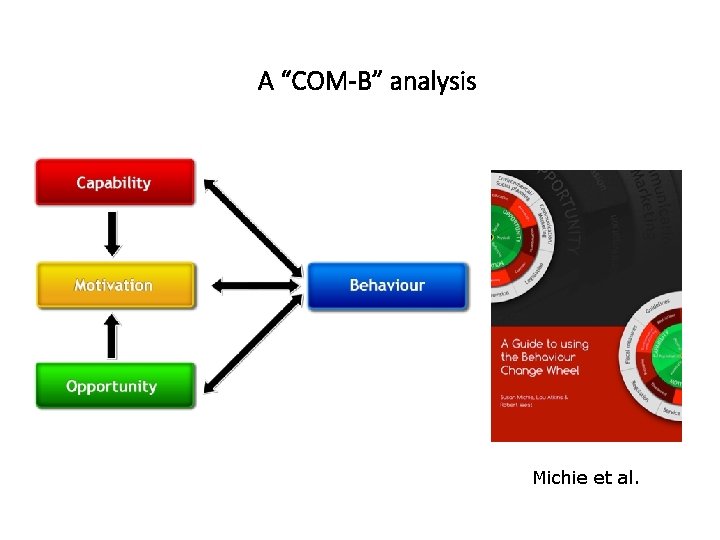
Summary • We need to be more caution about use of health technology than ever • Health technology assessment is important to ensure that the use of technology is safe, efficacious, and good value for money, and most importantly saying ‘NO’ to low-value technology • There is system failure to get out of this vicious cycle and doctor should lead medical professionals to take a paradigm shift unless we will fail our health care system • We need ‘capacity’, ‘motivation’ and ‘opportunity’… 46 46

Getting this kind of books and conference to Thai medical students & doctors 47

FOLLOW US AT

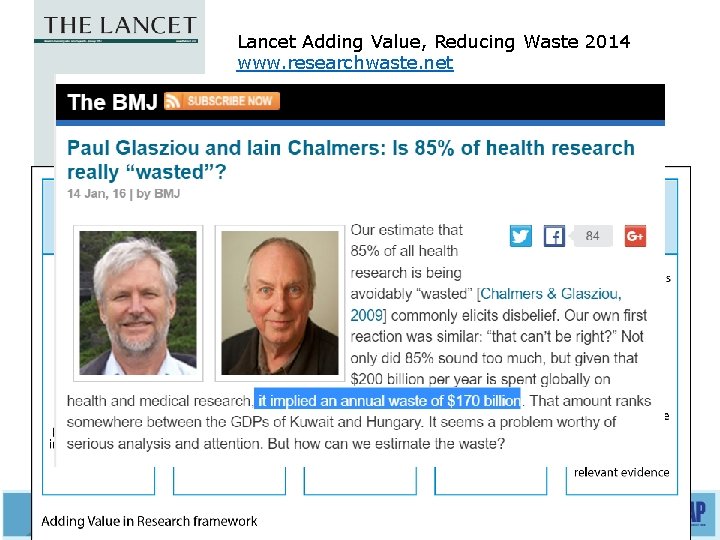
Lancet Adding Value, Reducing Waste 2014 www. researchwaste. net Five stages of waste in research

A “COM-B” analysis Michie et al.

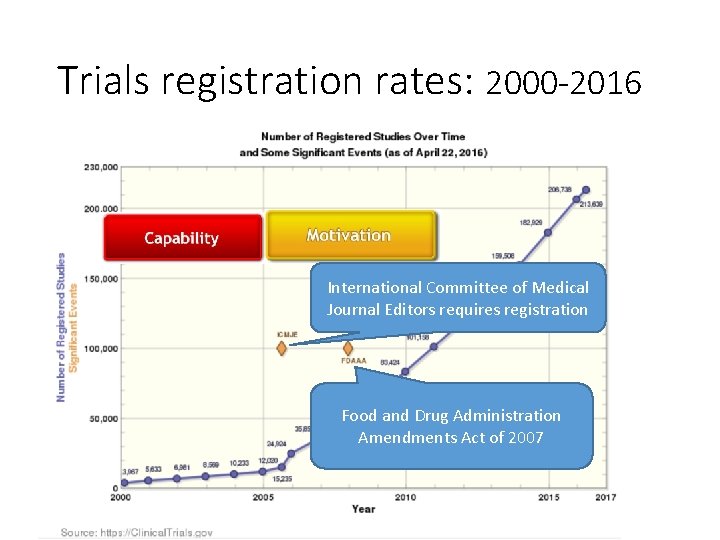
Trials registration rates: 2000 -2016 International Committee of Medical Journal Editors requires registration Food and Drug Administration Amendments Act of 2007

Trials registration Opportunities to check registration at: 1. Funding approval 2. Ethics approval 3. Final report to funder * (+ post summary results) 4. Publication (+ post summary results) * NIHR’s HTA program requires, and has 98% publication rate for their trials!