FAIR USE STATEMENT Please feel free to edit


































































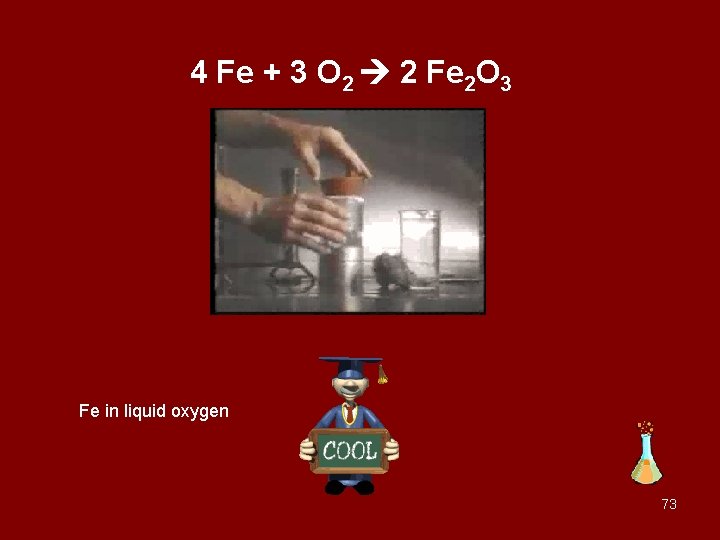



















- Slides: 95

FAIR USE STATEMENT: Please feel free to edit and use this presentation in your classroom. Please do not remove the credit line on the title page or republish the file in whole or in part as your own. Please do not distribute the file to individuals or at conferences or workshops. I am more than willing to share the presentation with anyone that contacts me at rhondaa@cox-internet. com. The images used in the presentation are not original and therefore the presentation is distributed freely and only for classroom instruction. Read the “read me file” on the disk if you have trouble viewing the animated GIFs or Movies. Rhonda Alexander 1

Click the test tube to check answer. Click the flask to return to the next questions. Click to begin Rhonda Alexander 2002 Robert E. Lee High School, Tyler, TX 2

potassium chromate is added to silver nitrate 3

sodium azide (Na. N 3) decomposes 4

a sodium bicarbonate tablet is dropped in a solution of dilute hydrochloric acid Sodium bicarbonate is one of the main ingredients in Alka-Seltzer 5

a piece of aluminum foil is placed in beaker of containing liquid bromine 6

ammonia vapors and hydrogen chloride gas come in contact 7

ammonium dichromate is ignited 8

chlorine gas is bubbled into a solution of potassium bromide 9

a sample of hydrogen gas is ignited 10

a solution of sodium sulfide and cadmium nitrate are mixed 11

calcium metal is placed in a beaker of water 12

calcium carbide is added to water 13

solutions of copper (II) nitrate and sodium carbonate are added to a test tube 14

a piece of copper is added to a solution of nitric acid 15

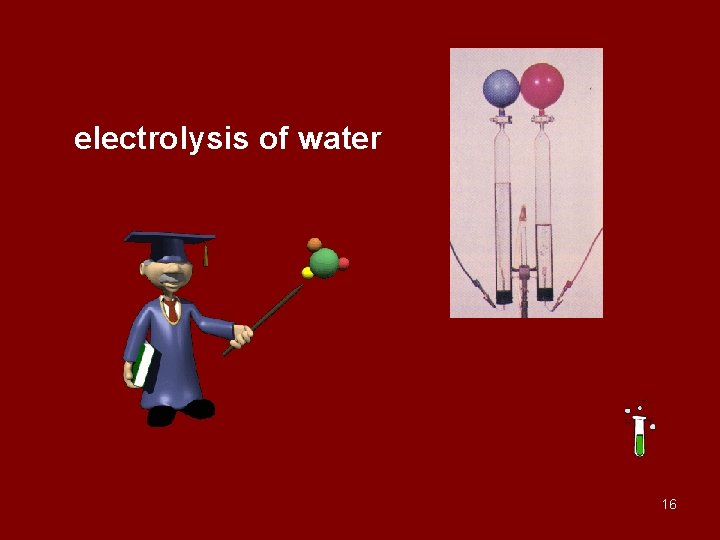
electrolysis of water 16

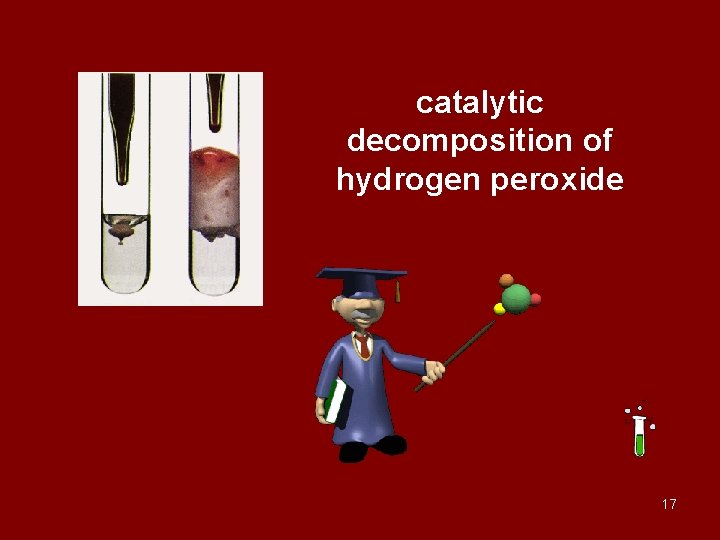
catalytic decomposition of hydrogen peroxide 17

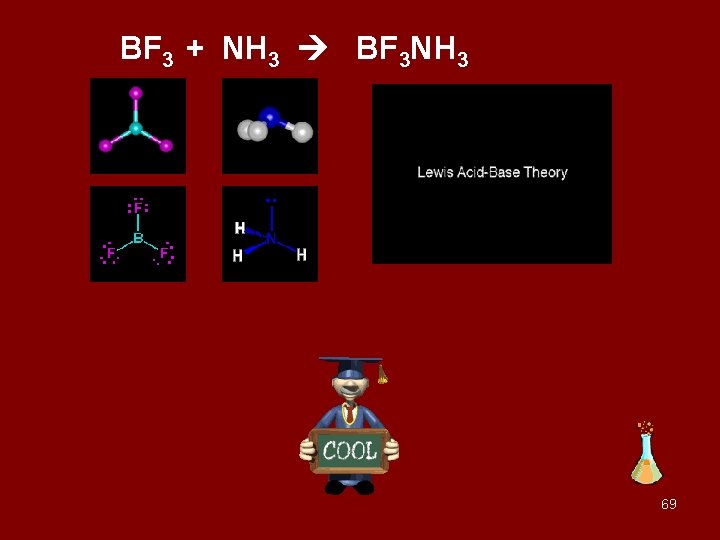
Boron trifluoride and ammonia gas are mixed 18

mercury (II) oxide is heated 19

an iron nail is placed in a beaker containing copper (II) sulfate 20

a solution of ferric nitrate is poured into a beaker containing a solution of sodium hydroxide 21

iron is placed in a beaker containing oxygen The iron must be hot and have a high surface area for the reaction to occur this quickly. 22

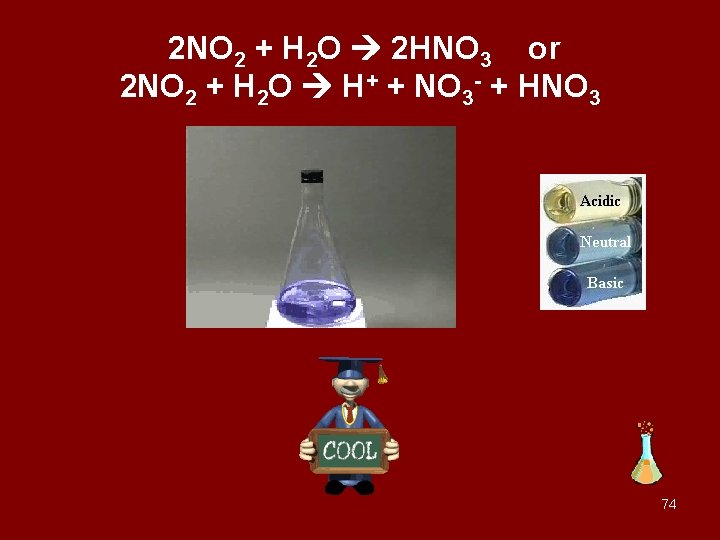
nitrogen dioxide and water react 23

a solution of lead nitrate is dropped into a test tube containing a solution of potassium iodide 24

lightning strikes and oxygen becomes ozone 25

solutions of barium nitrate and sodium sulfate are mixed 26

calcium carbonate chips are placed in a beaker of hydrochloric acid * Limestone is made primarily of calcium carbonate. This is a standard geological test for limestone. Any acid reacts in with calcium carbonate in the same way. 27

pieces of magnesium are placed in a test tube of hydrochloric acid 28

magnesium ribbon is burned 29

a solution of sodium iodide is added to a solution of mercury (II) chloride 30

a solution of magnesium hydroxide is added to a solution of hydrochloric acid 31

phosphorous is placed in a container of oxygen 32

sulfur dioxide is placed in a flask of water 33

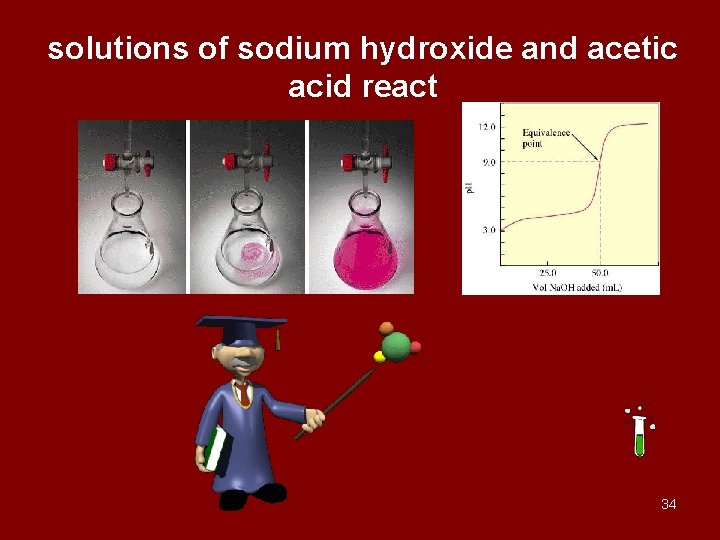
solutions of sodium hydroxide and acetic acid react 34

potassium is placed in a beaker containing liquid bromine 35

a piece of potassium is placed in water 36

calcium oxide is placed in water H 2 O 37

zinc and iodine are placed in an evaporation dish 38

solutions of nickel nitrate and sodium hydroxide are mixed 39

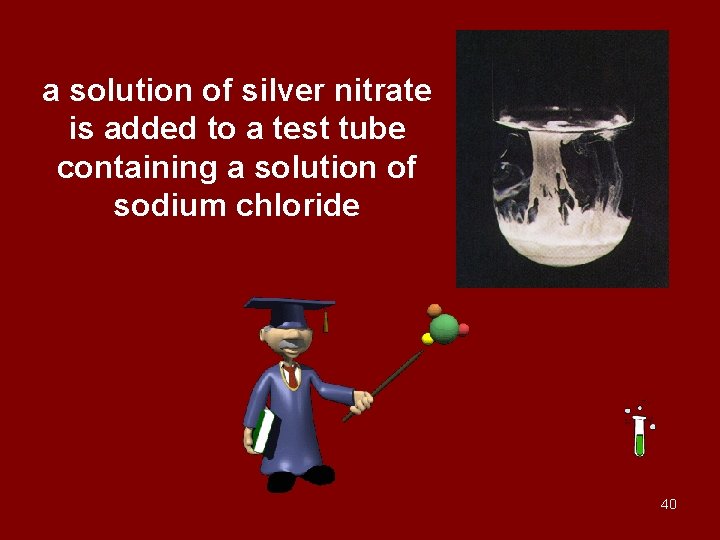
a solution of silver nitrate is added to a test tube containing a solution of sodium chloride 40

sodium is added to a flask containing chlorine gas 41

solutions of sodium iodide and lead (II) nitrate are mixed 42

ferric oxide reacts with aluminum 43

a solution of potassium permanganate reacts with a solution of iron (II) nitrate 44

45

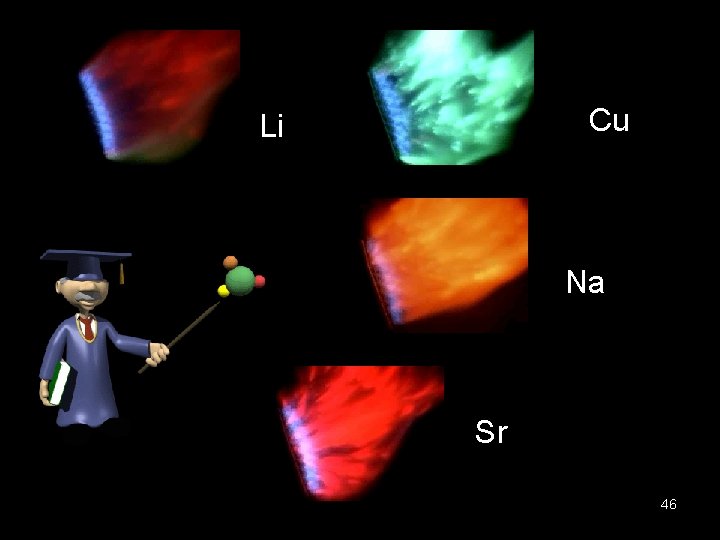
Cu Li Na Sr 46

47

48

49

Which of the following compound of silver is soluble? What is the color of the solution? Note the color of each precipitant. Ag. NO 3, Ag. Cl, Ag. OH 50

Which of the following sulfide is soluble? What is the color of the solution? Note the color of each precipitant. (NH 4)2 S, Cd. S, Sb 2 S 3, Pb. S 51

Which of the following hydroxide is soluble? What is the color of the solution? Note the color of each precipitant. Na. OH, Ca(OH)2, Fe(OH)3, Ni(OH)2 52

53

Ag+ + Cr 2 O 72 - Ag 2 Cr 2 O 7 54

2 Na. N 3 2 Na + 3 N 2 55

Na. HCO 3 + H+ Na+ + H 2 O + CO 2 56

2 Al + 3 Br 2 2 Al. Br 3 57

NH 3+ HCl NH 4 Cl 58

(NH 4)2 Cr 2 O 7 Cr 2 O 3 + N 2+ 4 H 2 O 59

2 Br- + Cl 2 2 Cl- + Br 2 60

2 H 2 + O 2 2 H 2 O 61

S-2 + Cd+ 2 Cd. S 62

Ca + 2 H 2 O Ca(OH)2 + H 2 63

Ca. C 2 + 2 H 2 O C 2 H 2 + Ca(OH)2 ADDING CALCIUM CARBIDE TO WATER C 2 H 2 BURNING 64

Cu+2 + CO 3 -2 Cu. CO 3 * copper (II) carbonate is the green pigment in malachite. Occurs in Egyptian tomb paintings since the fourth dynasty, in European paintings it seems to have been of importance mainly in the 15 th and 16 th centuries. The solution of sodium carbonate is being added to the solution of copper (II) sulfate Gaseous carbon dioxide is being formed Resulting copper (II) carbonate is being filtered off 65

Cu + 4 H+ +2 NO 3 - 2 NO 2 +2 H 2 O + Cu 2+ 66

2 H 2 O 2 H 2 + O 2 67

2 H 2 O 2 2 H 2 O + O 2 68

BF 3 + NH 3 BF 3 NH 3 69

2 Hg. O 2 Hg + O 2 70

Fe + Cu 2+ Fe 2+ + Cu 71

Fe+3 + 3 OH- Fe(OH)3 72
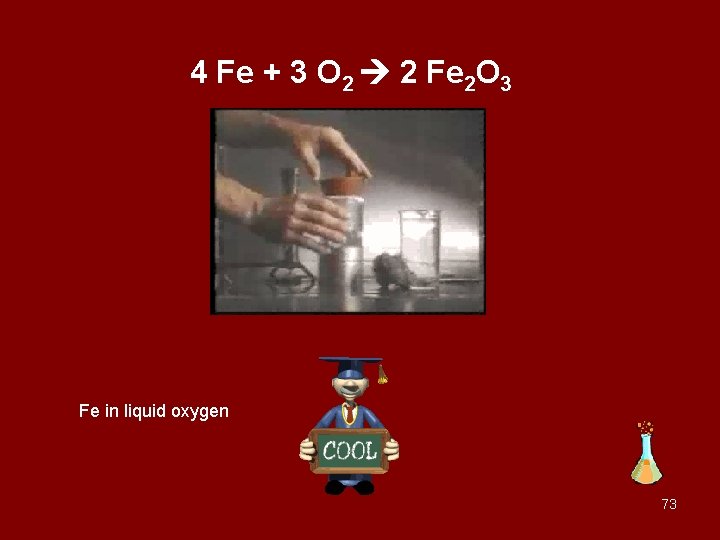
4 Fe + 3 O 2 2 Fe 2 O 3 Fe in liquid oxygen 73

2 NO 2 + H 2 O 2 HNO 3 or 2 NO 2 + H 2 O H+ + NO 3 - + HNO 3 74

Pb+2 + 2 I- Pb. I 2 75

O + O 2 O 3 76

Ba 2+ + SO 42 - Ba. SO 4 77

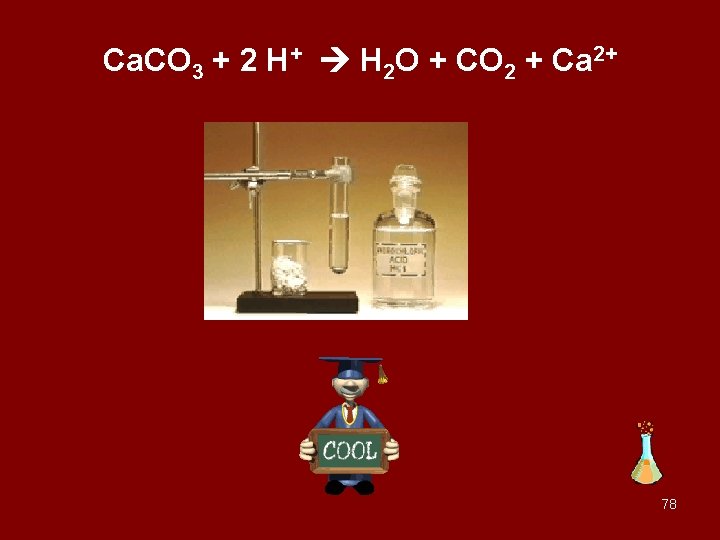
Ca. CO 3 + 2 H+ H 2 O + CO 2 + Ca 2+ 78

Mg + 2 H+ Mg 2+ + H 2 79

2 Mg + O 2 2 Mg. O 80

2 I- + Hg 2+ Hg. I 2 81

OH- + H+ H 2 O 82

4 P + 5 O 2 P 4 O 10 83

SO 2 + H 2 O H 2 SO 3 84

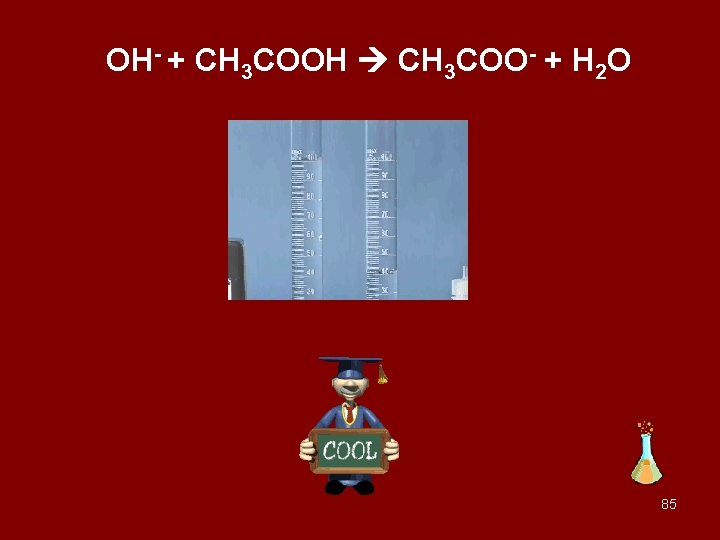
OH- + CH 3 COOH CH 3 COO- + H 2 O 85

K + Br 2 2 KBr 86

2 K + 2 H 2 O 2 KOH + H 2 87

Ca. O + H 2 O Ca(OH)2 88

Zn + I 2 Zn. I 2 89

Ni 2+ + 2 OH- Ni(OH)2 90

Ag+ + Cl- Ag. Cl 91

2 Na + Cl 2 2 Na. Cl 92

2 I- + Pb 2+ Pb. I 2 93

Fe 2 O 3 + 3 Al Al 2 O 3 + 2 Fe 94

5 Fe 2+ Mn. O 4 - + 8 H+ 5 Fe 3+ Mn 2+ + 4 H 2 O 95