Handling of Missing Data A regulatory view Ferran













- Slides: 25

Handling of Missing Data. A regulatory view Ferran Torres, MD, Ph. D IDIBAPS. Hospital Clinic Barcelona Autonomous University of Barcelona (UAB) 1

Documentation http: //ferran. torres. name/edu/eacpt • Power Point presentation • Direct links to guidelines • List of selected relevant references 2

Disclaimer • The opinions expressed today are my personal views and should not be understood or quoted as being made on behalf of any organization. – Regulatory • Spanish Medicines Agency (AEMPS) • European Medicines Agency (EMA) – Scientific Advice Working Party (SAWP) – Biostatistics Working Party (BSWP) – Hospital - Academic - Independent Research • IDIBAPS. Hospital Clinic Barcelona • Autonomous University of Barcelona (UAB) • CAIBER. Spanish Clinical Trials Platform 3

Best way to deal with Missing Data? ? Don’t have any!!! • Methods for imputation: – Many techniques – No gold standard for every situation 4

Regulatory guidance concerning MD • 1998: : ICHE 9. Statistical Principles for Clinical Trials • 2001: : Pt. C on Missing Data (rapporteurs: Gonzalo Calvo & Ferran Torres) • Dec-2007: Recommendation for the Revision of the Pt. C on MD • 2009: : Release for consultation • 2010: Adopted new guideline (rapporteurs: David Wright & Ferran Torres) 5

Status in early 2000 s • In general, MD was not seen as a source of bias: – considered mostly as a loss of power issue – little efforts in avoiding MD • Importance of the methods for dealing with: – Handling of missingness: Mostly LOCF, Worst Case 6

Status in early 2000 s • Very few information on the handling of MD in protocols and SAP (little prespecification) • Lack of Sensitivity analysis, or only one, and no justification • Lack (little) identification and description of missingness in reports 7

Key Points • Avoidance of MD • Bias: specially when MD was related to the outcome • Methods: – Warning on the LOCF – Open the door to other methods: • Multiple imputation, Mixed Models… • Sensitivity analyses 8

Current status in 2008 -9 Missing data remains a problem in protocols and final reports: • Little or no critical discussion on pattern of MD data and withdrawals • None / only one sensitivity analysis • Methods: – Inappropriate methods for the handling of MD – LOCF: Still used as a general approach for too many situations – Methods with very little use in early 2000 are now common (Mixed Models) 9

New Draft Pt. C 1. Executive Summary 2. Introduction 3. The Effect of MD on the Analysis & the Interpretation 4. General Recommendations 4. 1 Avoidance of Missing Data 4. 2 Design of the Study. Relevance Of Predefinition 4. 3 Final Report 5. Handling of Missing Data 5. 1 Theoretical Framework 5. 2 Complete Case Analysis 5. 3 Methods for Handling Missing Data 6. Sensitivity Analyses 10

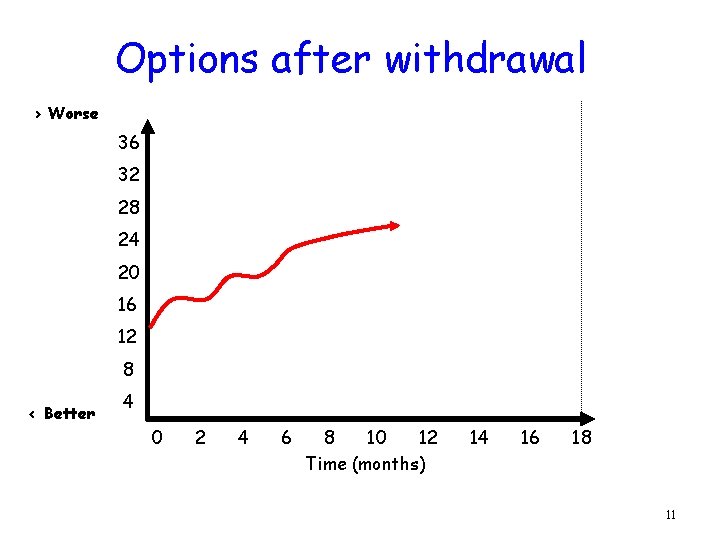
Options after withdrawal > Worse 36 32 28 24 20 16 12 8 < Better 4 0 2 4 6 8 10 12 Time (months) 14 16 18 11

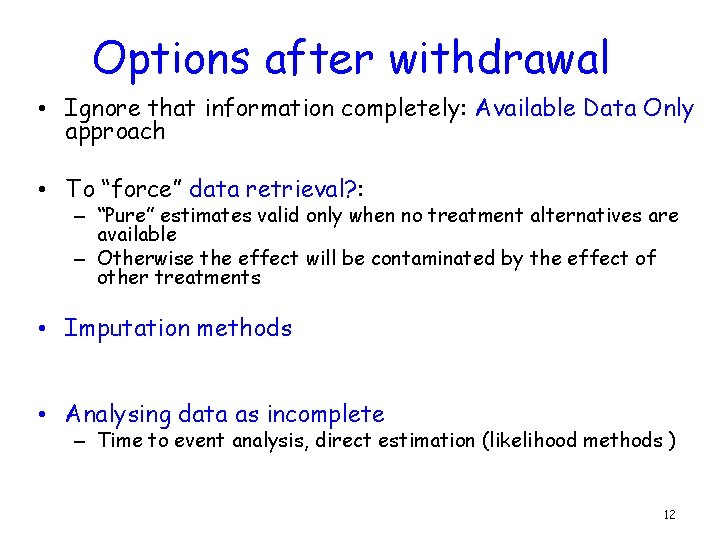
Options after withdrawal • Ignore that information completely: Available Data Only approach • To “force” data retrieval? : – “Pure” estimates valid only when no treatment alternatives are available – Otherwise the effect will be contaminated by the effect of other treatments • Imputation methods • Analysing data as incomplete – Time to event analysis, direct estimation (likelihood methods ) 12

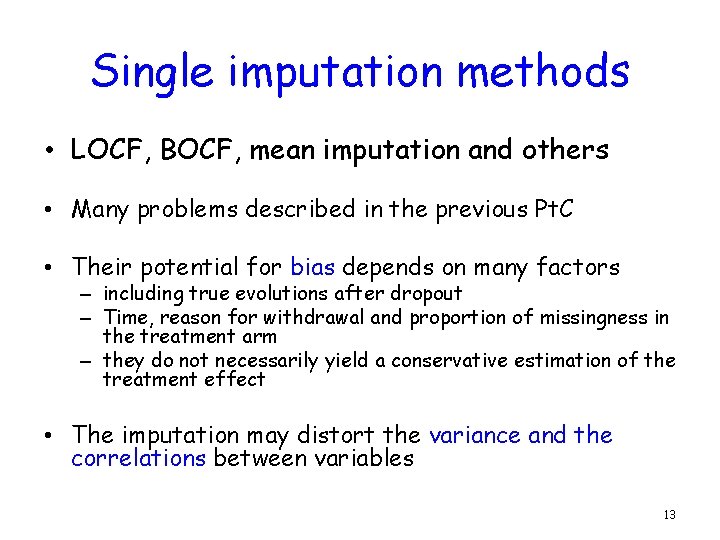
Single imputation methods • LOCF, BOCF, mean imputation and others • Many problems described in the previous Pt. C • Their potential for bias depends on many factors – including true evolutions after dropout – Time, reason for withdrawal and proportion of missingness in the treatment arm – they do not necessarily yield a conservative estimation of the treatment effect • The imputation may distort the variance and the correlations between variables 13

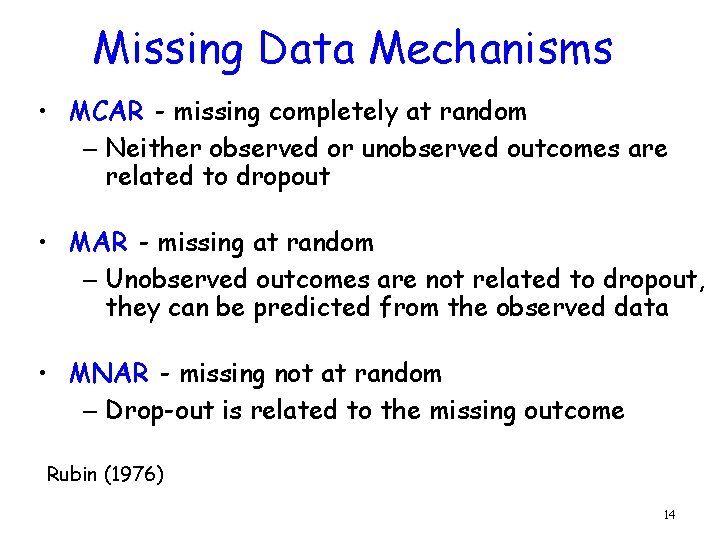
Missing Data Mechanisms • MCAR - missing completely at random – Neither observed or unobserved outcomes are related to dropout • MAR - missing at random – Unobserved outcomes are not related to dropout, they can be predicted from the observed data • MNAR - missing not at random – Drop-out is related to the missing outcome Rubin (1976) 14

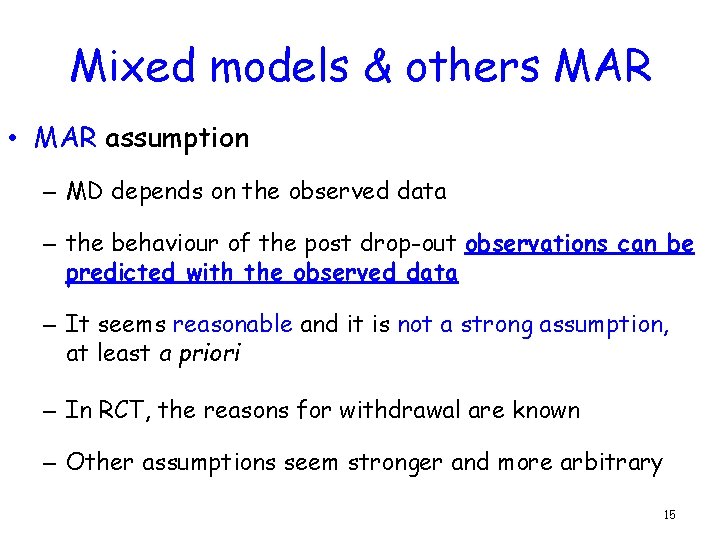
Mixed models & others MAR • MAR assumption – MD depends on the observed data – the behaviour of the post drop-out observations can be predicted with the observed data – It seems reasonable and it is not a strong assumption, at least a priori – In RCT, the reasons for withdrawal are known – Other assumptions seem stronger and more arbitrary 15

However… • It is reasonable to consider that the treatment effect will somehow cease/attenuate after withdrawal • If there is a good response, MAR will not “predict” a bad response • =>MAR assumption not suitable for early drop-outs because of safety issues • In this context MAR seems likely to be anticonservative 16

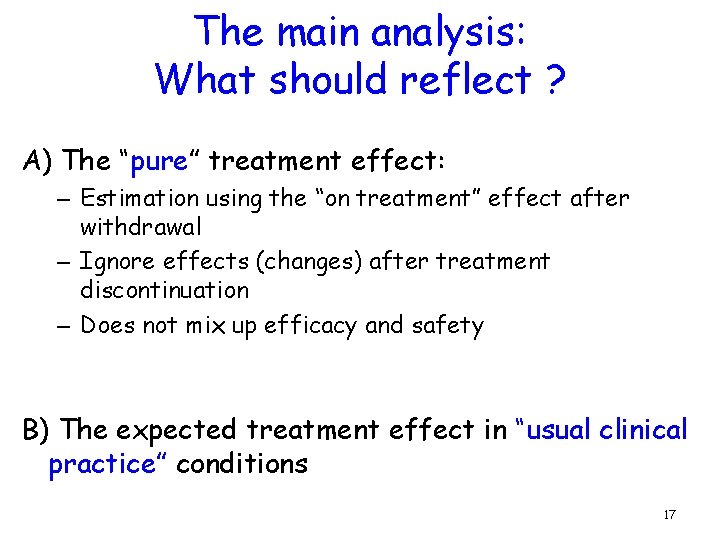
The main analysis: What should reflect ? A) The “pure” treatment effect: – Estimation using the “on treatment” effect after withdrawal – Ignore effects (changes) after treatment discontinuation – Does not mix up efficacy and safety B) The expected treatment effect in “usual clinical practice” conditions 17

MAR • Estimate the treatment effect that would be seen if patients had continued on the study as planned. • . . . results could be seen as not fully compliant with the ITT principle 18

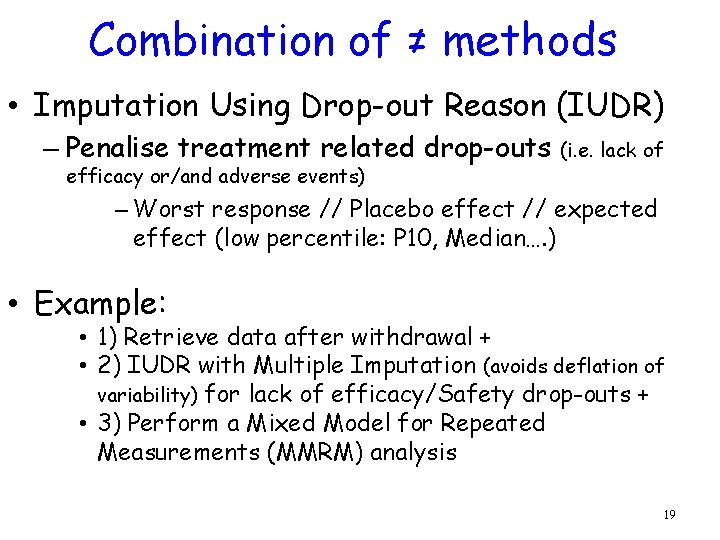
Combination of ≠ methods • Imputation Using Drop-out Reason (IUDR) – Penalise treatment related drop-outs efficacy or/and adverse events) (i. e. lack of – Worst response // Placebo effect // expected effect (low percentile: P 10, Median…. ) • Example: • 1) Retrieve data after withdrawal + • 2) IUDR with Multiple Imputation (avoids deflation of variability) for lack of efficacy/Safety drop-outs + • 3) Perform a Mixed Model for Repeated Measurements (MMRM) analysis 19

Key recommendations (1/4) • Design – Assume that MD is probably biased – Avoidance of MD – Relevance of predefinition (avoid data-driven methods) – Detailed description. . – and justification of absence of bias in favour of experimental treatment • Final Report – Detailed description of the planned and amendments of the predefined methods 20

Key recommendations (2/4) Detailed description (numerical & graphical) • Pattern of MD • Rate and time of withdrawal – By reason, time/visit and treatment – Some withdrawals will occur between visits: use survival methods • Outcome – By reason of withdrawal and also for completers 21

Key recommendations (3/4) Sensitivity Analyses • a set of analyses showing the influence of different methods of handling missing data on the study results • Pre-defined and designed to assess the repercussion on the results of the particular assumptions made in the handling of missingness • Responder analysis 22

Key recommendations (4/4) • No universally best method • Analysis must be tailored to the specific situation at hand • Better methods than LOCF: • But still useful for sensitivity analyses and as an anchor to compare with previous trials • Methods: – MCAR: almost any method is valid but difficult to assume – MAR: More likely to occur • Likelihood (Mixed Models MMRM, E-M) / weighted-GEE • Multiple imputation – MNAR: model drop-out as well as response • Theoretically more useful, in practice highly dependent on drop-out assumptions which are un-checkable • For sensibility analysis. 23

Concluding Remarks • Avoid and foresee MD • Sensitivity analyses • Methods for handling: – No gold standard for every situation – In principle, “almost any method may be valid”: – =>But their appropriateness has to be justified 24

http: //ferran. torres. name/edu/eacpt 25