FLUIDS ELECTROLYTES DR OMAR MANSOUR FRCSI UEMS MSC




























- Slides: 41

FLUIDS & ELECTROLYTES DR OMAR MANSOUR FRCSI UEMS MSC CONSULTANT COLORECTAL SURGEON BAU

• Total Body Water: BODY FLUIDS • Water constitutes approximately 50% to 60% of total body weight. • In an average young adult male, TBW accounts for 60% of total body weight, whereas in an average young adult female, it is 50%. • The highest percentage of TBW is found in newborns, with approximately 80%, which decreases to about 65% by the age of 1 year.

• FLUID COMPARTMENTS TBW is divided into three functional fluid compartments: • Extravascular ( 20%) One third • Plasma (5%) • Extravascular interstitial fluid (15%) • Intracellular fluid (40%) Two third.

FLUID COMPARTMENTS

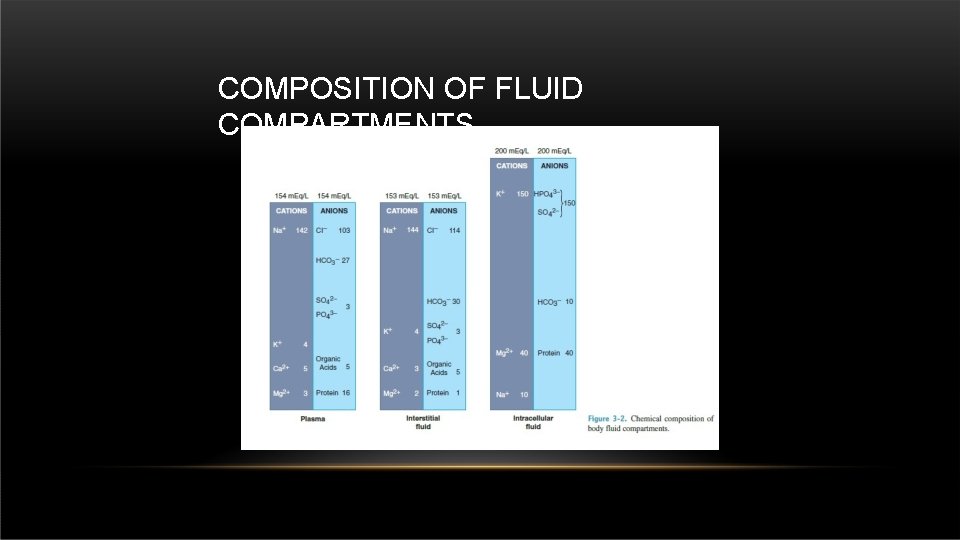
COMPOSITION OF FLUID COMPARTMENTS

• COMPOSITION OF FLUID COMPARTMENTS The concentration gradient between compartments is maintained by adenosine triphosphate– driven sodium-potassium pumps located within the cell membranes. • Gibbs-Donnan equilibrium equation : describes the unequal distribution of permeant charged ions on either side of a semipermeable membrane which occurs in the presence of impermeant charged ions.

• OSMOTIC PRESSURE The physiologic activity of electrolytes in solution depends on: • mmol/L : the number of particles per unit volume • m. Eq/L : the number of electric charges per unit volume • m. Osm/L : the number of osmotically active ions per unit volume • Calculated serum osmolality = 2 sodium + (glucose/18) + (BUN/2. 8) • The osmolality of the intracellular and extracellular fluids is maintained between 290 and 310 m. Osm in each compartment.

BODY FLUID CHANGES • The healthy person consumes an average of 2000 m. L of water per day, approximately 75% from oral intake and the rest extracted from solid foods.

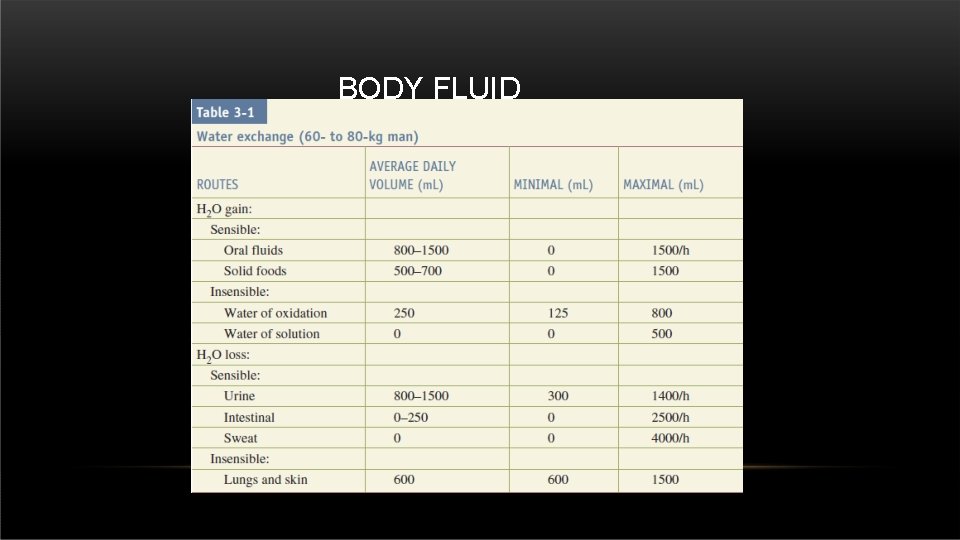
• Daily water losses include : BODY FLUID CHANGES • 800 to 1200 m. L in urine. • 250 m. L in stool. • 600 m. L in insensible losses. • Insensible losses of water occur through both the skin (75%) and lungs (25%) and can be increased by such factors as fever, hypermetabolism, and hyperventilation. • To clear the products of metabolism, the kidneys must excrete a minimum of 500 to 800 m. L of urine per day, regardless of the amount of oral intake.

BODY FLUID CHANGES

• BODY FLUID CHANGES The typical individual consumes 3 to 5 g of dietary salt per day, with the balance maintained by the kidneys (not sweat or GI). • With hyponatremia or hypovolemia, sodium excretion can be reduced to as little as 1 m. Eq/d or maximized to as much as 5000 m. Eq/d.

• CLASSIFICATION OF BODY FLUID CHANGES Disorders in fluid balance may be classified into three general categories: • disturbances in • (a) volume • (b) concentration • (c) composition.

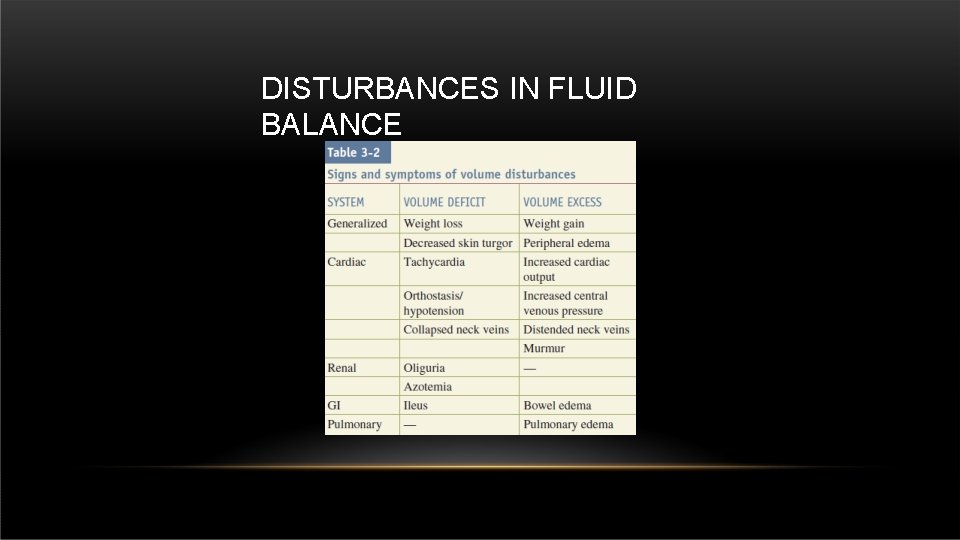
• DISTURBANCES IN FLUID BALANCE Extracellular volume deficit is the most common fluid disorder in surgical patients and can be either acute or chronic. • Laboratory, urine osmolality usually will be higher than serum osmolality, and urine sodium will be low, typically <20 m. Eq/L , but serum sodium concentration does not reflect volume status.

DISTURBANCES IN FLUID BALANCE

• DISTURBANCES IN FLUID BALANCE The most common cause of volume deficit in surgical patients is a loss of GI fluids from nasogastric suction, vomiting, diarrhea, or enterocutaneous fistula. • In addition, sequestration secondary to soft tissue injuries, burns, and intra-abdominal processes such as peritonitis, obstruction, or prolonged surgery can also lead to massive volume deficits.

DISTURBANCES IN FLUID BALANCE

• VOLUME CHANGES Volume changes are sensed by both osmoreceptors and baroreceptors. • Osmoreceptors are specialized sensors that detect even small changes in fluid osmolality and drive changes in thirst and diuresis through the kidneys. • For example, when plasma osmolality is increased, thirst is stimulated and water consumption increases. Additionally, the hypothalamus is stimulated to secrete vasopressin, which increases water reabsorption in the kidneys.

• VOLUME CHANGES Baroreceptors also modulate volume in response to changes in pressure and circulating volume through specialized pressure sensors located in the aortic arch and carotid sinuses. • Baroreceptor responses are both: • neural, through sympathetic and parasympathetic pathways • hormonal, through substances including renin-angiotensin, aldosterone, atrial natriuretic peptide, and renal prostaglandins

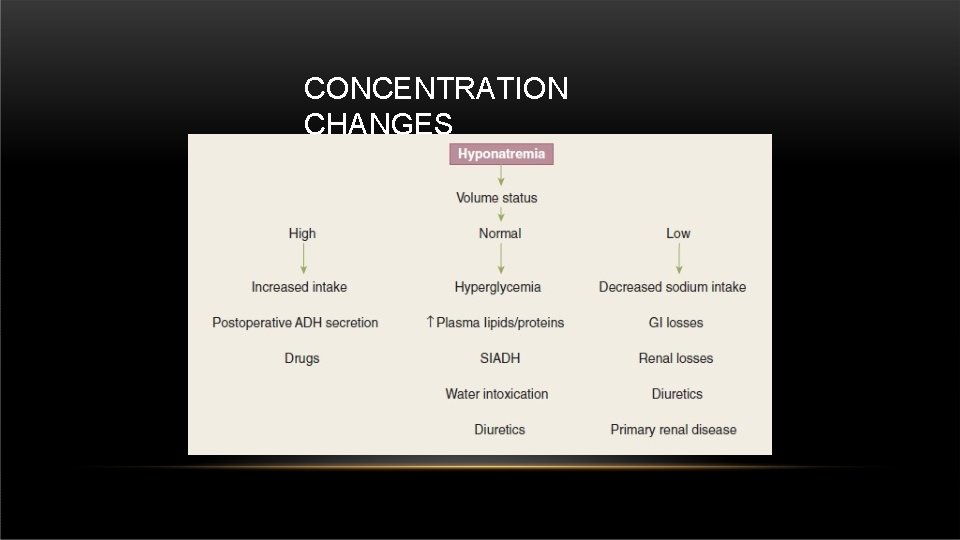
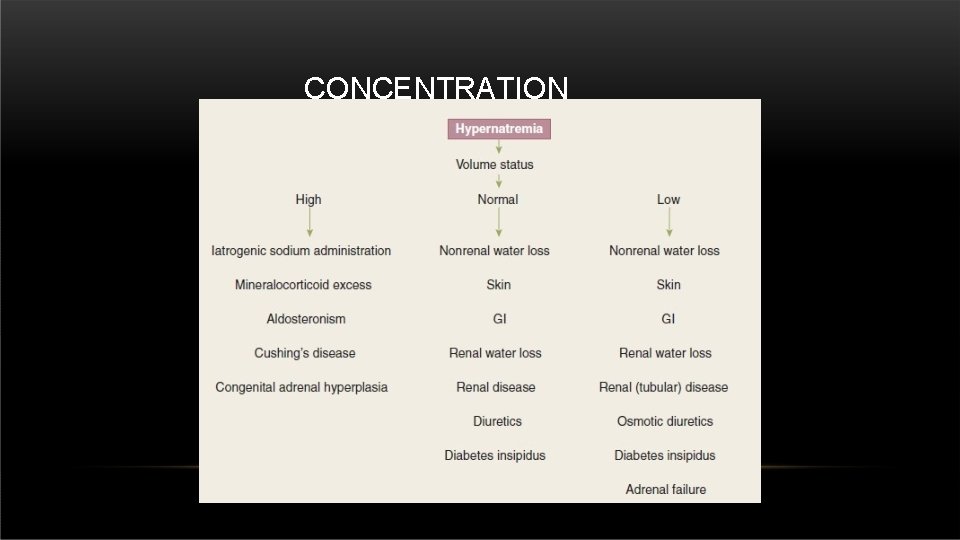
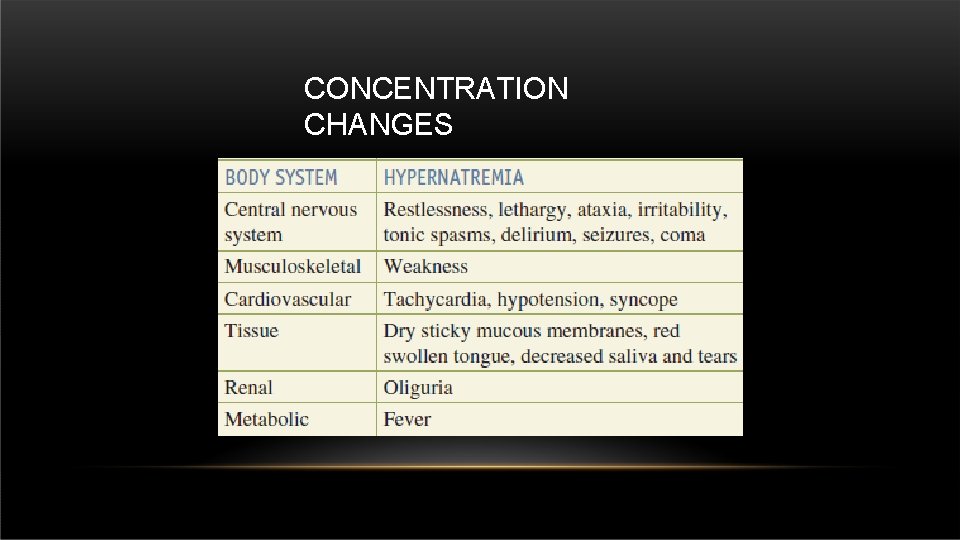
CONCENTRATION CHANGES • Sodium normal level in the body : 135 – 153 m. Eq/L.

CONCENTRATION CHANGES

• CONCENTRATION CHANGES Calculated hyponatremia with hyperglycemia: • For every 100 -mg/d. L increment in plasma glucose above normal, the plasma sodium should decrease by 1. 6 m. Eq/L

CONCENTRATION CHANGES

CONCENTRATION CHANGES

CONCENTRATION CHANGES

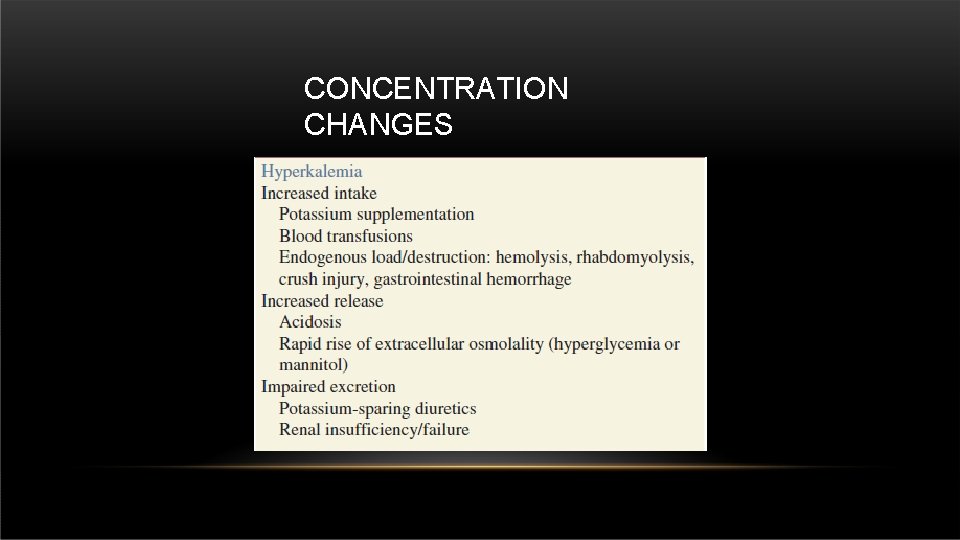
CONCENTRATION CHANGES • Potassium normal level in the body : 3. 5 – 5. 3 m. Eq/L.

CONCENTRATION CHANGES

CONCENTRATION CHANGES

CONCENTRATION CHANGES

CONCENTRATION CHANGES • Calcium normal level in the body : 8. 5 – 10. 5 mg/dl. • Magnesium normal level in the body : 1. 7 – 2. 2 mg/dl. • Phosphorus normal level in the body : 2. 5 – 4. 5 mg/dl.

• • Hypercalcemia causes: • Primary hyperparathyroidism • Malignancy CONCENTRATION CHANGES Hypocalcemia causes: • Pancreatitis • Massive soft tissue infections such as necrotizing fasciitis • Renal failure • Pancreatic and small bowel fistulas • Hypoparathyroidism • Toxic shock syndrome • Abnormalities in magnesium levels • Tumor lysis syndrome. • After removal of a parathyroid adenoma • Malignancies associated with increased osteoblastic activity • Calcium precipitation with organic • Massive blood transfusion with citrate binding is another mechanism.

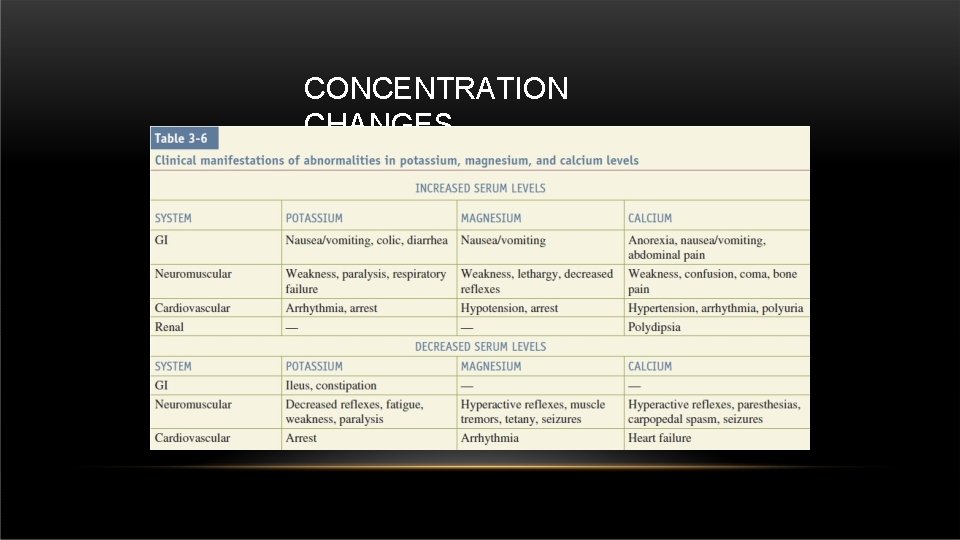
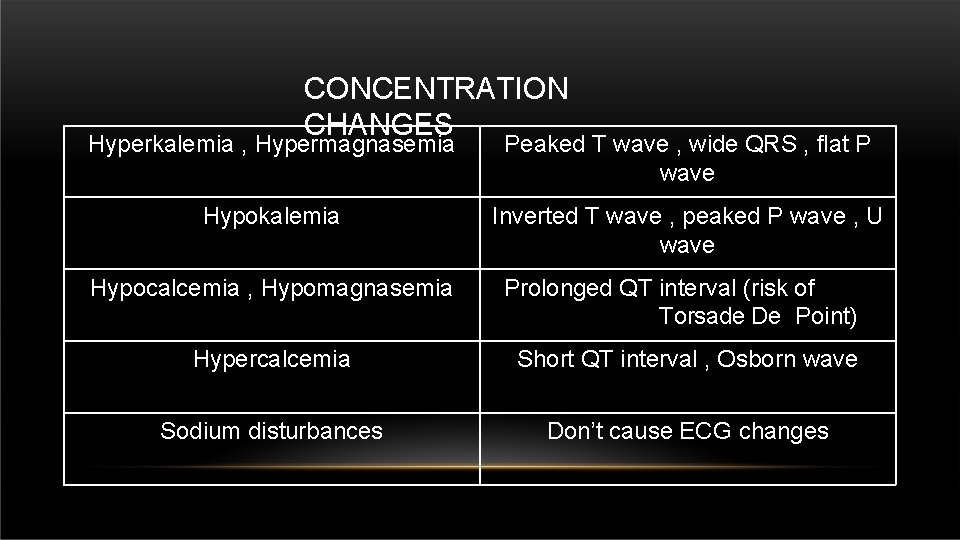
CONCENTRATION CHANGES Hyperkalemia , Hypermagnasemia Peaked T wave , wide QRS , flat P wave Hypokalemia Inverted T wave , peaked P wave , U wave Hypocalcemia , Hypomagnasemia Prolonged QT interval (risk of Torsade De Point) Hypercalcemia Short QT interval , Osborn wave Sodium disturbances Don’t cause ECG changes

CONCENTRATION CHANGES

ACID-BASE BALANCE

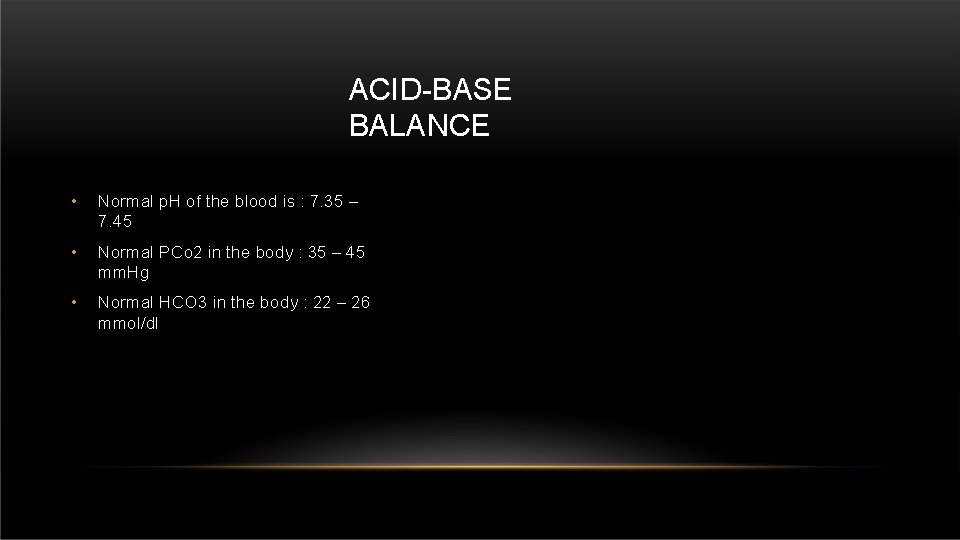
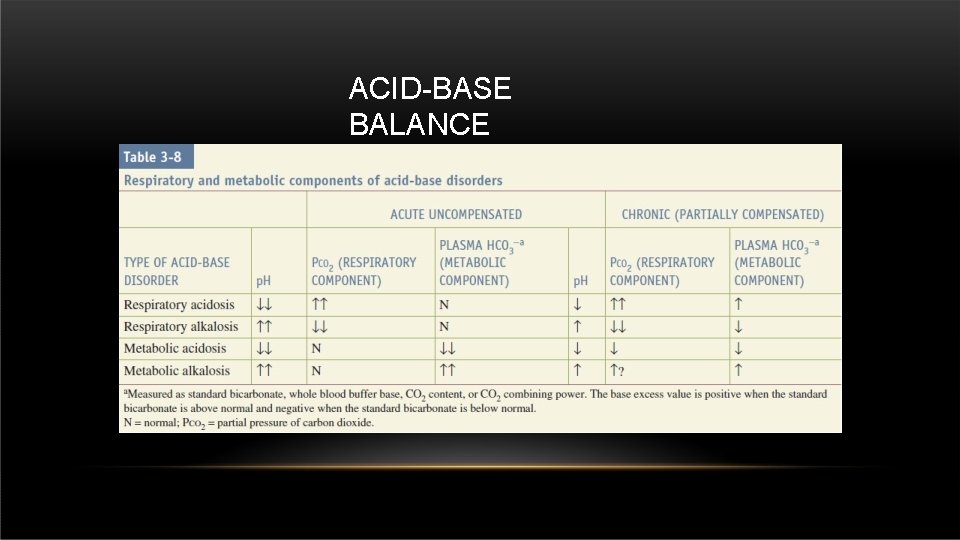
ACID-BASE BALANCE • Normal p. H of the blood is : 7. 35 – 7. 45 • Normal PCo 2 in the body : 35 – 45 mm. Hg • Normal HCO 3 in the body : 22 – 26 mmol/dl

• ACID-BASE BALANCE The normal AG is <12 mmol/L and is due primarily to the albumin effect, so that the estimated AG must be adjusted for albumin (hypoalbuminemia reduces the AG). • AG = (Na) – (Cl + HCO 3) • Corrected AG = actual AG – [2. 5(4. 5 – albumin)]

ACID-BASE BALANCE

ACID-BASE BALANCE

ACID-BASE BALANCE

ACID-BASE BALANCE

ACID-BASE BALANCE

TO BE CONTINUED. . THANK