Grade Level Content Expectations 6 Grade Science Teacher





























- Slides: 60

Grade Level Content Expectations 6 Grade Science Teacher Workshops Potential & Kinetic Energy Developed by: Mr. P. A. Klozik & Dr. M. H. Suckley Email: MAP@Science. Scene. com Visit our Website: http: //www. Science. Scene. com (The MAPs Co. )

Matrix for Physical Science Grade 6 Process Inquiry Analysis and Communication Reflection and Social Implications Content Energy Kinetic and Potential Energy Transfer Changes in Matter Changes in State

Inquiry Process S. IP. M. 1 K-7 Standard S. IP: Develop an understanding that scientific inquiry and reasoning involves observing, questioning, investigating, recording, and developing solutions to problems. Inquiry involves generating questions, conducting investigations, and developing solutions to problems through reasoning and observation. S. IP. 06. 11 Generate scientific questions based on observations, investigations, and research S. IP. 06. 12 Design and conduct scientific investigations. S. IP. 06. 13 Use tools and equipment (spring scales, stop watches, meter sticks and tapes, models, hand lens, thermometer, models, sieves, microscopes) appropriate to scientific investigations. S. IP. 06. 14 Use metric measurement devices in an investigation. S. IP. 06. 15 Construct charts and graphs from data and observations. S. IP. 06. 16 Identify patterns in data. Back

Inquiry Analysis K-7 Standard S. IA: Develop an understanding that scientific inquiry and investigations require analysis and communication of findings, using Communication appropriate technology. S. IA. M. 1 Inquiry includes an analysis and presentation of findings that lead to future questions, research, and investigations. S. IA. 06. 11 Analyze information from data tables and graphs to answer scientific questions. S. IA. 06. 12 Evaluate data, claims, and personal knowledge through collaborative science discourse. S. IA. 06. 13 Communicate and defend findings of observations and investigations using evidence. S. IA. 06. 14 Draw conclusions from sets of data from multiple trials of a scientific investigation. S. IA. 06. 15 Use multiple sources of information to evaluate strengths and weaknesses of claims, arguments, or data. Back

Reflection And Social Implications S. RS. M. 1 K-7 Standard S. RS: Develop an understanding that claims and evidence for their scientific merit should be analyzed. Understand how scientists decide what constitutes scientific knowledge. Develop an understanding of the importance of reflection on scientific knowledge and its application to new situations to better understand the role of science in society and technology. Reflecting on knowledge is the application of scientific knowledge to new and different situations. Reflecting on knowledge requires careful analysis of evidence that guides decision-making and the application of science throughout history and within society. S. RS. 06. 11 Evaluate the strengths and weaknesses of claims, arguments, and data. S. RS. 06. 12 Describe limitations in personal and scientific knowledge. S. RS. 06. 13 Identify the need for evidence in making scientific decisions. S. RS. 06. 14 S. RS. 06. 15 Evaluate scientific explanations based on current evidence and scientific principles. Demonstrate scientific concepts through various illustrations, performances, models, exhibits, and activities. S. RS. 06. 16 Design solutions to problems using technology. S. RS. 06. 17 Describe the effect humans and other organisms have on the balance of the natural world. S. RS. 06. 18 Describe what science and technology can and cannot reasonably contribute to society. S. RS. 06. 19 Describe how science and technology have advanced because of the contributions of many people throughout history and across cultures. Back

P. EN: Energy Develop an understanding that there are many forms of energy (such as heat, light, sound, and electrical) and that energy is transferable by convection, conduction, or radiation. Understand energy can be in motion, called kinetic; or it can be stored, called potential. Develop an understanding that as temperature increases, more energy is added to a system. Understand nuclear reactions in the sun produce light and heat for the Earth. P. EN. M. 1 Kinetic and Potential Energy- Objects and substances in motion have kinetic energy. Objects and substances may have potential energy due to their relative positions in a system. Gravitational, elastic, and chemical energy are all forms of potential energy. P. EN. 06. 11 Identify kinetic or potential energy in everyday situations (for example: stretched rubber band, objects in motion, ball on a hill, food energy). P. EN. 06. 12 Demonstrate the transformation between potential and kinetic energy in simple mechanical systems (for example: roller coasters, pendulums). P. EN. M. 4 Energy Transfer- Energy is transferred from a source to a receiver by radiation, conduction, and convection. When energy is transferred from a source to a receiver, the quantity of energy before the transfer is equal to the quantity of energy after the transfer. P. EN. 06. 41 Explain how different forms of energy can be transferred from one place to another by radiation, conduction, or convection. P. EN. 06. 42 Illustrate how energy can be transferred while no energy is lost or gained in the transfer. Back

P. CM: Changes in Matter P. CM. M. 1 Develop an understanding of changes in the state of matter in terms of heating and cooling, and in terms of arrangement and relative motion of atoms and molecules. Understand the differences between physical and chemical changes. Develop an understanding of the conservation of mass. Develop an understanding of products and reactants in a chemical change. Changes in State- Matter changing from state to state can be explained by using models which show that matter is composed of tiny particles in motion. When changes of state occur, the atoms and/or molecules are not changed in structure. When the changes in state occur, mass is conserved because matter is not created or destroyed. P. CM. 06. 11 Describe and illustrate changes in state, in terms of the arrangement and relative motion of the atoms or molecules. P. CM. 06. 12 Explain how mass is conserved as it changes from state to state in a closed system. Back


Potential & Kinetic Energy Presented By: The MAPs Team Meaningful Applications of Physical Science Email: MAP@Science. Scene. com Visit Our Website: http: //www. Science. Scene. com

Potential & Kinetic Energy A. What Is Energy? B. Seven Forms Of Energy. C. Two Types Of Energy. D. Conservation of Energy. E. Work and Power.

Potential & Kinetic Energy A. What Is Energy? 1. Naive Ideas Concerning Energy. 2. Does Energy (Light) Have Either Weight Or Volume? . . 8 3. What Makes It Move? . . . . 9 4. Defining Energy. . . . 10 5. How Is Energy / Work Measured? . . . . 12 6. How Is the Strength of Energy Measured? . . . 13

Potential & Kinetic Energy B. Forms Of Energy 1. The Seven Forms Of Energy. . . . 16 2. Energy and the Human Body. . . . 18 3. Transfer Of Chemical To Heat - Food Burning. . . 23 4. Can Heat Make Things Move? . . . 24 5. Can Chemicals Make Things Move? . . 26 6. Using A Radio Speaker Electrical to Mechanical Energy. . . . 29 7. Changing Mechanical Energy To Heat Energy. . . 30 8. Energy Conversion Smorgasbord (Demonstrating Energy Conversions). 32 9. Energy Conversion Summary. . . . 36 10. Energy Chains. . . . . 38

Potential & Kinetic Energy C. There Are Two Types Of Energy. 1. Two types of Energy. . . . . 42 2. Ah-La-Bounce. . . . . 43 3. The weight of A Body and Its Gravitational Potential Energy. . . 44 4. Galilean Cannon. . . . . 46 5. Storing Energy. . . . . 48 6. Investigating Potential / Kinetic Energy a. Does Potential / Kinetic Energy depend on Force? . . 52 b. Does Potential / Kinetic Energy depend on Mass? . . 54

Potential & Kinetic Energy D. Conservation of Energy 1. Is Energy Conserved When Converted to Work? a. Is Energy Conserved When Force is Changed? . . 57 b. Is Energy Conserved When Mass is Changed? . . 58 2. Energy Transformations And The Pendulum. . . 59 3. Stop And Go Balls. . . . . 62 4. Coat Hanger Cannon. . . . 63

Potential & Kinetic Energy E. Work and Power 1. Determining your Horsepower. . . . 70 2. Hot Rod Power. . . . . 72 3. Comparing Work, Kinetic and Potential Energy Summary


We Had A Great Time


Naive Ideas - Energy 1. Energy is a "thing". This is kind of a fuzzy notion, probably because of the way we think about newton-meters or joules. 2. The terms "energy" and "force" are interchangeable. 3. From the non-scientific point of view, "work" is synonymous with "labor. " 4. An object at rest has no energy. 5. Doubling the speed of a moving object doubles the kinetic energy. 6. Energy can be changed from one form to another with no energy losses. 7. Things "use up" energy. 8. Energy is confined to some particular origin, such as what we get from food, or what the electric company sells. 9. There is no relationship between matter and energy. 10. If energy is conserved, why are we running out of it? 10

Does Light Have Either Weight Or Volume? 1. Place a box on a scale. 2. Tare (zero) the scale. 3. Add light energy to the box. 4. Observe any increase in mass.

What Makes It Move? 1. 2. 4. 6. 5. 7. 9 3. 8. 9.

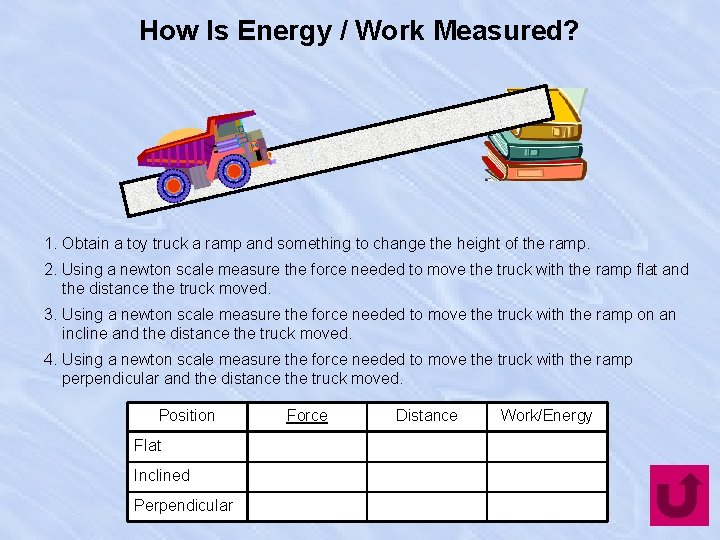
How Is Energy / Work Measured? 1. Obtain a toy truck a ramp and something to change the height of the ramp. 2. Using a newton scale measure the force needed to move the truck with the ramp flat and the distance the truck moved. 3. Using a newton scale measure the force needed to move the truck with the ramp on an incline and the distance the truck moved. 4. Using a newton scale measure the force needed to move the truck with the ramp perpendicular and the distance the truck moved. Position Flat Inclined Perpendicular Force Distance Work/Energy

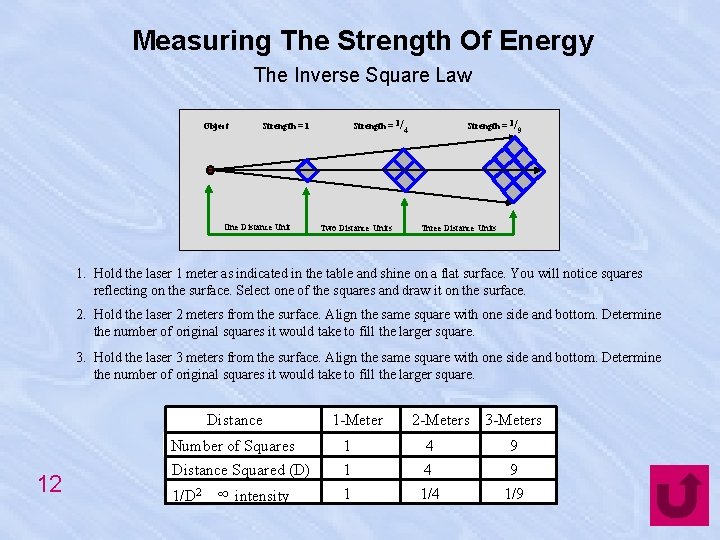
Measuring The Strength Of Energy The Inverse Square Law Object Strength = 1/4 Strength = 1 One Distance Unit Two Distance Units Strength = 1/9 Three Distance Units 1. Hold the laser 1 meter as indicated in the table and shine on a flat surface. You will notice squares reflecting on the surface. Select one of the squares and draw it on the surface. 2. Hold the laser 2 meters from the surface. Align the same square with one side and bottom. Determine the number of original squares it would take to fill the larger square. 3. Hold the laser 3 meters from the surface. Align the same square with one side and bottom. Determine the number of original squares it would take to fill the larger square. Distance 12 Number of Squares Distance Squared (D) 1/D 2 ∞ intensity 1 -Meter 1 1 1 2 -Meters 3 -Meters 4 4 1/4 9 9 1/9

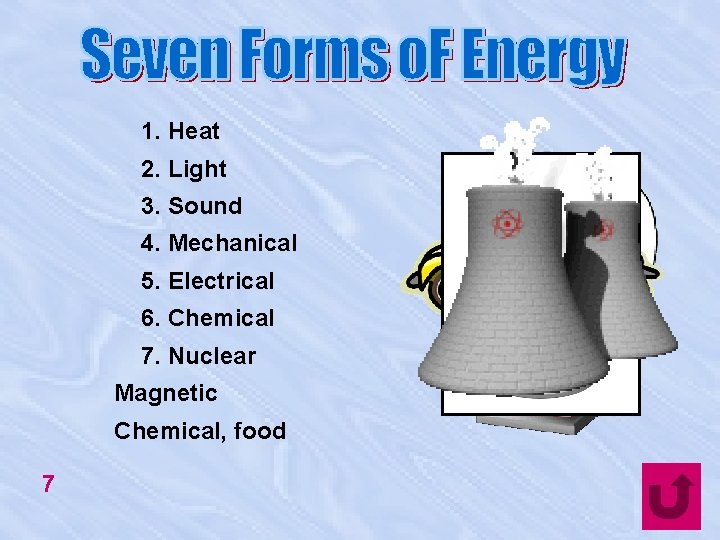
1. Heat 2. Light 3. Sound 4. Mechanical 5. Electrical 6. Chemical 7. Nuclear Magnetic Chemical, food 7

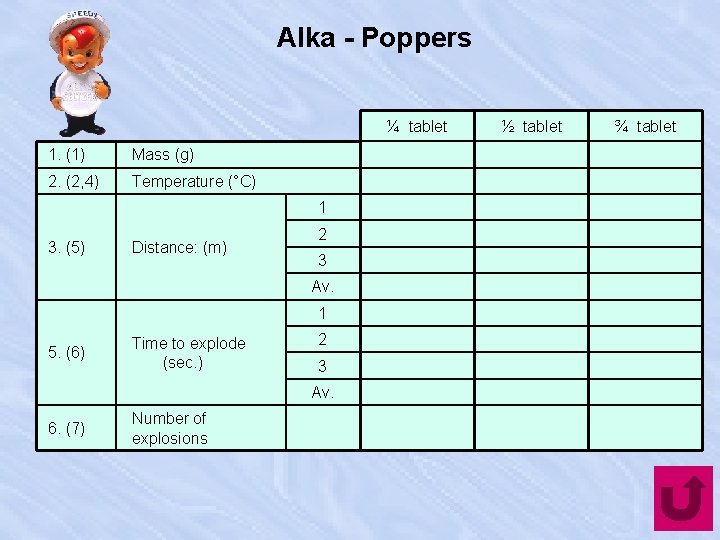
Alka - Poppers ¼ tablet 1. (1) Mass (g) 2. (2, 4) Temperature (°C) 1 3. (5) Distance: (m) 2 3 Av. 1 5. (6) Time to explode (sec. ) 2 3 Av. 6. (7) Number of explosions ½ tablet ¾ tablet

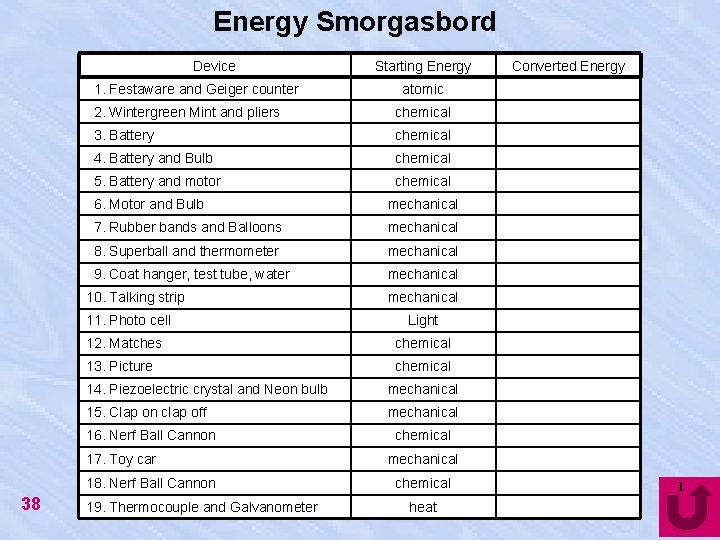
Energy Smorgasbord Device 1. Festaware and Geiger counter chemical 3. Battery chemical 4. Battery and Bulb chemical 5. Battery and motor chemical 6. Motor and Bulb mechanical 7. Rubber bands and Balloons mechanical 8. Superball and thermometer mechanical 9. Coat hanger, test tube, water mechanical 11. Photo cell mechanical Light 12. Matches chemical 13. Picture chemical 14. Piezoelectric crystal and Neon bulb mechanical 15. Clap on clap off mechanical 16. Nerf Ball Cannon 17. Toy car 18. Nerf Ball Cannon 19. Thermocouple and Galvanometer Converted Energy atomic 2. Wintergreen Mint and pliers 10. Talking strip 38 Starting Energy chemical mechanical chemical heat 1

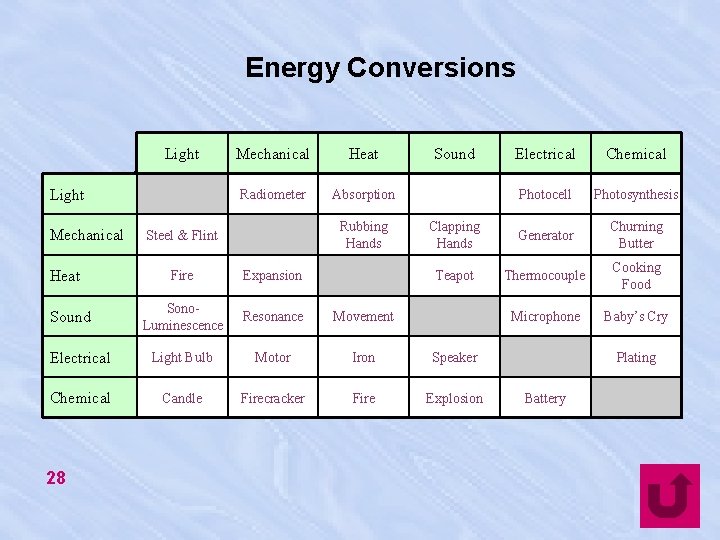
Energy Conversions Light Mechanical Heat Radiometer Absorption Rubbing Hands Steel & Flint Sound Electrical Chemical Photocell Photosynthesis Clapping Hands Generator Churning Butter Teapot Thermocouple Cooking Food Microphone Baby’s Cry Fire Expansion Sono. Luminescence Resonance Movement Electrical Light Bulb Motor Iron Speaker Chemical Candle Firecracker Fire Explosion Sound 28 Plating Battery

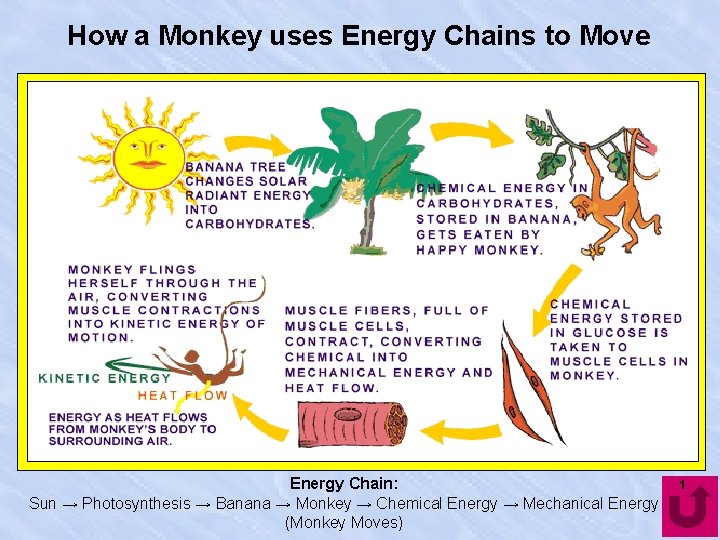
How a Monkey uses Energy Chains to Move Energy Chain: Sun → Photosynthesis → Banana → Monkey → Chemical Energy → Mechanical Energy (Monkey Moves) 1

Where Does The Energy Come From That is Needed For A Fisherman To Fish? Where does the energy needed for a fisherman to fish come from? 0

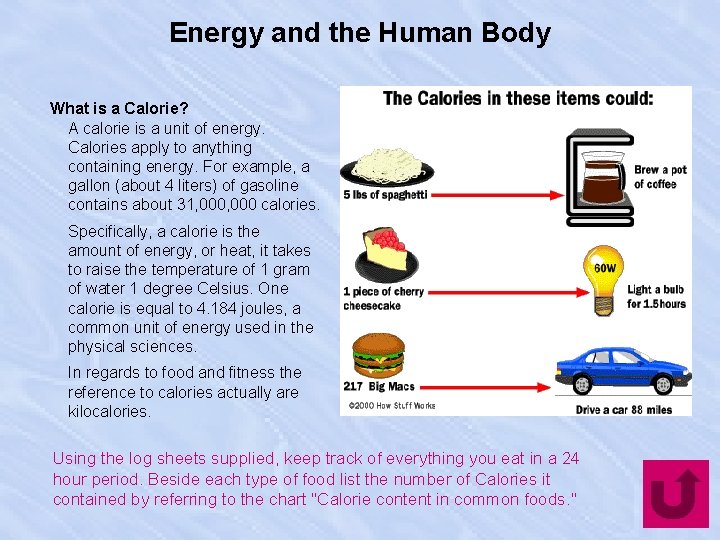
Energy and the Human Body What is a Calorie? A calorie is a unit of energy. Calories apply to anything containing energy. For example, a gallon (about 4 liters) of gasoline contains about 31, 000 calories. Specifically, a calorie is the amount of energy, or heat, it takes to raise the temperature of 1 gram of water 1 degree Celsius. One calorie is equal to 4. 184 joules, a common unit of energy used in the physical sciences. In regards to food and fitness the reference to calories actually are kilocalories. Using the log sheets supplied, keep track of everything you eat in a 24 hour period. Beside each type of food list the number of Calories it contained by referring to the chart "Calorie content in common foods. "

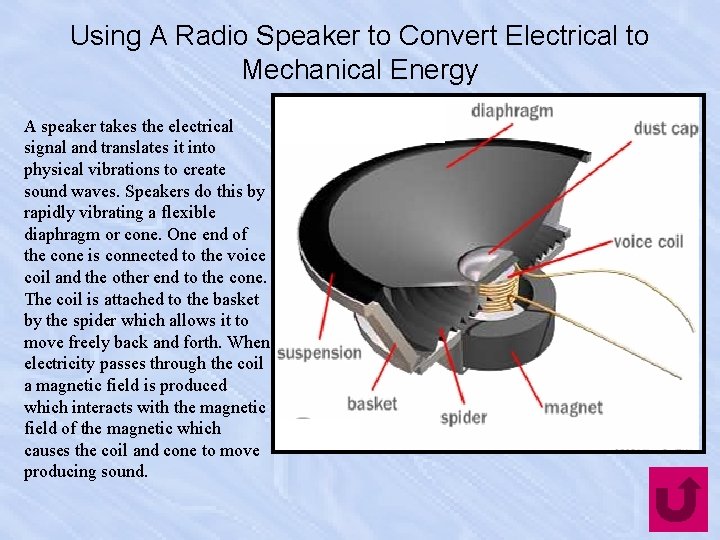
Using A Radio Speaker to Convert Electrical to Mechanical Energy A speaker takes the electrical signal and translates it into physical vibrations to create sound waves. Speakers do this by rapidly vibrating a flexible diaphragm or cone. One end of the cone is connected to the voice coil and the other end to the cone. The coil is attached to the basket by the spider which allows it to move freely back and forth. When electricity passes through the coil a magnetic field is produced which interacts with the magnetic field of the magnetic which causes the coil and cone to move producing sound.

Can Heat Make Things Move? Chewing gum wrapper and a source of heat such as an incandescent light bulb and socket

Mechanical To Heat With Elastic Bands Obtain a rubber band While holding the rubber band in both hands stretch it across your lips. You will notice that while the rubber band is being stretched it will feel warmer. When you “un-stretch” the rubber band it will feel cooler to your lips. The heat is caused by the rubber molecules rubbing against one another. 2

Superball Heat Energy Drill a hole into a super ball large enough to fit a thermometer. Insert thermometer, record the temperature and remove thermometer. Bounce the ball for about 5 -minutes. Insert thermometer, record the temperature and remove thermometer. 1

Hammer, Nail and Block of Wood When hammering a nail into wood, the hammer is raised ready to fall and hit the nail. The mass of the hammer is acted upon by gravity and the hammer contains potential energy. When the hammer is moving towards the nail the potential energy changes to kinetic energy. More energy can be added to the hammer by muscle power, the hammer accelerates and its velocity increases. The nail has zero velocity. On impact with the nail, the velocity of the hammer retards to zero and the energy contained within the hammer transfers to the nail. 1. Hold a nail to sense its temperature. 2. Pound the nail 4 to 5 -cm into the wood. Identify the work needed to do this. 3. Using the claw part of the hammer pull out the nail. Immediately touch the part of the nail that was in the wood. Describe the sensation. 4. Identify the energy transformations that took place. 0

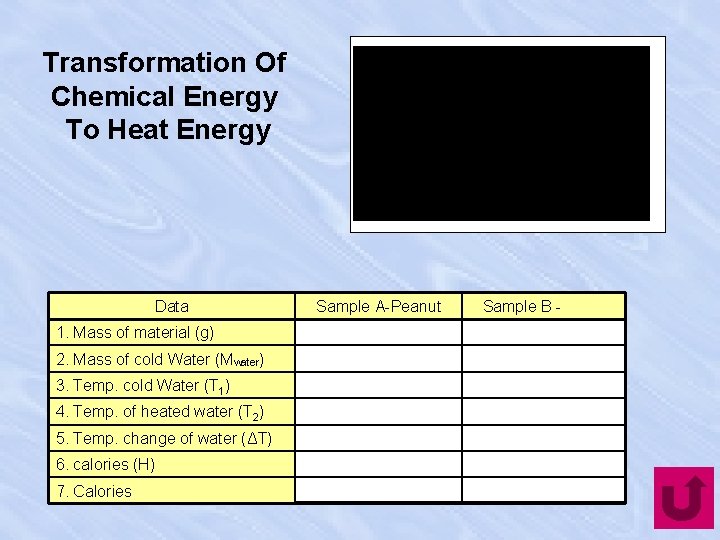
Transformation Of Chemical Energy To Heat Energy Data 1. Mass of material (g) 2. Mass of cold Water (Mwater) 3. Temp. cold Water (T 1) 4. Temp. of heated water (T 2) 5. Temp. change of water (ΔT) 6. calories (H) 7. Calories Sample A-Peanut Sample B -

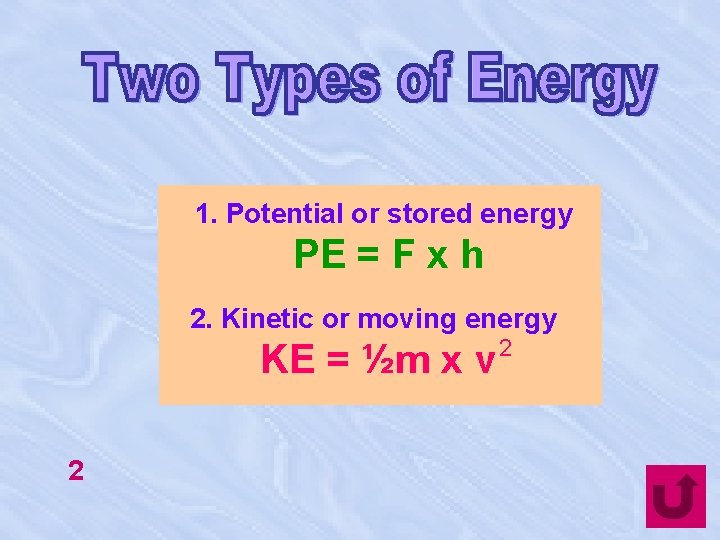
1. Potential or stored energy PE = F x h 2. Kinetic or moving energy KE = ½m x v 2 2

Kinetic Potential Pull Back Car 1

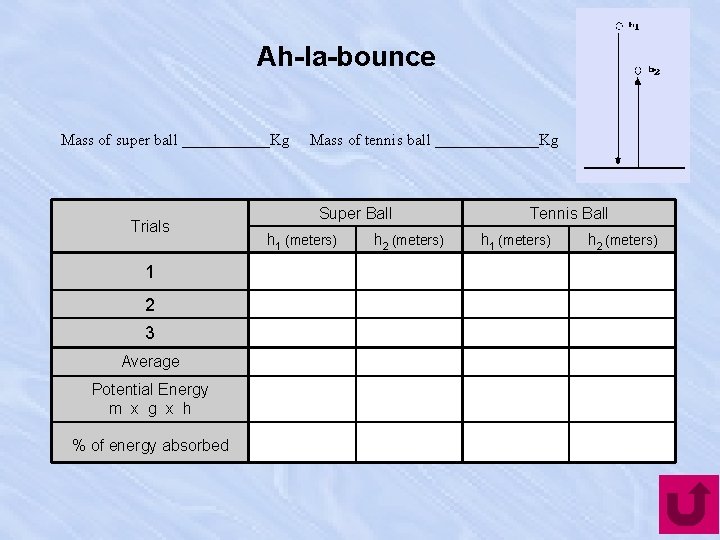
Ah-la-bounce Mass of super ball ______Kg Trials 1 2 3 Average Potential Energy m x g x h % of energy absorbed Mass of tennis ball _______Kg Super Ball h 1 (meters) h 2 (meters) Tennis Ball h 1 (meters) h 2 (meters)

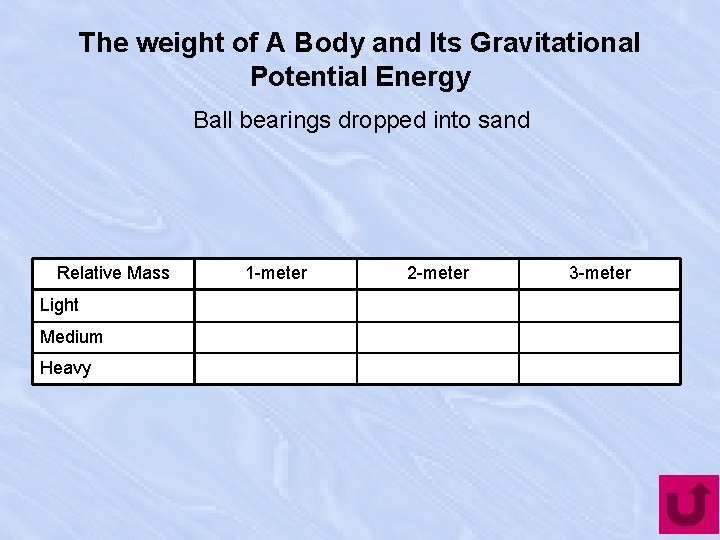
The weight of A Body and Its Gravitational Potential Energy Ball bearings dropped into sand Relative Mass Light Medium Heavy 1 -meter 2 -meter 3 -meter

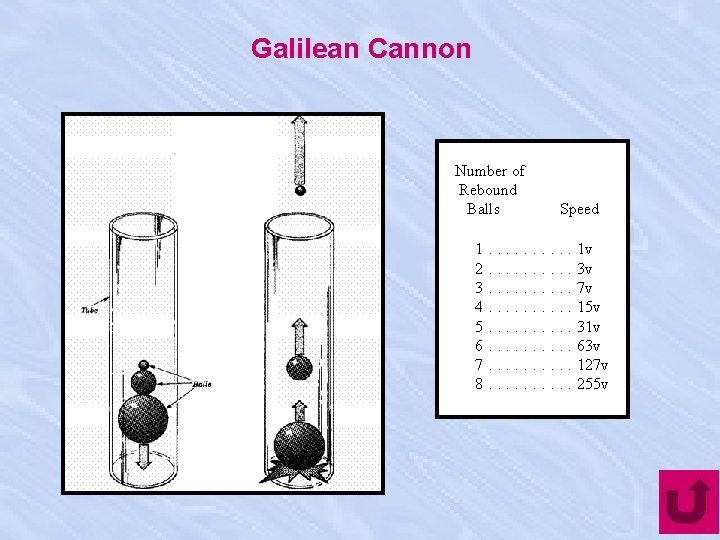
Galilean Cannon Number of Rebound Balls Speed 1. . 1 v 2. . 3 v 3. . 7 v 4. . 15 v 5. . 31 v 6. . 63 v 7. . 127 v 8. . 255 v

INVESTIGATING POTENTIAL AND KINETIC ENERGY Equipment Set-up . 380 -m Finish – t 2 Start - t 1 1

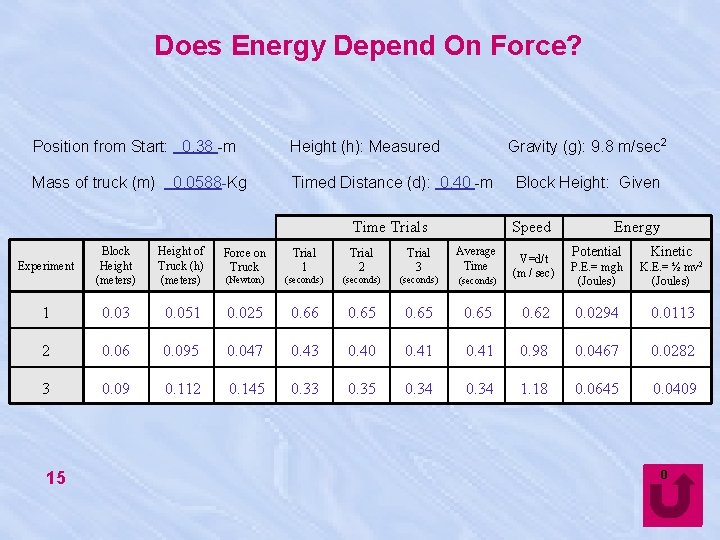
Does Energy Depend On Force? Gravity (g): 9. 8 m/sec 2 Position from Start: 0. 38 -m Height (h): Measured Mass of truck (m) Timed Distance (d): 0. 40 -m 0. 0588 -Kg Time Trials Speed Potential Kinetic K. E. = ½ mv 2 (Joules) 0. 62 0. 0294 0. 0113 0. 41 0. 98 0. 0467 0. 0282 0. 34 1. 18 0. 0645 0. 0409 Height of Truck (h) (meters) Force on Truck Trial 1 Trial 2 Trial 3 Average Time (Newton) (seconds) 1 0. 03 0. 051 0. 025 0. 66 0. 65 2 0. 06 0. 095 0. 047 0. 43 0. 40 0. 41 3 0. 09 0. 112 0. 145 0. 33 0. 35 0. 34 15 Energy P. E. = mgh (Joules) Block Height (meters) Experiment Block Height: Given V=d/t (m / sec) 0

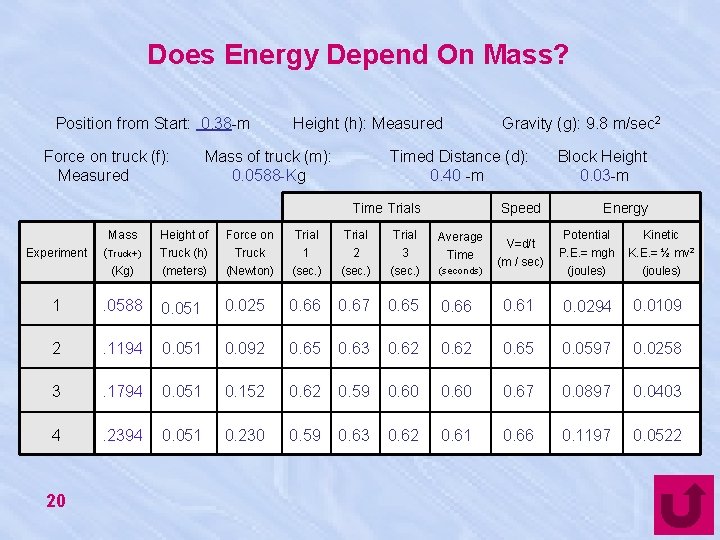
Does Energy Depend On Mass? Position from Start: 0. 38 -m Force on truck (f): Measured Height (h): Measured Mass of truck (m): 0. 0588 -Kg Gravity (g): 9. 8 m/sec 2 Timed Distance (d): 0. 40 -m Time Trials Speed Mass Height of Force on Trial Average (Truck+) (Kg) Truck (h) (meters) Truck (Newton) 1 (sec. ) 2 (sec. ) 3 (sec. ) Time (seconds) 1 . 0588 0. 051 0. 025 0. 66 0. 67 0. 65 2 . 1194 0. 051 0. 092 0. 65 0. 63 3 . 1794 0. 051 0. 152 0. 62 4 . 2394 0. 051 0. 230 0. 59 Experiment 20 V=d/t Block Height 0. 03 -m Energy Potential Kinetic K. E. = ½ mv 2 (m / sec) P. E. = mgh (joules) 0. 66 0. 61 0. 0294 0. 0109 0. 62 0. 65 0. 0597 0. 0258 0. 59 0. 60 0. 67 0. 0897 0. 0403 0. 62 0. 61 0. 66 0. 1197 0. 0522 (joules)

IS ENERGY CONSERVED WHEN CONVERTED TO WORK? Equipment Set-up Finish – t 2 Start - t 1 Work = force (newtons) x distance (meters) 1

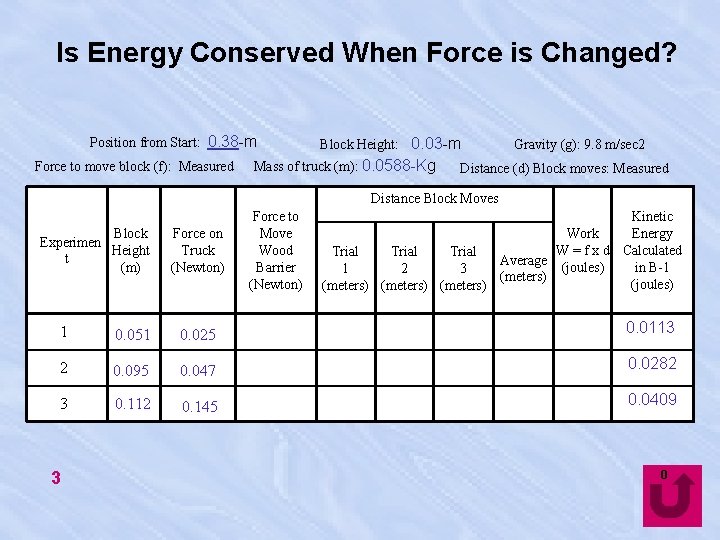
Is Energy Conserved When Force is Changed? Position from Start: 0. 38 -m Force to move block (f): Measured Block Height: 0. 03 -m Mass of truck (m): 0. 0588 -Kg Gravity (g): 9. 8 m/sec 2 Distance (d) Block moves: Measured Distance Block Moves Block Experimen Height t (m) Force on Truck (Newton) Force to Move Wood Barrier (Newton) Kinetic Work Energy W = f x d Calculated Trial Average (joules) in B-1 1 2 3 (meters) (joules) (meters) 1 0. 051 0. 025 0. 0113 2 0. 095 0. 047 0. 0282 3 0. 112 0. 145 0. 0409 3 0

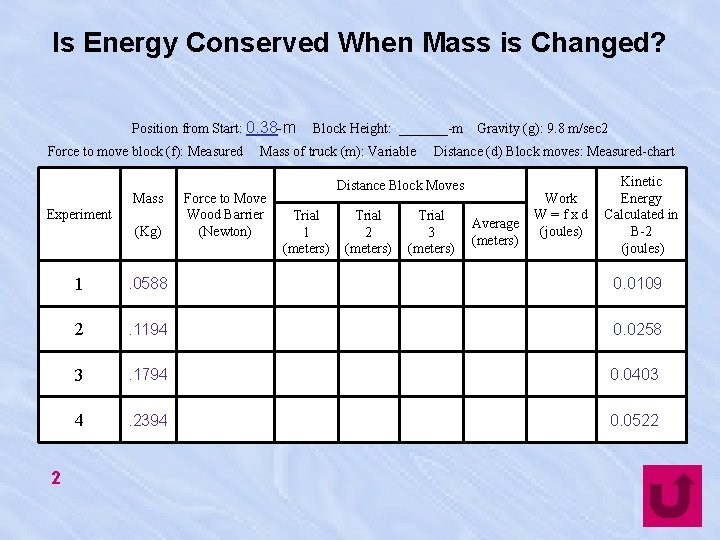
Is Energy Conserved When Mass is Changed? Position from Start: 0. 38 -m Force to move block (f): Measured Mass Experiment (Kg) 2 Block Height: _______-m Mass of truck (m): Variable Force to Move Wood Barrier (Newton) Gravity (g): 9. 8 m/sec 2 Distance (d) Block moves: Measured-chart Distance Block Moves Trial 1 (meters) Trial 2 (meters) Trial 3 (meters) Average (meters) Work W=fxd (joules) Kinetic Energy Calculated in B-2 (joules) 1 . 0588 0. 0109 2 . 1194 0. 0258 3 . 1794 0. 0403 4 . 2394 0. 0522

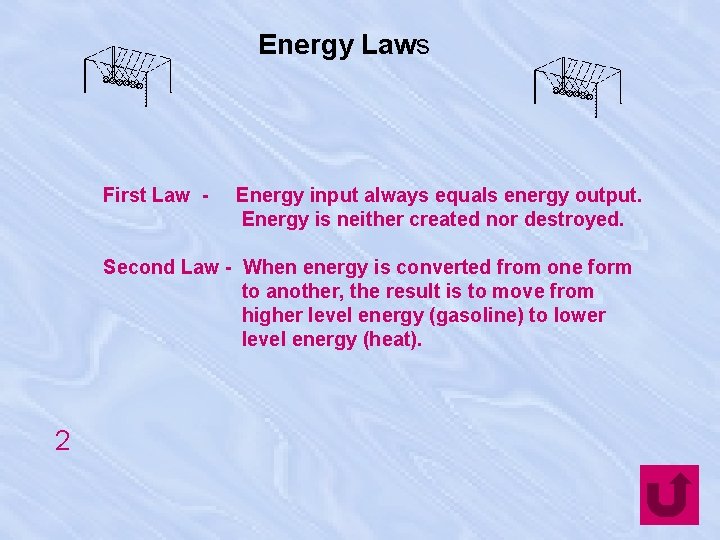
Energy Laws First Law - Energy input always equals energy output. Energy is neither created nor destroyed. Second Law - When energy is converted from one form to another, the result is to move from higher level energy (gasoline) to lower level energy (heat). 2

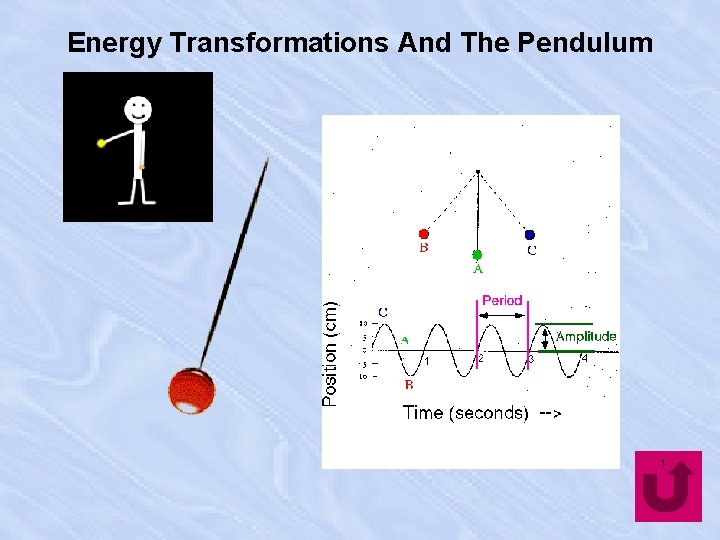
Energy Transformations And The Pendulum 1

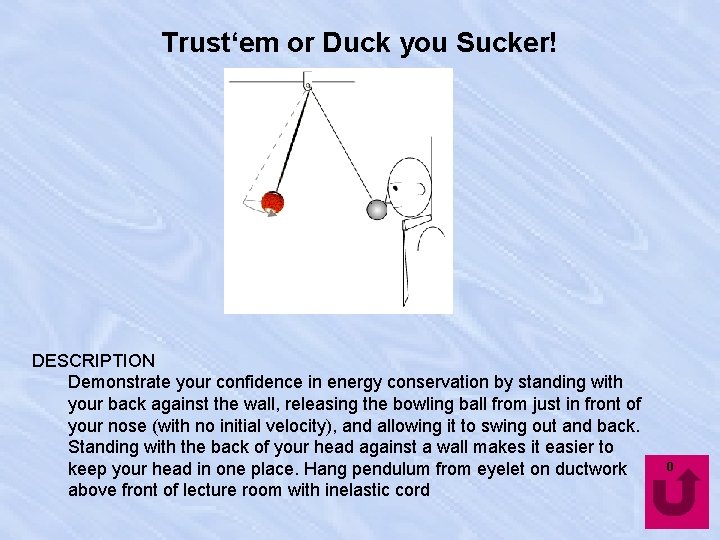
Trust‘em or Duck you Sucker! DESCRIPTION Demonstrate your confidence in energy conservation by standing with your back against the wall, releasing the bowling ball from just in front of your nose (with no initial velocity), and allowing it to swing out and back. Standing with the back of your head against a wall makes it easier to keep your head in one place. Hang pendulum from eyelet on ductwork above front of lecture room with inelastic cord 0

Stop And Go Balls Tie about 1 -meter of string between two supports. Suspend two balls from the string using pieces of string approximately 0. 5 -meter long. Start one of the ball swinging. Note that the other ball begins to respond to this motion, and its amplitude of swing increases. The ball that was initially moving loses some of its energy and begins to slow down. This continues until the ball that was originally at rest is swinging with full amplitude, and the other ball comes to a stop. This cycle continues with the balls transferring energy from one ball to the other.

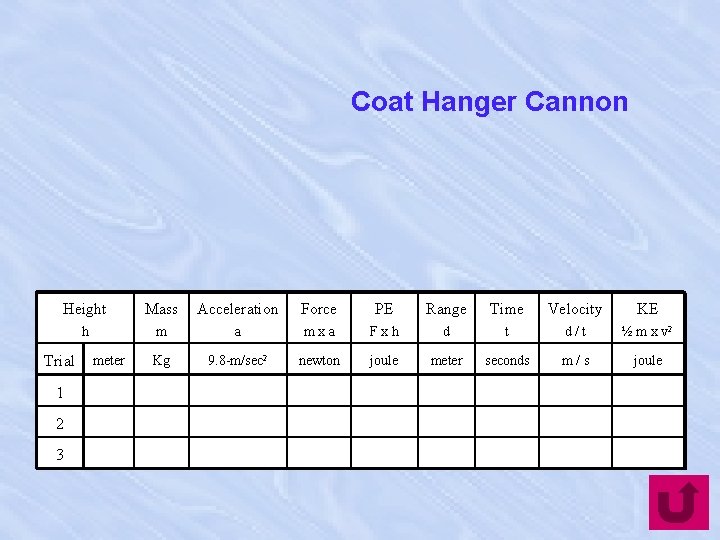
Coat Hanger Cannon Height Mass Acceleration Force PE Range Time Velocity KE h m a mxa Fxh d t d/t ½ m x v 2 Kg 9. 8 -m/sec 2 newton joule meter seconds m/s joule Trial 1 2 3 meter

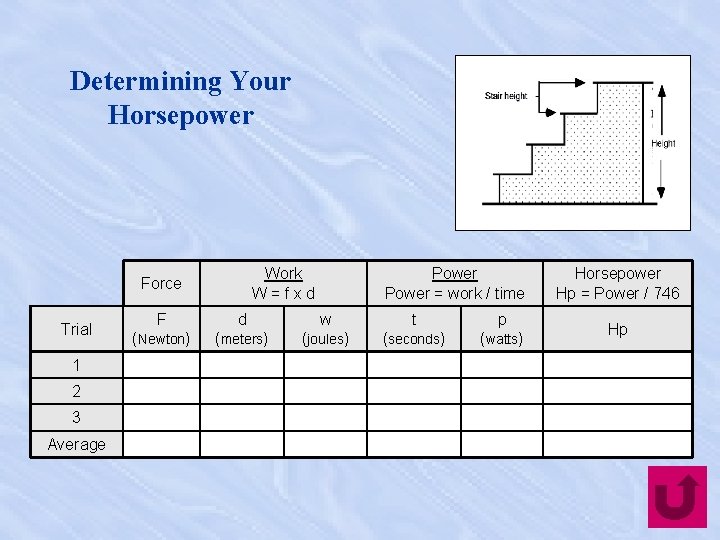
Determining Your Horsepower Work W=fxd Force Trial 1 2 3 Average Power = work / time F d w t p (Newton) (meters) (joules) (seconds) (watts) Horsepower Hp = Power / 746 Hp

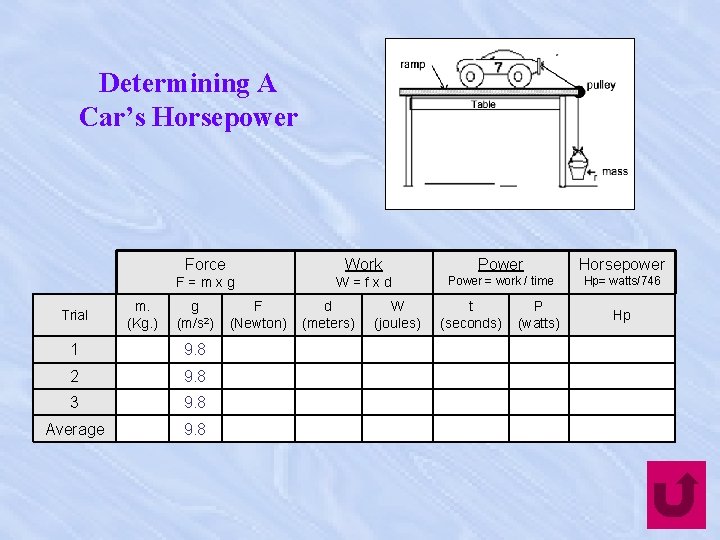
Determining A Car’s Horsepower Trial m. (Kg. ) Force Work Power Horsepower F=mxg W=fxd Power = work / time Hp= watts/746 g (m/s 2) 1 9. 8 2 9. 8 3 9. 8 Average 9. 8 F (Newton) d (meters) W (joules) t (seconds) P (watts) Hp

Force (newton) ÷ Distance (meters) 1

Work (joules) ÷ Time (seconds) 0

4

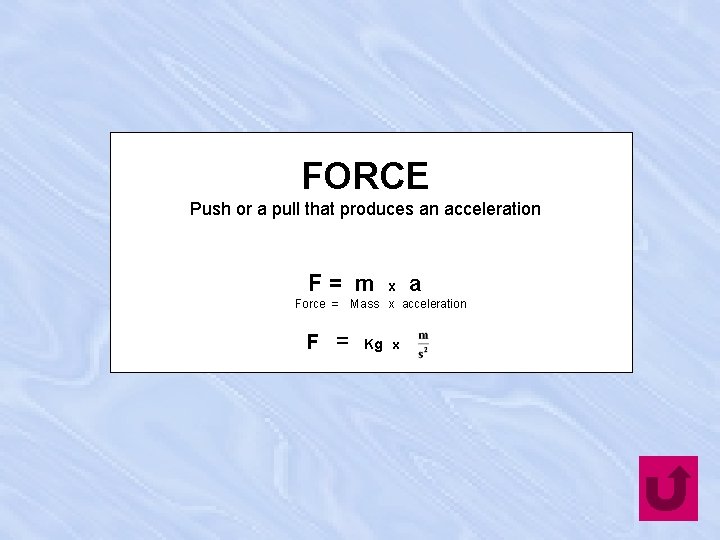
FORCE Push or a pull that produces an acceleration F= m x a Force = Mass x acceleration F = Kg x


We Had A Great Time