Georgia Immunization Requirements for School and Childcare Attendance





























- Slides: 51

Georgia Immunization Requirements for School and Childcare Attendance Presentation to: Presented by: Date:

Disclosure Statements • Neither the planners of this session nor I have any financial relationship with pharmaceutical companies, biomedical device manufacturers, or corporations whose products and services are related to the vaccines we discuss. • There is no commercial support being received for this event. • The mention of specific brands of vaccines in this presentation is for the purpose of providing education and does not constitute endorsement. • The GA Immunization Program utilizes ACIP recommendations as the basis for this presentation and for our guidelines, policies, and recommendations. • For certain vaccines this may represent a slight departure from or offlabel use of the vaccine package insert guidelines.

Disclosure Statement • To obtain nursing contact hours for this session, you must be present for the entire hour and complete an evaluation. • Continuing education will be provided through the Georgia Department of Public Health • Georgia Department of Public Health is an approved provider of continuing nursing education by the Alabama State Nurses Association, an accredited approver by the American Nurses Credentialing Center’s Commission of Accreditation

Objectives At the end of this presentation, participants will be able to: • Recall the role vaccines have played in preventing diseases • Discuss GA Immunization law and DPH rules and regulations for schools and child care attendance • Review process for maintaining immunization certificates • Discuss reliable immunization resources

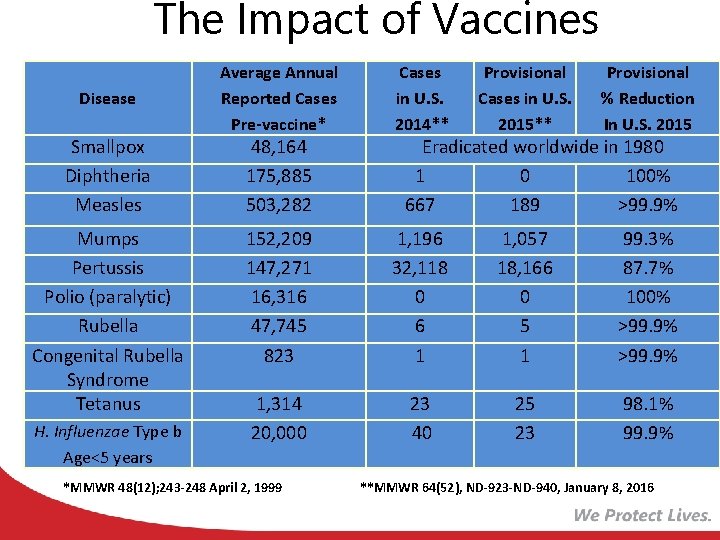
The Impact of Vaccines Disease Smallpox Diphtheria Measles Mumps Pertussis Polio (paralytic) Rubella Congenital Rubella Syndrome Tetanus H. Influenzae Type b Age<5 years Average Annual Reported Cases Pre-vaccine* Cases in U. S. 2014** Provisional Cases in U. S. 2015** Provisional % Reduction In U. S. 2015 152, 209 147, 271 16, 316 47, 745 823 1, 196 32, 118 0 6 1 1, 057 18, 166 0 5 1 99. 3% 87. 7% 100% >99. 9% 1, 314 20, 000 23 40 25 23 98. 1% 99. 9% 48, 164 175, 885 503, 282 *MMWR 48(12); 243 -248 April 2, 1999 Eradicated worldwide in 1980 1 0 100% 667 189 >99. 9% **MMWR 64(52), ND-923 -ND-940, January 8, 2016

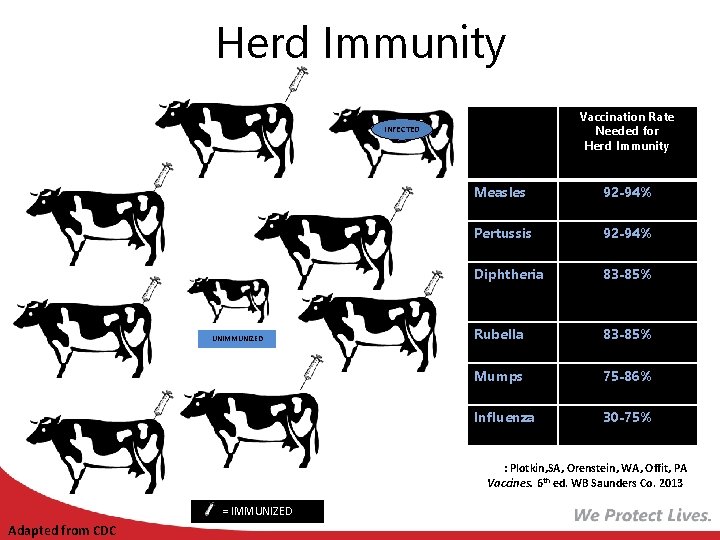
Herd Immunity Immunized individuals block infection from reaching those who are unimmunized Vaccination Rate Needed for Herd Immunity INFECTED UNIMMUNIZED Measles 92 -94% Pertussis 92 -94% Diphtheria 83 -85% Rubella 83 -85% Mumps 75 -86% Influenza 30 -75% Ref: Plotkin, SA, Orenstein, WA, Offit, PA Vaccines. 6 th ed. WB Saunders Co. 2013 = IMMUNIZED Adapted from CDC

Advisory Committee on Immunization Practices (ACIP) • 15 voting members with expertise in one or more of the following: Ø Vaccinology Ø Immunology Ø Infectious diseases Ø Pediatrics Ø Internal Medicine Ø Preventive medicine Ø Public health Ø Consumer perspectives and/or social and community aspects of immunization programs • ACIP develops recommendations and schedules for the use of licensed vaccines

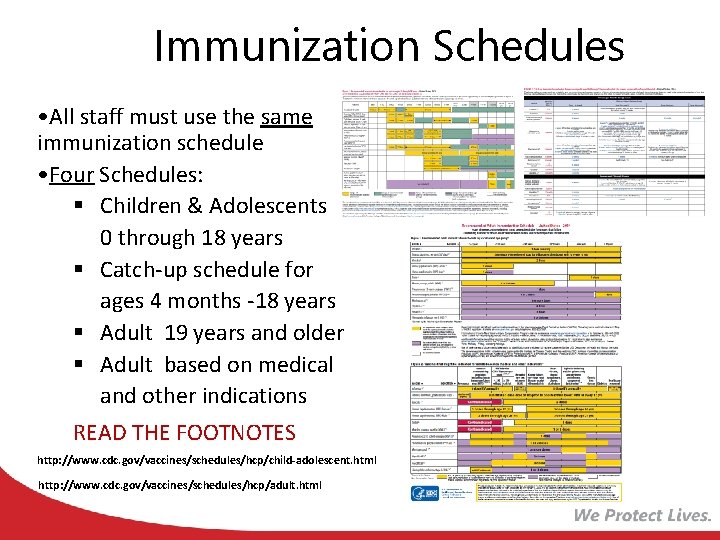
Immunization Schedules • All staff must use the same immunization schedule • Four Schedules: § Children & Adolescents 0 through 18 years § Catch-up schedule for ages 4 months -18 years § Adult 19 years and older § Adult based on medical and other indications READ THE FOOTNOTES http: //www. cdc. gov/vaccines/schedules/hcp/child-adolescent. html http: //www. cdc. gov/vaccines/schedules/hcp/adult. html

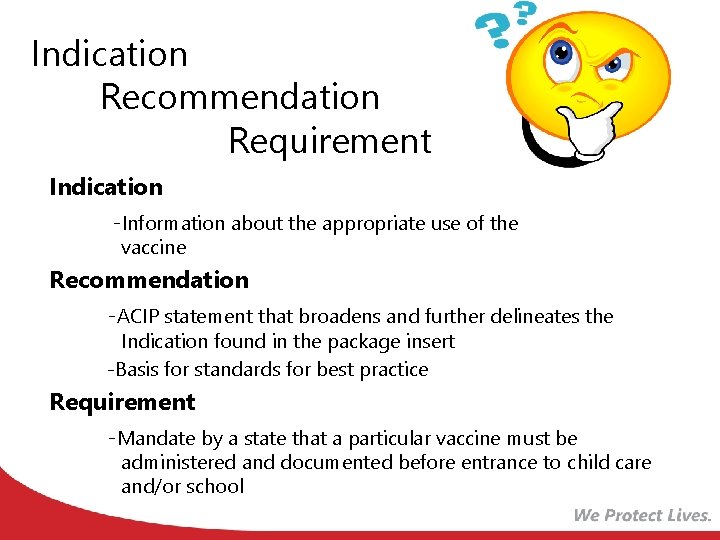
Indication Recommendation Requirement Indication • -Information about the appropriate use of the vaccine Recommendation • -ACIP statement that broadens and further delineates the • Indication found in the package insert -Basis for standards for best practice Requirement • -Mandate by a state that a particular vaccine must be administered and documented before entrance to child care and/or school

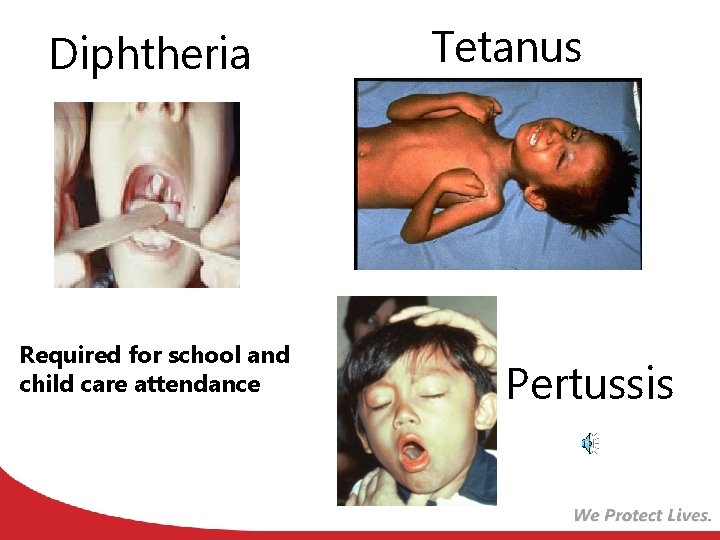
Diphtheria Required for school and child care attendance Tetanus Pertussis

Diphtheria, Tetanus and Pertussis Vaccines for Children, Adolescents, and Adults ACIP recommends: A 5 dose series of DTa. P: Administered at 2, 4, 6, 15 -18 months and 4 -6 years (Do not administer after age 6) one dose of Tdap: For children and adolescents starting at 11 or 12 years of age For all adults aged 19 years and older who have not had Tdap previously Boostrix is licensed for persons 10 years and older Adacel is licensed for persons 10 through 64 years For adults 65 years and older Boostrix should be used, when feasible; however, either vaccine product provides protection and is considered valid. There is no minimal interval between the last dose of Td and Tdap. MMWR, September 23, 2011, Vol 60, #37 MMWR, January 14, 2011, Vol 60, #01 MMWR, June 29, 2012 Vol 61, #25

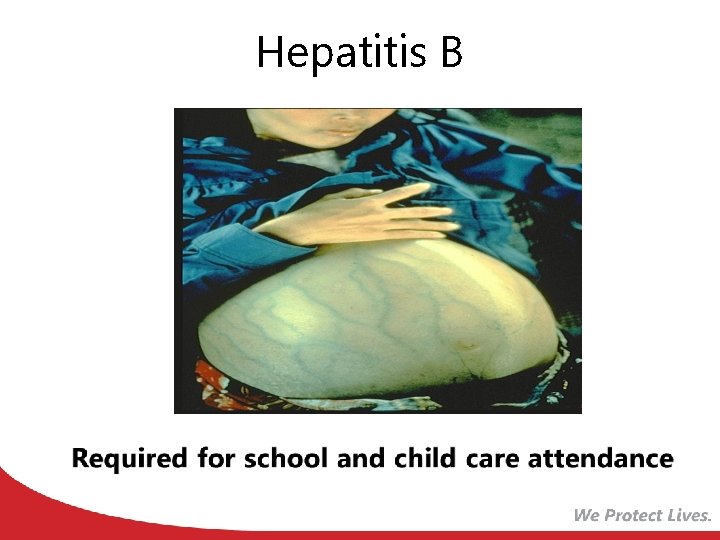
Hepatitis B

Hepatitis A Vaccine for Children ACIP recommends 2 doses of hepatitis A vaccine for: • All children 12 through 23 months of age (Separate the 2 doses by 6 to 18 months) • Any child or adolescent 2 through 18 years, who has not previously received the vaccine, can receive the vaccine at subsequent visits. Required for school and child care attendance MMWR, May 19, 2006, Vol 55, #RR-07

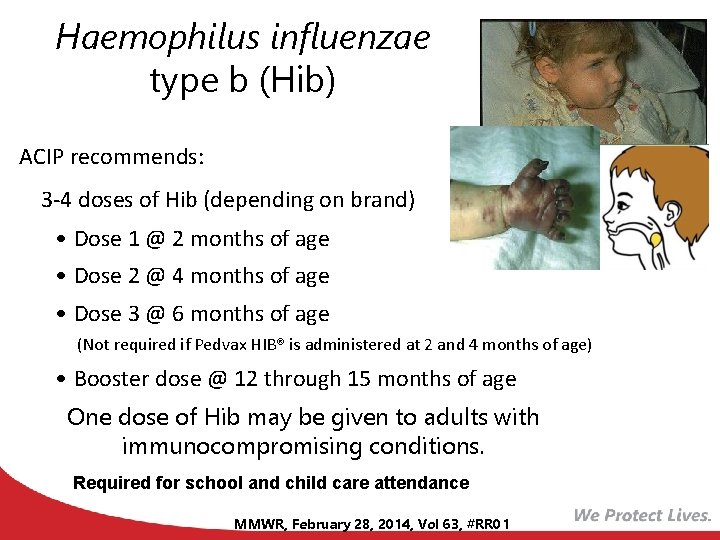
Haemophilus influenzae type b (Hib) ACIP recommends: 3 -4 doses of Hib (depending on brand) • Dose 1 @ 2 months of age • Dose 2 @ 4 months of age • Dose 3 @ 6 months of age (Not required if Pedvax HIB® is administered at 2 and 4 months of age) • Booster dose @ 12 through 15 months of age One dose of Hib may be given to adults with immunocompromising conditions. Required for school and child care attendance MMWR, February 28, 2014, Vol 63, #RR 01

Polio ACIP recommends: Four dose series of IPV at : 2, 4, 6 through 18 months and 4 through 6 years of age. • Minimum interval from dose 3 to dose 4 is six months • Final dose at 4 years of age or older regardless of the number of previous doses A booster dose may be recommended, depending on length of stay and the timing of the last dose of polio vaccine, for persons traveling to countries experiencing polio outbreaks. Required for school and child care attendance MMWR, August 7, 2009, Vol 58, #30 MMWR, July 11, 2014, Vol 63, # 27 Source: World Health Organization

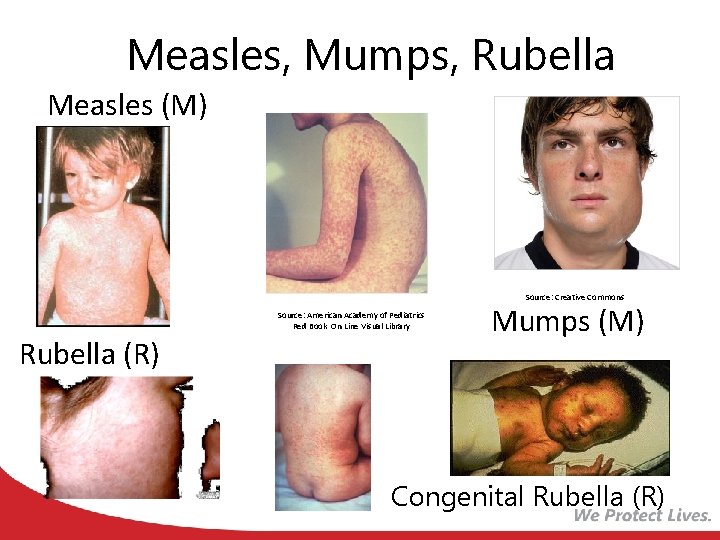
Measles, Mumps, Rubella Measles (M) Source: Creative Commons Source: American Academy of Pediatrics Red Book On Line Visual Library Rubella (R) Mumps (M) Congenital Rubella (R)

MMR Vaccine ACIP recommends 2 doses of MMR: – Dose 1 @ 12 through 15 months of age – Dose 2 @ 4 through 6 years of age – Second dose can be administered at an earlier age provided the interval between the first and second dose is at least 28 days Required for school and child care attendance Acceptable presumptive evidence of MMR immunity • Documentation of age appropriate vaccination with MMR vaccine • Laboratory evidence of immunity • Laboratory confirmation of disease • Birth before 1957 Birth date not acceptable evidence of rubella immunity for women who could become pregnant MMWR, June 14, 2013, Vol 62, #RR-04

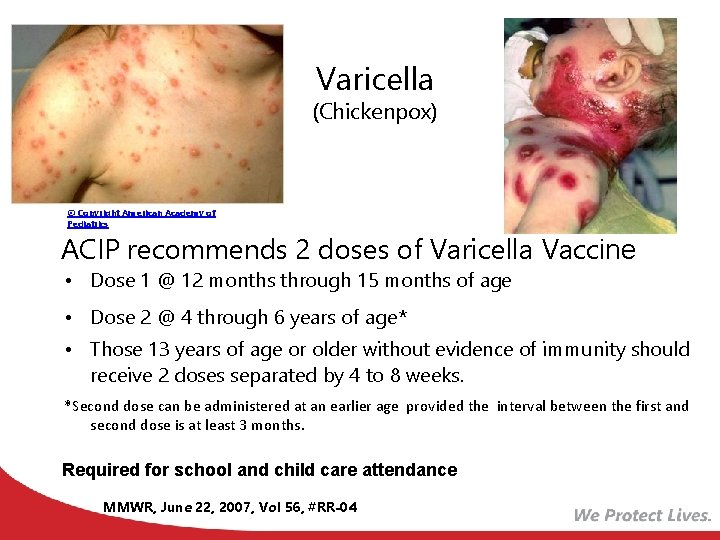
Varicella (Chickenpox) © Copyright American Academy of Pediatrics ACIP recommends 2 doses of Varicella Vaccine • Dose 1 @ 12 months through 15 months of age • Dose 2 @ 4 through 6 years of age* • Those 13 years of age or older without evidence of immunity should receive 2 doses separated by 4 to 8 weeks. *Second dose can be administered at an earlier age provided the interval between the first and second dose is at least 3 months. Required for school and child care attendance MMWR, June 22, 2007, Vol 56, #RR-04

Pneumococcal Conjugate Vaccine for Children (PCV 13) ACIP recommends PCV 13 for: – All children 2 through 59 months of age – Children 60 through 71 months who have underlying medical conditions that increase their risk of pneumococcal disease or complications – Children and adolescents aged 6 through 18 years with: • • • immunocompromising conditions functional or anatomic asplenia CSF leaks or cochlear implants, solid organ transplants or chronic renal failure those who have not previously received PCV 13 Required for school and child care attendance MMWR, December 10, 2010, Vo 1 59, #RR-11 MMWR, June 28, 2013, Vol 62, #25

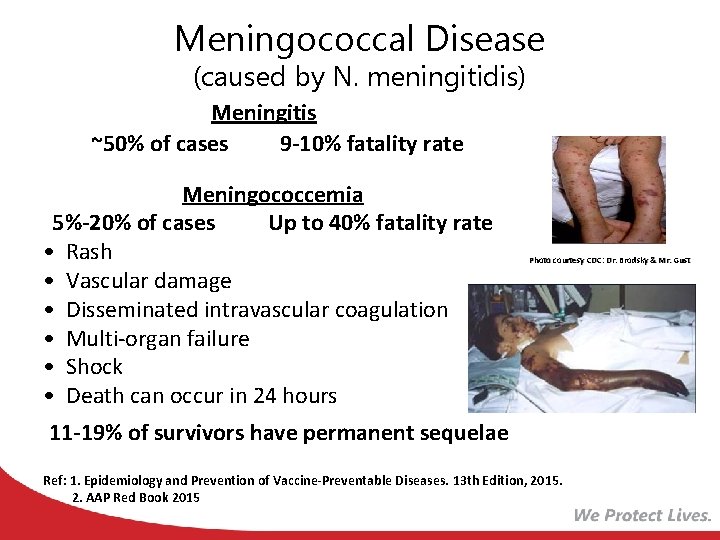
Meningococcal Disease (caused by N. meningitidis) Meningitis ~50% of cases 9 -10% fatality rate Meningococcemia 5%-20% of cases Up to 40% fatality rate • Rash • Vascular damage • Disseminated intravascular coagulation • Multi-organ failure • Shock • Death can occur in 24 hours Photo courtesy CDC: Dr. Brodsky & Mr. Gust 11 -19% of survivors have permanent sequelae Ref: 1. Epidemiology and Prevention of Vaccine-Preventable Diseases. 13 th Edition, 2015. 2. AAP Red Book 2015

Meningococcal Conjugate Vaccine (MCV 4) (Men A, C, Y, W-135) Menactra licensed for 9 mos. through 55 years Menveo® licensed for ages 2 mos. through 55 years ACIP recommends: • First dose at age 11 or 12 years and a booster dose at 16 years. • If first dose is at 13 -15 years, give booster dose 5 years after the first dose or sooner if entering college or technical school. • If first dose is received ≥ 16 years of age, a 2 nd dose is not needed. • Persons aged 21 years or younger attending school or college should have documentation of one dose of MVC 4 not more than 5 years before enrollment. For persons 56 years and older who need meningococcal vaccine, use MPSV 4. If MCV 4 has been given previously and a booster is needed MCV 4 is preferred. Required for school attendance MMWR, March 22, 2013, Vol 62, #RR 02

Requirements for School and Childcare Attendance

Vaccines Required for School and Childcare Attendance: • Consistent with the Recommended Childhood and Adolescent Immunization Schedule • Require children to be age appropriately immunized against each of the specified vaccine preventable diseases

Goal • Vaccines work Goal 100 % compliance rate • Immunization Laws work • Partnerships work

DPH Rules and Regulations • Provide definition of terms • Stipulate the specific requirements – List required vaccines or – Acceptable proof of immunity – Define medical and religious exemptions • Provide directions for issuing, maintaining, and inspecting certificates GA Rules and Regulations updated for the 2014 -2015 school year to reflect the new school requirements. (511 -2 -2)

Job Aids for Assessing Certificates of Immunization

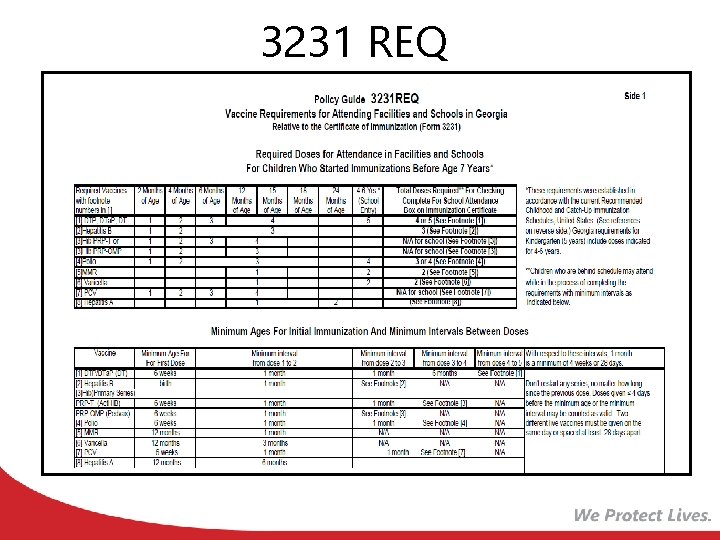
3231 REQ

3231 INS

Form 3231

Certificate of Immunization (Form 3231) • A child must have a certificate on file at each facility or school he attends • Photocopies of appropriately completed and signed certificates are acceptable • If a certificate is not on file for each child attending, the facility is held legally responsible • A licensed Georgia physician, APRN, PA or public health official is responsible for the interpretation of and compliance with the requirements for vaccines and completing the certificate • Only physician offices and health clinics can obtain blank certificates

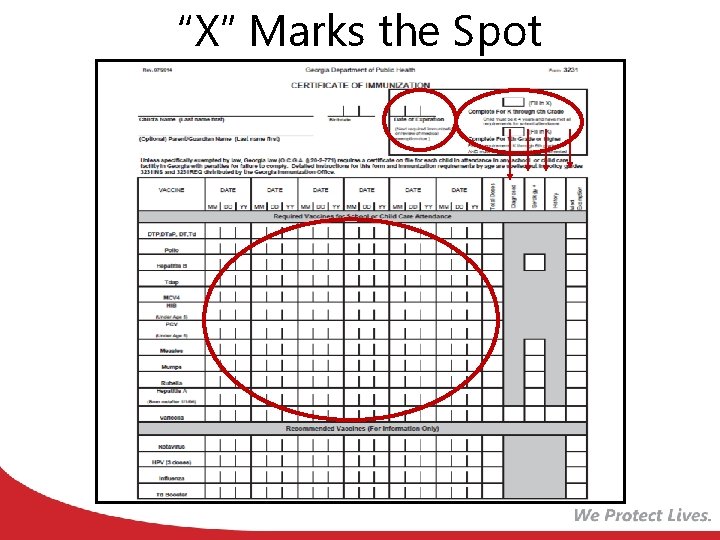
Valid Certificates • All certificates must be marked with : – Child’s name – Birth date – Name and Address of Physician, APRN, PA, Qualified Board of Health official or State Immunization Office Official – Certified Signature – Date of Issue

“X” Marks the Spot

Expiration Date • Expires on the date entered as “Expiration Date” • Must be replaced with a current certificate within 30 days after the expiration date, or child is excluded from attending • Allows for a child who does not meet all the immunization requirements to attend child care or school while he is catching up • Required for all children four years and older who have not completed vaccine requirement for 7 th grade • Required if a medical exemption for a vaccine(s) is marked • Should not be completed if “Complete for 7 th Grade or higher” is marked

“Complete for School Attendance” • Do not expire • Issued only to children who: – Are four years of age or older; and – Have met all the requirements for school attendance as outlined in the Policy Guide 3231 REQ; and – Have all the required vaccine administration dates or natural immunity dates filled in; and – Do not have a “Date of Expiration”

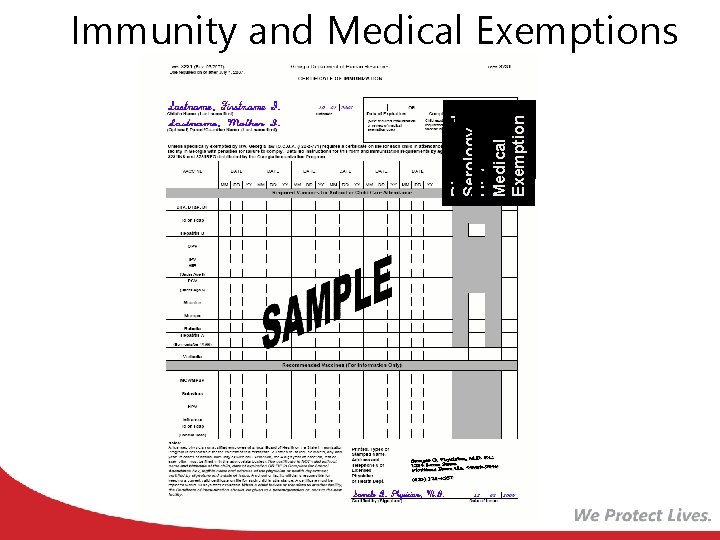
Diagnosed Serology History Medical Exemption Immunity and Medical Exemptions

Exemptions Medical: • Physical disability or condition that contraindicates immunization for that specific vaccine • Documented in the medical exemption box indicated for each vaccine • Reviewed annually

Exemptions Religious: • Documented on form 2208 • Form kept on file by the school or facility in lieu of a Certificate of Immunization (form 3231) • Do not expire

Child Care Requirements • Number of vaccine doses • Always need more doses • Must have a current “expiration date”

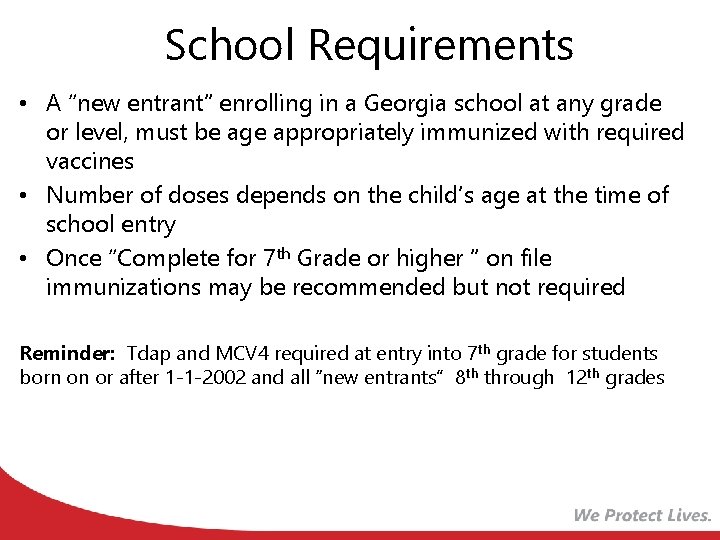
School Requirements • A “new entrant” enrolling in a Georgia school at any grade or level, must be age appropriately immunized with required vaccines • Number of doses depends on the child’s age at the time of school entry • Once “Complete for 7 th Grade or higher ” on file immunizations may be recommended but not required Reminder: Tdap and MCV 4 required at entry into 7 th grade for students born on or after 1 -1 -2002 and all “new entrants” 8 th through 12 th grades

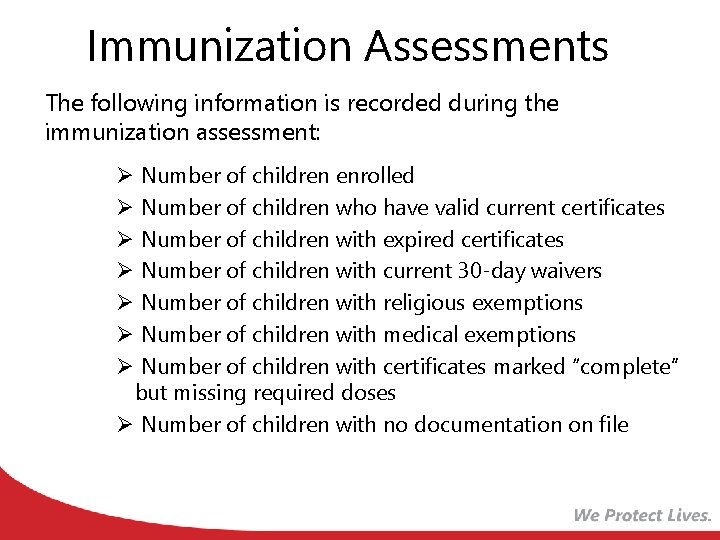
Immunization Assessments The following information is recorded during the immunization assessment: Ø Number of children enrolled Ø Number of children who have valid current certificates Ø Number of children with expired certificates Ø Number of children with current 30 -day waivers Ø Number of children with religious exemptions Ø Number of children with medical exemptions Ø Number of children with certificates marked “complete” but missing required doses Ø Number of children with no documentation on file


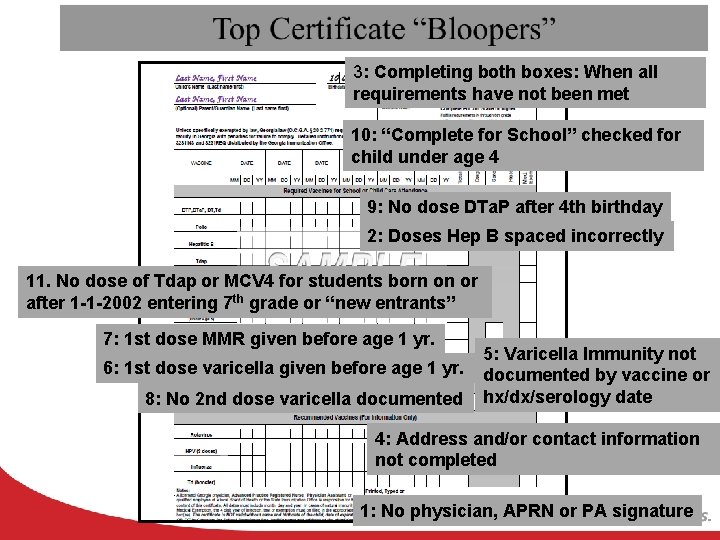
3: Completing both boxes: When all requirements have not been met 10: “Complete for School” checked for child under age 4 9: No dose DTa. P after 4 th birthday 2: Doses Hep B spaced incorrectly 11. No dose of Tdap or MCV 4 for students born on or after 1 -1 -2002 entering 7 th grade or “new entrants” 7: 1 st dose MMR given before age 1 yr. 6: 1 st dose varicella given before age 1 yr. 8: No 2 nd dose varicella documented 5: Varicella Immunity not documented by vaccine or hx/dx/serology date 4: Address and/or contact information not completed 1: No physician, APRN or PA signature

Maintenance of Certificates To be valid, certificates must be current – Certificate becomes invalid on the “Date of Expiration” – Child must submit a current certificate within 30 days after the expiration date or be excluded from attendance – Facility or school is responsible for notifying parent/guardian of an upcoming expiration date and requesting a current certificate be submitted – Any school/facility official who does not enforce the requirements shall be guilty of a misdemeanor

Filing of Certificates • All children enrolled must have a valid Certificate of Immunization on file • Certificates must be available for inspection by health officials • If child attends more than one facility, a photocopy to the second facility is acceptable • If child leaves or transfers to another school or facility, the certificate should be given to a parent/guardian or sent to the new school/facility • In the case of religious exemption, form 2208 must be on file after 8/2015

Tickler Filing System • “How to” instructions located in the Immunization Guidelines for Child Care Facility Operators & School Personnel (Form 3258) • Tickler system (set up by month and year) • Remind parents or care givers • Give parents information sheet about requirements • Document follow-up • Enforce requirements

Georgia Registry of Immunization Transactions and Services (GRITS)

Responsibilities • Physicians and Public Health Clinics: – Know current legal requirements for attendance and accurately completing the certificate – Administering immunizations according to the current Recommended Childhood and Adolescent Immunization Schedule – Report the occurrence of any disease listed on the “Notifiable Disease List” – Report any adverse event following the administration of a vaccine to VAERS • Child Care and School : – – Review the certificates for validity prior to accepting Develop a system for immunization certificate management/Tickler system Have certificates available for inspection and audit by health officials Report the occurrence of any disease listed on the “Notifiable Disease List” • Parent/Caregiver: - Stay on immunization schedule – Obtain immunization certificate – Provide copy of certificate to each facility

VAERS

Healthcare Personnel (HCP) and Other Adults Need These Immunizations: • Annual influenza vaccine • Tdap or Td • Hepatitis B (exposure risk) Check immunity Validate immune status of: • Varicella • Measles, Mumps & Rubella(MMR) Are YOU up to date?

Resources • Local health department • District Immunization Coordinator • GA Immunization Program Office – On call Help line: 404 -657 -3158 – GRITS Help Line: 1 -866 -483 -2958 – VFC Help Line: 1 -800 -848 -3868 – Website http: //dph. georgia. gov/immunization-section to access school and child care forms https: //dph. georgia. gov/schools-andchildcare – Your local Immunization Program Consultant (IPC) – Epidemiology: 1 -866 -782 -4584 • GA Chapter of the AAP • GA Academy of Family Physicians

It’s a Team Effort! Let’s make sure all children attending schools and child care facilities in Georgia are up-to-date on immunizations.
 Ohio immunization summary for school attendance
Ohio immunization summary for school attendance Grits ga registry of immunization
Grits ga registry of immunization Georgia requirements for high school graduation
Georgia requirements for high school graduation How good is our early learning and childcare examples
How good is our early learning and childcare examples Cache level 3 diploma in childcare and education
Cache level 3 diploma in childcare and education Mls lds
Mls lds Seminary attendance
Seminary attendance Little kingdom childcare
Little kingdom childcare Childcare jobs blackburn
Childcare jobs blackburn Whos entitled to 30 hours free childcare
Whos entitled to 30 hours free childcare Quality observation
Quality observation Pedagogical leadership skills
Pedagogical leadership skills Level 3 residential childcare
Level 3 residential childcare Sustainability checklist in childcare
Sustainability checklist in childcare Moe childcare leave
Moe childcare leave Homecare hertford
Homecare hertford Childcare
Childcare Online child care invoice
Online child care invoice Coral spring middle school
Coral spring middle school Coral springs middle school attendance line
Coral springs middle school attendance line Stagg attendance
Stagg attendance Wolftime eastlake
Wolftime eastlake Kyrene middle school attendance line
Kyrene middle school attendance line Sabbath school theme ideas
Sabbath school theme ideas Eaton high school attendance
Eaton high school attendance Global alliance for vaccines and immunization
Global alliance for vaccines and immunization Formuö
Formuö Novell typiska drag
Novell typiska drag Tack för att ni lyssnade bild
Tack för att ni lyssnade bild Ekologiskt fotavtryck
Ekologiskt fotavtryck Shingelfrisyren
Shingelfrisyren En lathund för arbete med kontinuitetshantering
En lathund för arbete med kontinuitetshantering Personalliggare bygg undantag
Personalliggare bygg undantag Tidbok för yrkesförare
Tidbok för yrkesförare Anatomi organ reproduksi
Anatomi organ reproduksi Vad är densitet
Vad är densitet Datorkunskap för nybörjare
Datorkunskap för nybörjare Boverket ka
Boverket ka Debatt artikel mall
Debatt artikel mall Magnetsjukhus
Magnetsjukhus Nyckelkompetenser för livslångt lärande
Nyckelkompetenser för livslångt lärande Påbyggnader för flakfordon
Påbyggnader för flakfordon Arkimedes princip formel
Arkimedes princip formel Publik sektor
Publik sektor Bo bergman jag fryser om dina händer
Bo bergman jag fryser om dina händer Presentera för publik crossboss
Presentera för publik crossboss Vad är ett minoritetsspråk
Vad är ett minoritetsspråk Bat mitza
Bat mitza Treserva lathund
Treserva lathund Epiteltyper
Epiteltyper Claes martinsson
Claes martinsson Centrum för kunskap och säkerhet
Centrum för kunskap och säkerhet










































































