Faculty Theo Hodge Jr MD Capital Medical Associates































- Slides: 57


Faculty Theo Hodge, Jr. , MD Capital Medical Associates, Washington, D. C. HOST Terrance Moore NASTAD (National Alliance of State and Territorial AIDS Directors), Washington DC

Learner Objectives ‣ Discuss the ongoing need for Pr. EP among Black men who have sex with men (MSM) ‣ Articulate the supportive evidence for Pr. EP efficacy and utilization found in real life research and demonstration projects ‣ Identify disparities in Pr. EP eligibility and utilization

Case Study: Jaquis ‣ Demographics: 22 Year Old Black MSM ‣ Chief Complaint: Rash on the palm of his hands ‣ Social History: Lives in Miami, hairdresser, identifies as gay, is monogamous to his partner of two-years, used to go out to clubs regularly, large social network ‣ Medical History: Had taken Pr. EP before his current relationship ‣ Family History: Raised as an only child by his grandmother. Out about his sexual orientation to his grandmother ‣ Mental Health and Substance Use: No history of mental health issues or substance use; social drinker; does not smoke ‣ Sexual Health History: Age at first sexual intercourse at age 16, has sex with men only, receptive and insertive partner, does not currently use condoms or Pr. EP, last HIV test 18 months ago was HIV-negative

Video Blog (Part 1 of 3) Look at this rash! I am a hot mess. I’m done with this toxic crap. Other countries are banning these chemicals in salons, but in the good old U. S. of A. we got no problem exposing stylists to formaldehyde and God knows what else. I’m calling OSHA on their asses. And while I’m at it, who can I call to get some insurance? I am stuck going to urgent care to get something for this and to get a note I can throw in their face. Hope there’s something that can clear this up quick. Jonathan has been hinting that he wants to ‘put a ring on it’. And nothing, I mean nothing, is going to spoil that special moment.

Goal of His Health To increase the capacity, quality and effectiveness of health care providers to screen, diagnose, link and retain Black MSM in HIV clinical care

Module Overview ‣ The Continued Need for Pr. EP among Black MSM ‣ Pr. EP Basics: A Review ‣ Pr. EP Findings in Real Life


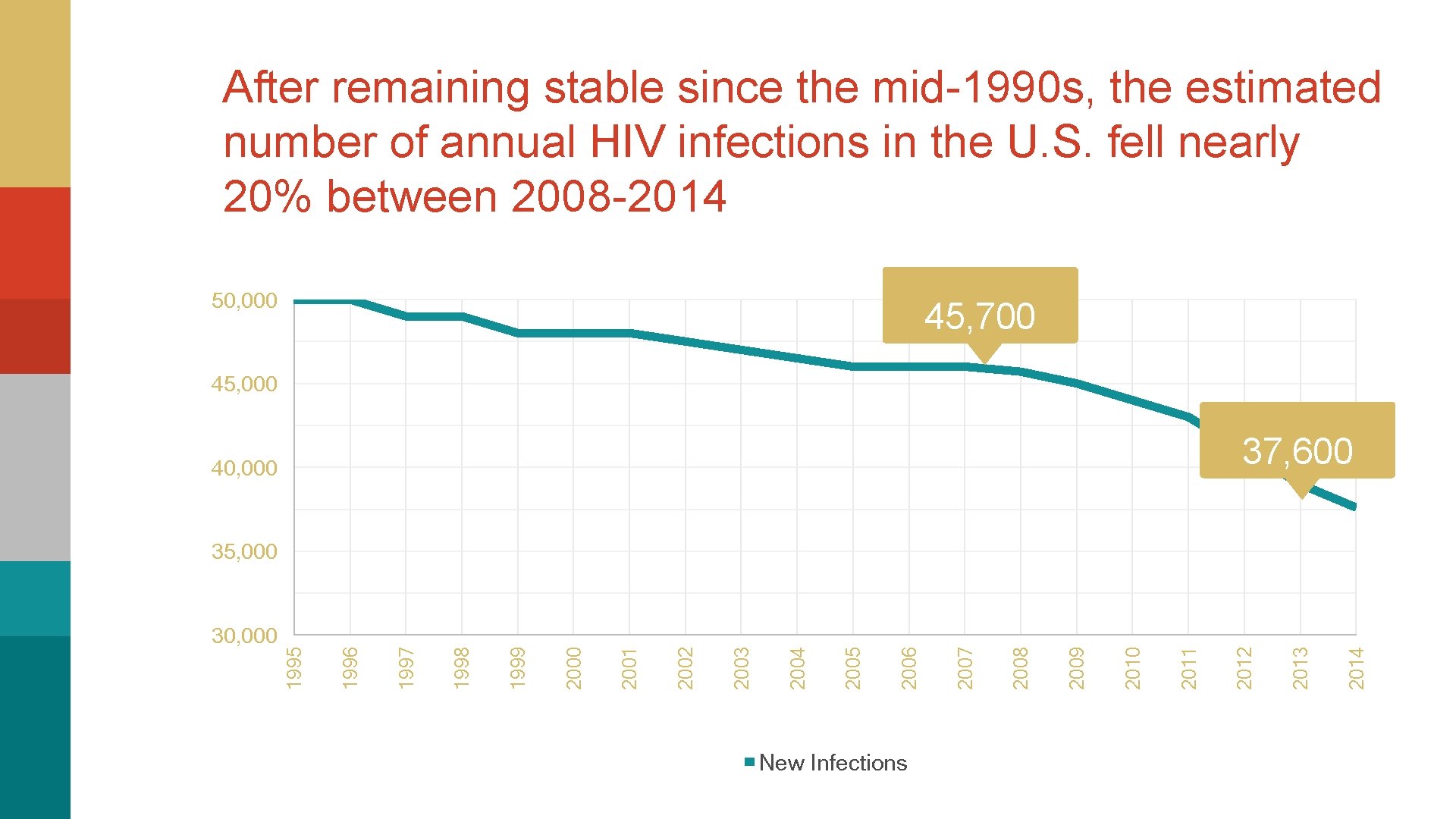
After remaining stable since the mid-1990 s, the estimated number of annual HIV infections in the U. S. fell nearly 20% between 2008 -2014 50, 000 45, 700 45, 000 37, 600 40, 000 New Infections 2014 2013 2012 2011 2010 2009 2008 2007 2006 2005 2004 2003 2002 2001 2000 1999 1998 1997 1996 30, 000 1995 35, 000

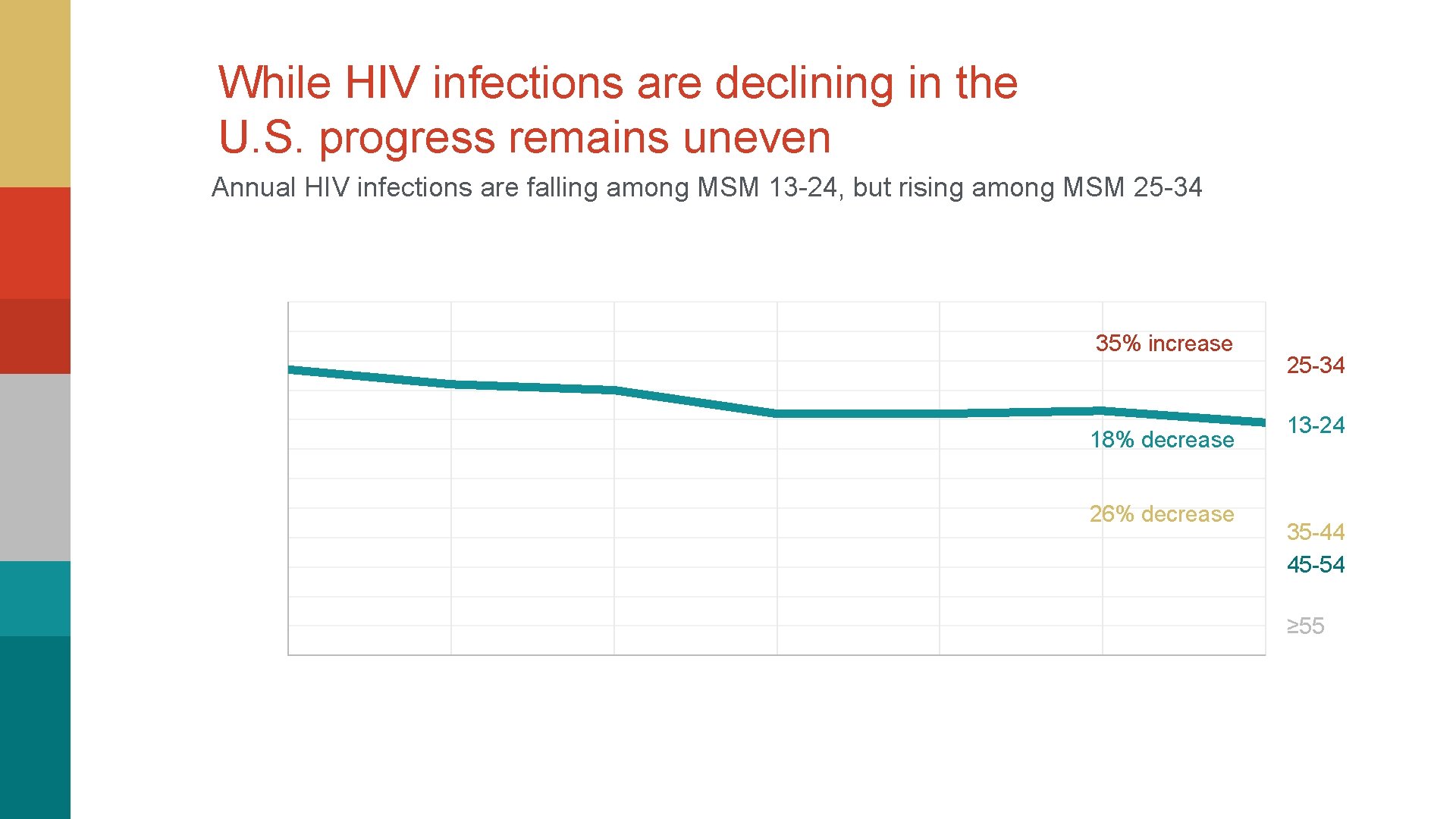
While HIV infections are declining in the U. S. progress remains uneven Annual HIV infections are falling among MSM 13 -24, but rising among MSM 25 -34 35% increase 18% decrease 26% decrease 25 -34 13 -24 35 -44 45 -54 ≥ 55

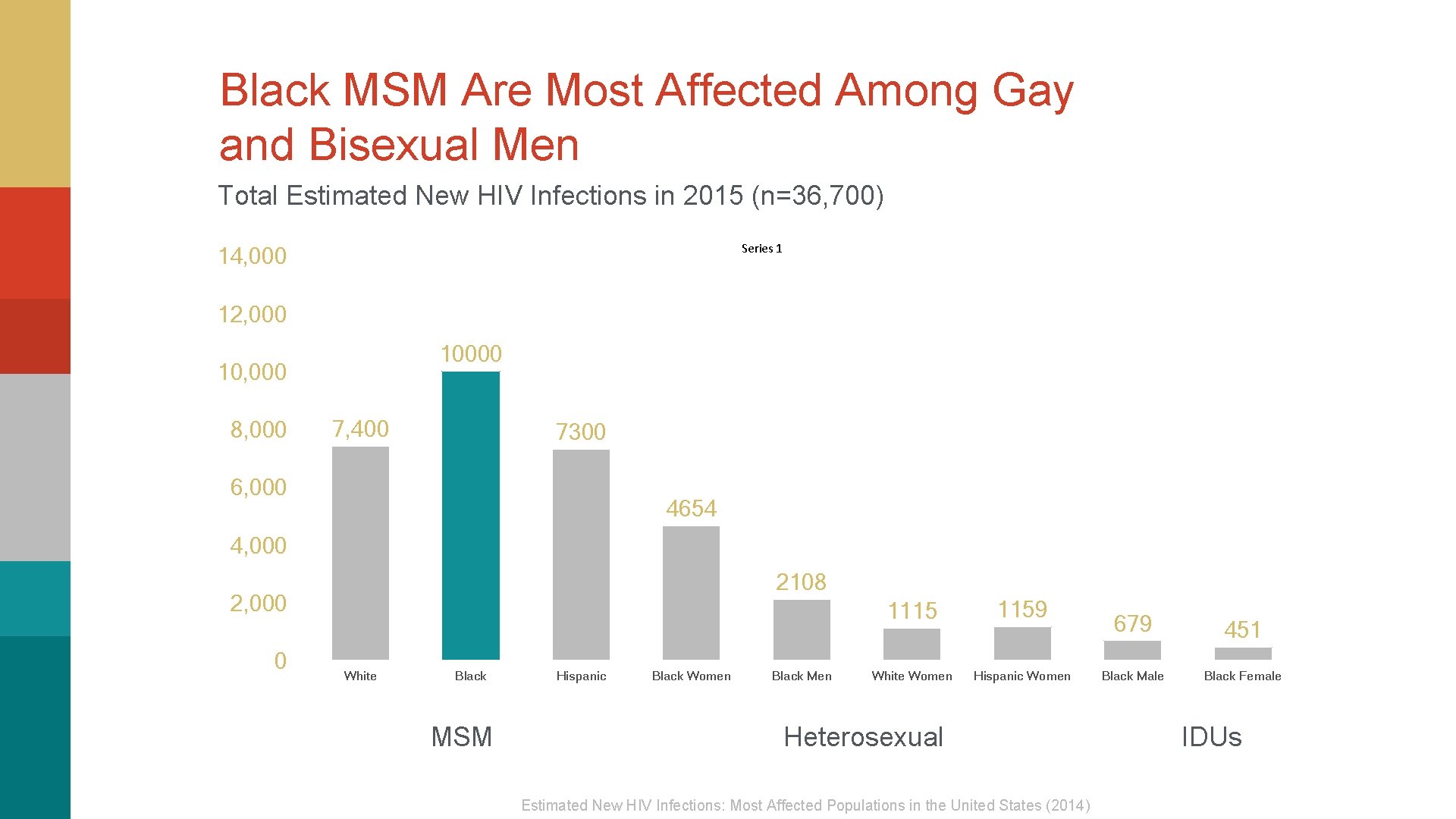
Black MSM Are Most Affected Among Gay and Bisexual Men Total Estimated New HIV Infections in 2015 (n=36, 700) Series 1 14, 000 12, 000 10, 000 8, 000 7, 400 7300 6, 000 4654 4, 000 2108 2, 000 0 White Black MSM Hispanic Black Women Black Men 1115 1159 White Women Hispanic Women Heterosexual Estimated New HIV Infections: Most Affected Populations in the United States (2014) 679 Black Male 451 Black Female IDUs

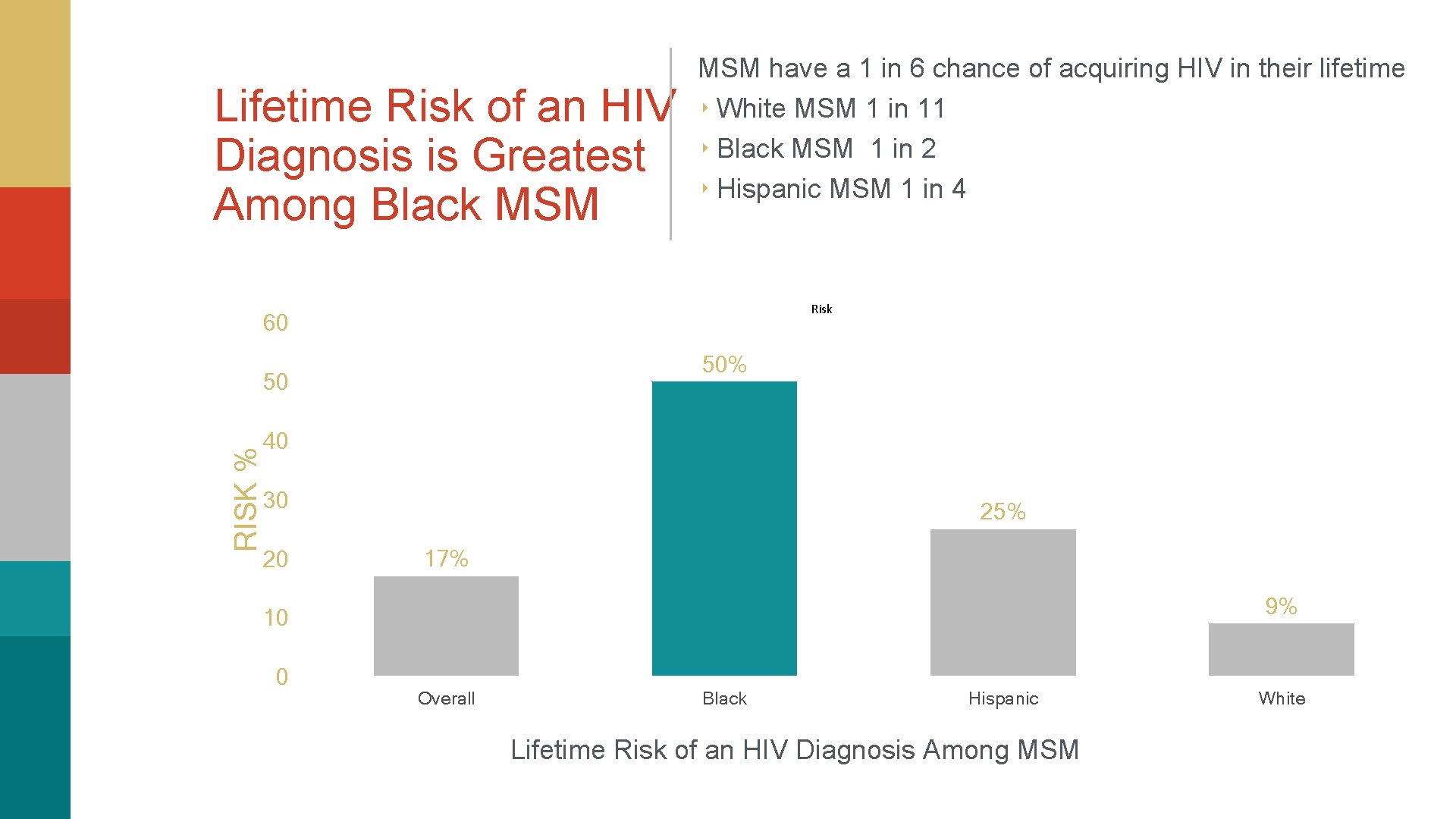
Lifetime Risk of an HIV Diagnosis is Greatest Among Black MSM have a 1 in 6 chance of acquiring HIV in their lifetime ‣ White MSM 1 in 11 ‣ Black MSM 1 in 2 ‣ Hispanic MSM 1 in 4 Risk 60 50% RISK % 50 40 30 20 25% 17% 9% 10 0 Overall Black Hispanic Lifetime Risk of an HIV Diagnosis Among MSM White

The Reasons for the High Lifetime HIV Risk Among Black MSMS are Multifactorial Sexual Networks Play a Key Role

Black MSM less likely to than other HIV-positive MSM ‣ Have health insurance ‣ Initiate combination ART ‣ Adhere to ART ‣ Be virally suppressed

Pop-Up Questions #1 What about Jaquis’ situation suggests the need for Pr. EP? (Check all that apply) A. He is a man who has sex with men B. As a Black gay man, his lifetime risk for HIV is high C. He may have a sexually transmitted infection D. He is in a monogamous relationship E. He may have greater exposure to sexual networks with high levels of HIV

Pr. EP Basics

Truvada emtricitabine (FTC) 200 mg + tenofovir disoproxil fumarate (TDF) 300 mg Approved for Pr. EP in combination with safer sex practices

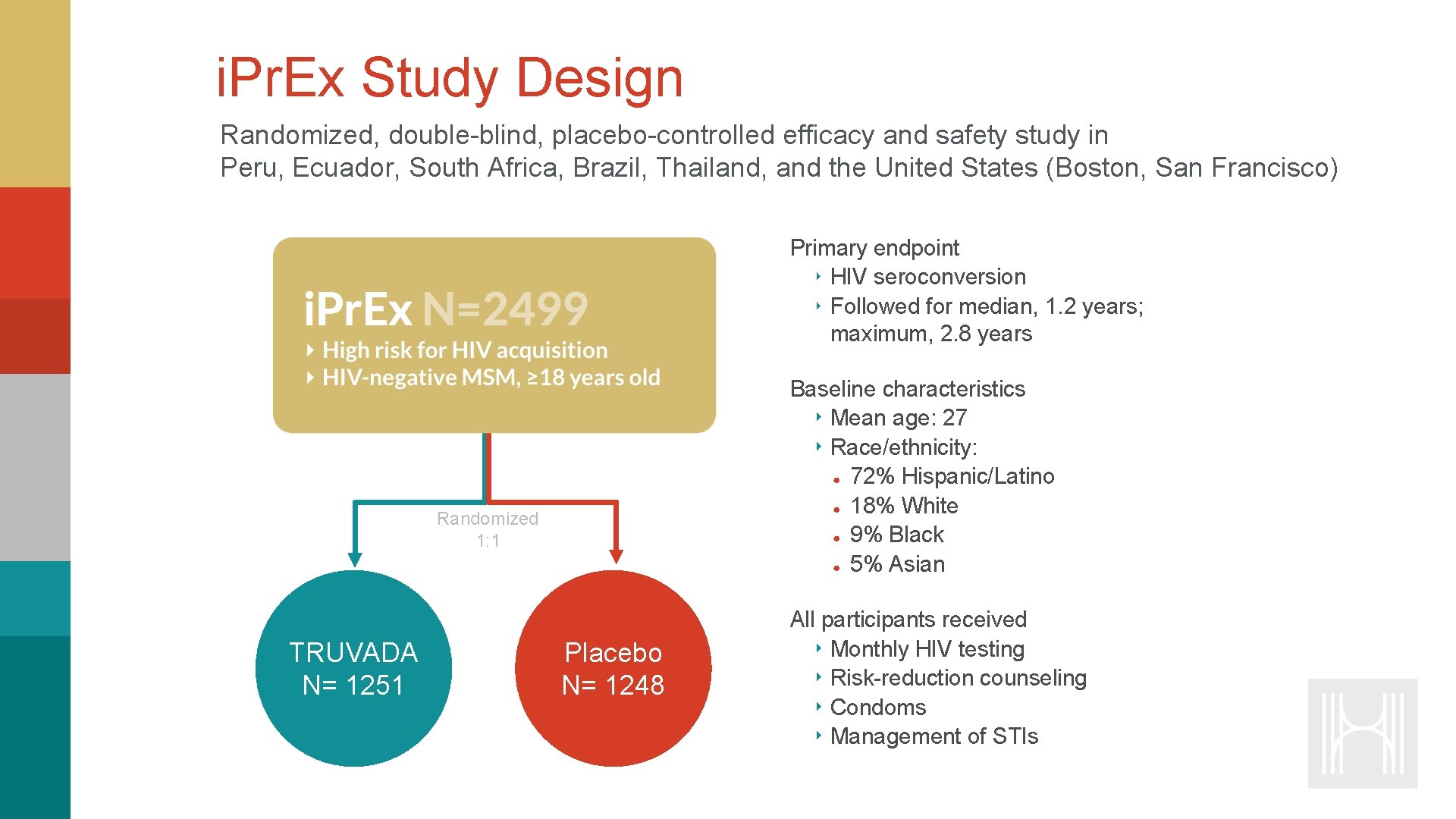
i. Pr. Ex Study Design Randomized, double-blind, placebo-controlled efficacy and safety study in Peru, Ecuador, South Africa, Brazil, Thailand, and the United States (Boston, San Francisco) Primary endpoint ‣ HIV seroconversion ‣ Followed for median, 1. 2 years; maximum, 2. 8 years Baseline characteristics ‣ Mean age: 27 ‣ Race/ethnicity: ● 72% Hispanic/Latino ● 18% White ● 9% Black ● 5% Asian Randomized 1: 1 TRUVADA N= 1251 Placebo N= 1248 All participants received ‣ Monthly HIV testing ‣ Risk-reduction counseling ‣ Condoms ‣ Management of STIs

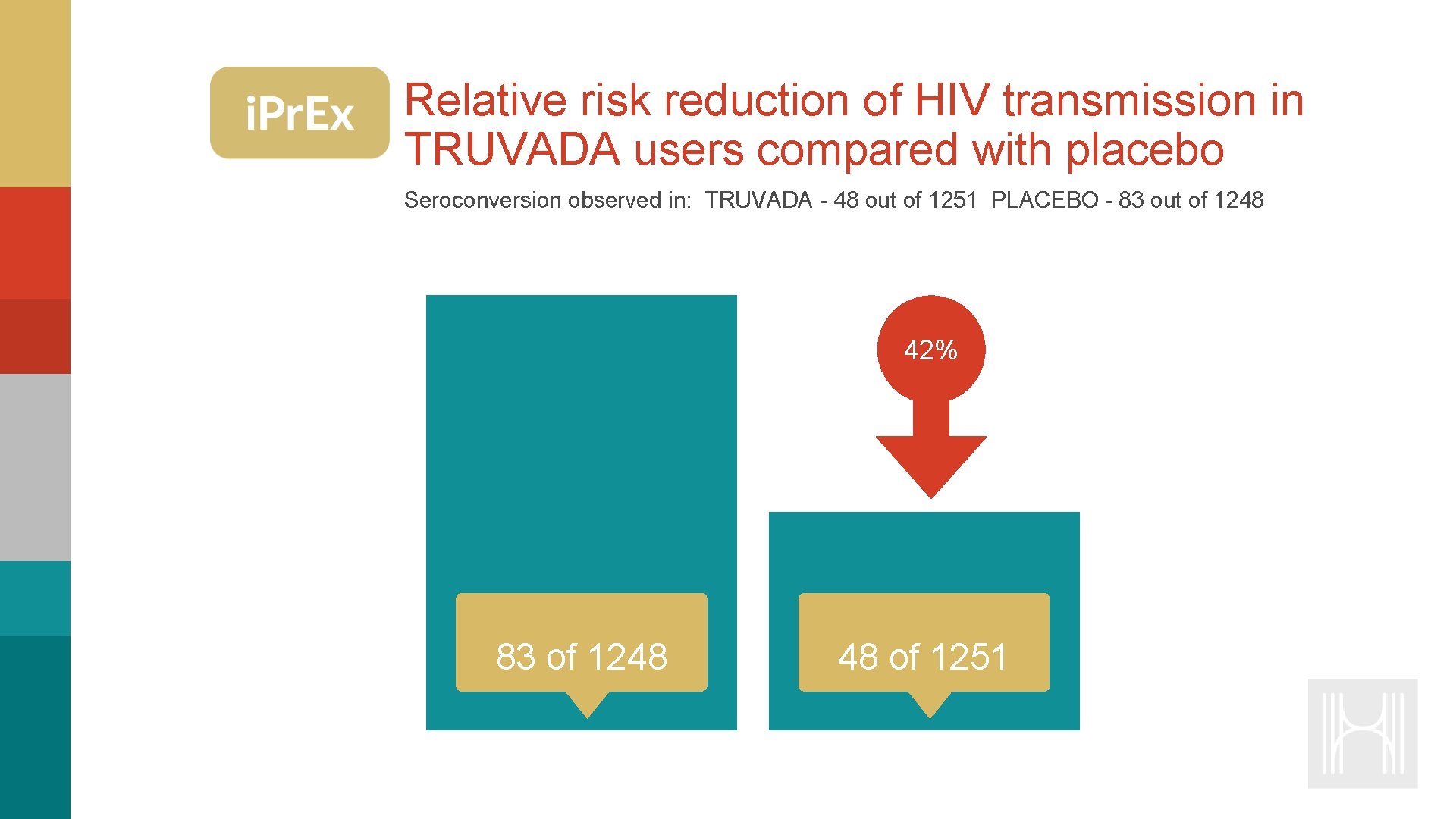
Relative risk reduction of HIV transmission in TRUVADA users compared with placebo Seroconversion observed in: TRUVADA - 48 out of 1251 PLACEBO - 83 out of 1248 42% 83 of 1248 48 of 1251

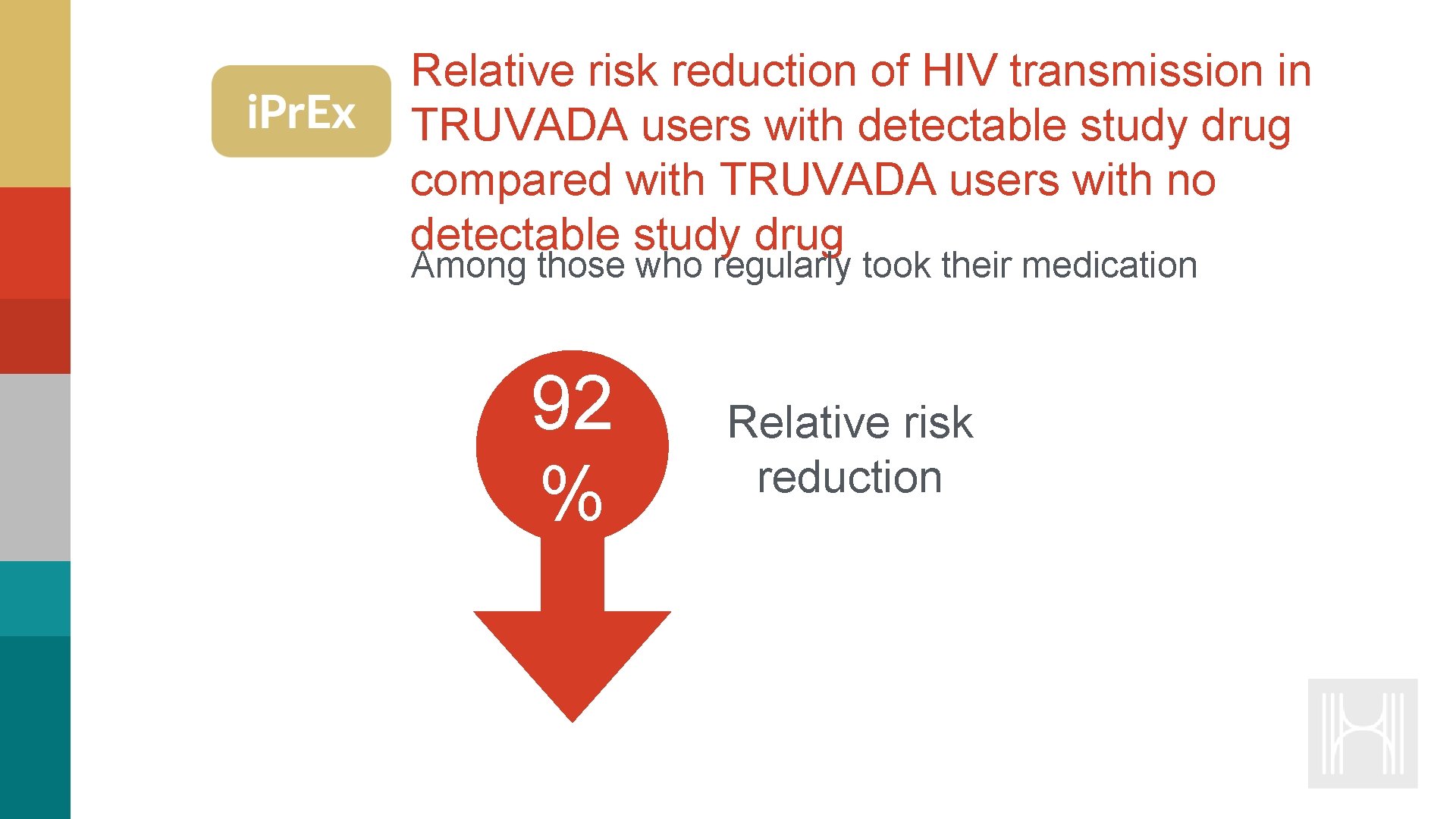
Relative risk reduction of HIV transmission in TRUVADA users with detectable study drug compared with TRUVADA users with no detectable study drug Among those who regularly took their medication 92 % Relative risk reduction

Pr. EP provided 100% protection in those taking four or more doses a week i. Pr. Ex Open-Label Extension (OLE): HIV Incidence and Risk Reduction by Detectable Drug Tablets/week <2 2 - 3 4 to 6 7 Risk Reduction 44% 84% 100% HIV Incidence (per 100 person-years) On Pr. EP Off Pr. EP 0 350 500 700 1000 1250 1500 Tenofovir Diphosphate From Dried Blood Spots (fmol/punch)


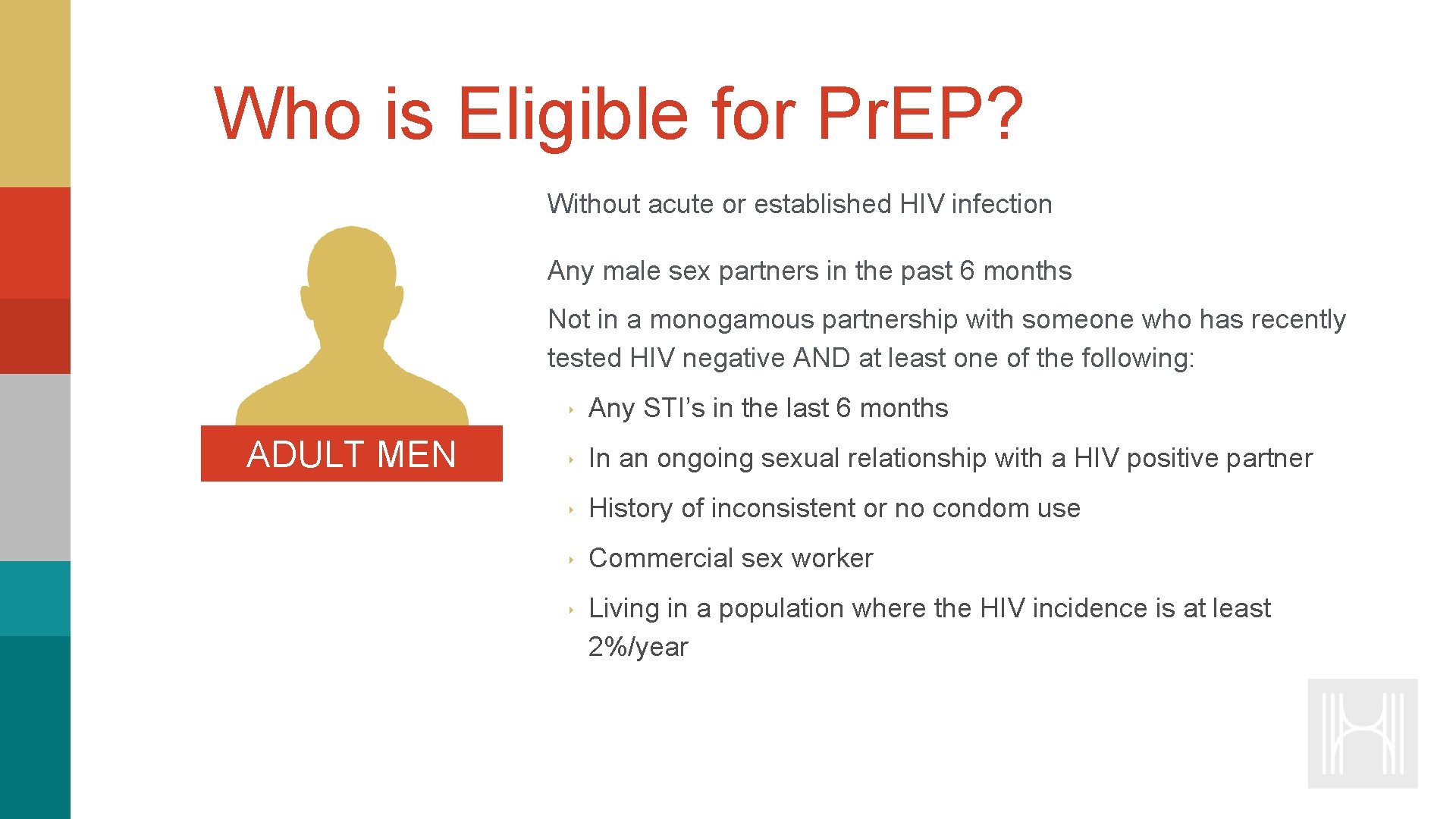
Who is Eligible for Pr. EP? Without acute or established HIV infection Any male sex partners in the past 6 months Not in a monogamous partnership with someone who has recently tested HIV negative AND at least one of the following: ADULT MEN ‣ Any STI’s in the last 6 months ‣ In an ongoing sexual relationship with a HIV positive partner ‣ History of inconsistent or no condom use ‣ Commercial sex worker ‣ Living in a population where the HIV incidence is at least 2%/year

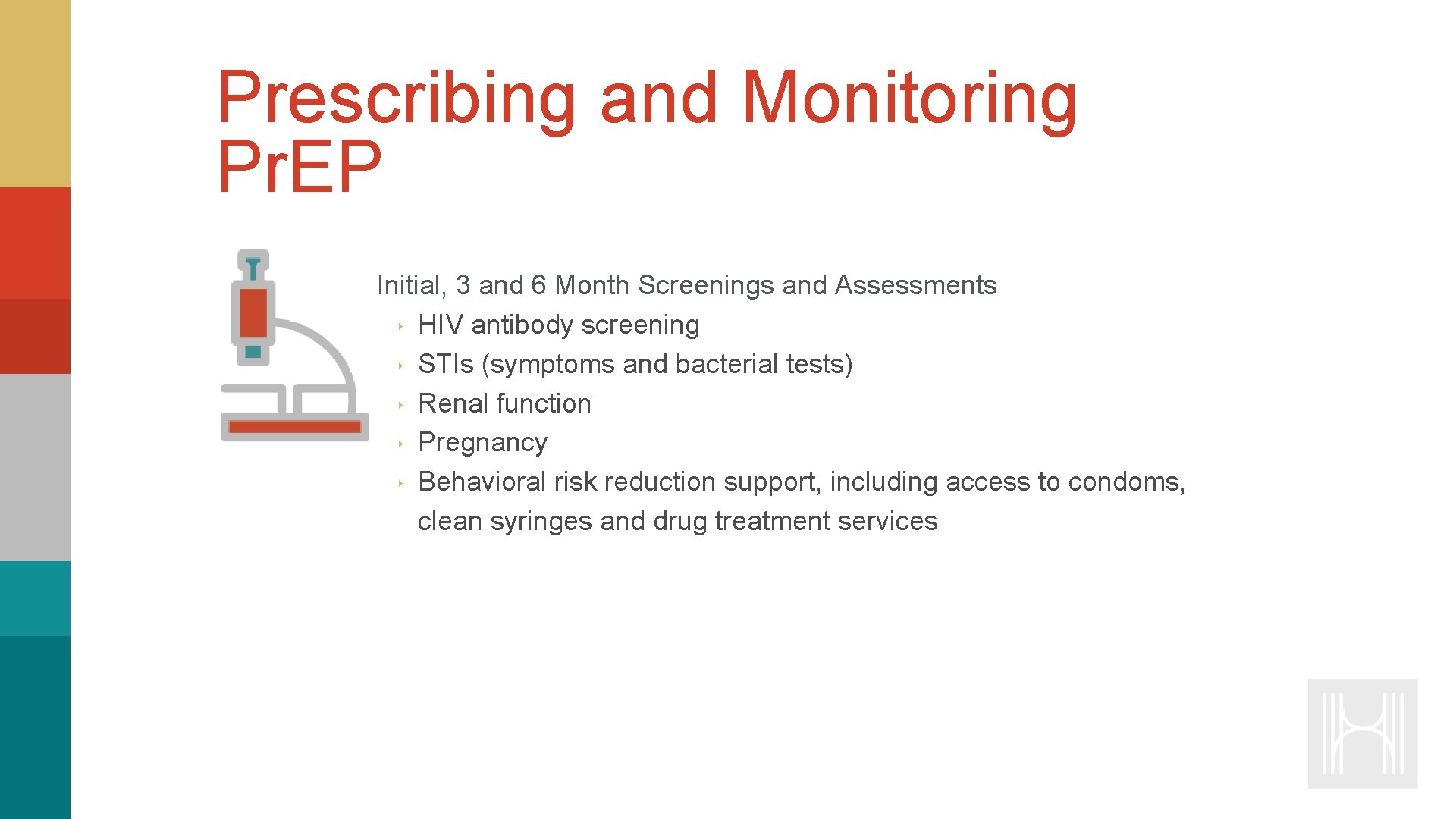
Prescribing and Monitoring Pr. EP Initial, 3 and 6 Month Screenings and Assessments ‣ HIV antibody screening ‣ STIs (symptoms and bacterial tests) ‣ Renal function ‣ Pregnancy ‣ Behavioral risk reduction support, including access to condoms, clean syringes and drug treatment services

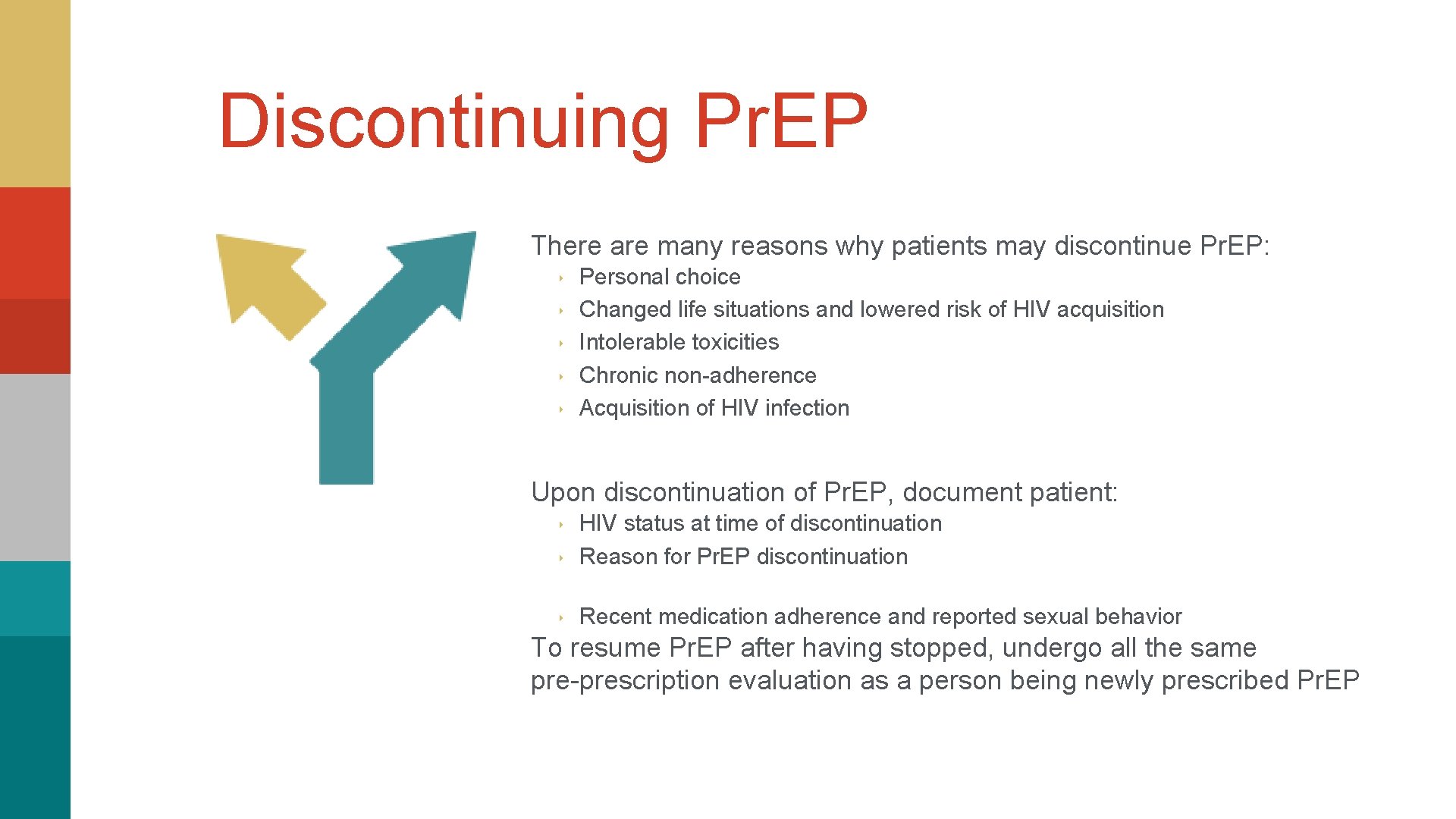
Discontinuing Pr. EP There are many reasons why patients may discontinue Pr. EP: ‣ ‣ ‣ Personal choice Changed life situations and lowered risk of HIV acquisition Intolerable toxicities Chronic non-adherence Acquisition of HIV infection Upon discontinuation of Pr. EP, document patient: ‣ HIV status at time of discontinuation Reason for Pr. EP discontinuation ‣ Recent medication adherence and reported sexual behavior ‣ To resume Pr. EP after having stopped, undergo all the same pre-prescription evaluation as a person being newly prescribed Pr. EP

Pop-Up Questions #2 Which of the following facts would you use to explain to Jaquis why condoms are used in conjunction with Pr. EP? A. The CDC guidance for Pr. EP recommends that condoms and other prevention interventions be used with Pr. EP B. Pr. EP does not provide protection for other sexually transmitted infections C. There have been 2 cases of HIV infection while on Pr. EP with strains of HIV that were resistant to the drugs in Pr. EP D. B and C only E. All of the above F. None of the above

Video Blog (Part 2 of 3) Oh my God – I’m so mad. Why couldn’t they just give me something, anything. They’re always trying to over-complicate things. Said that it might be syphilis – something to with my feet – I don’t think so! That is dirty. They ran a bunch of tests - took blood and samples from everywhere – and I do mean everywhere – Now I have to wait for those come back before I can FINALLY get something for my hands. As I’m leaving they asked if Pr. EP was something I would be interested in? Ha! Please! I have been there, done that - before Jonathan. I don’t even know if Pr. EP worked. They kept asking me, reminding - condoms, use condoms, condoms - blah, blah. I always used condoms before Jonathan, okay. These doctors, they’re always pushing pills – even when you feel fine – use a pill. Just like that Tuskegee nightmare - they are using us for their experiments. Data, statistics - I am not a statistic. Swallowing that stuff is unnatural – probably more toxic than the stuff that’s messing with my hands.


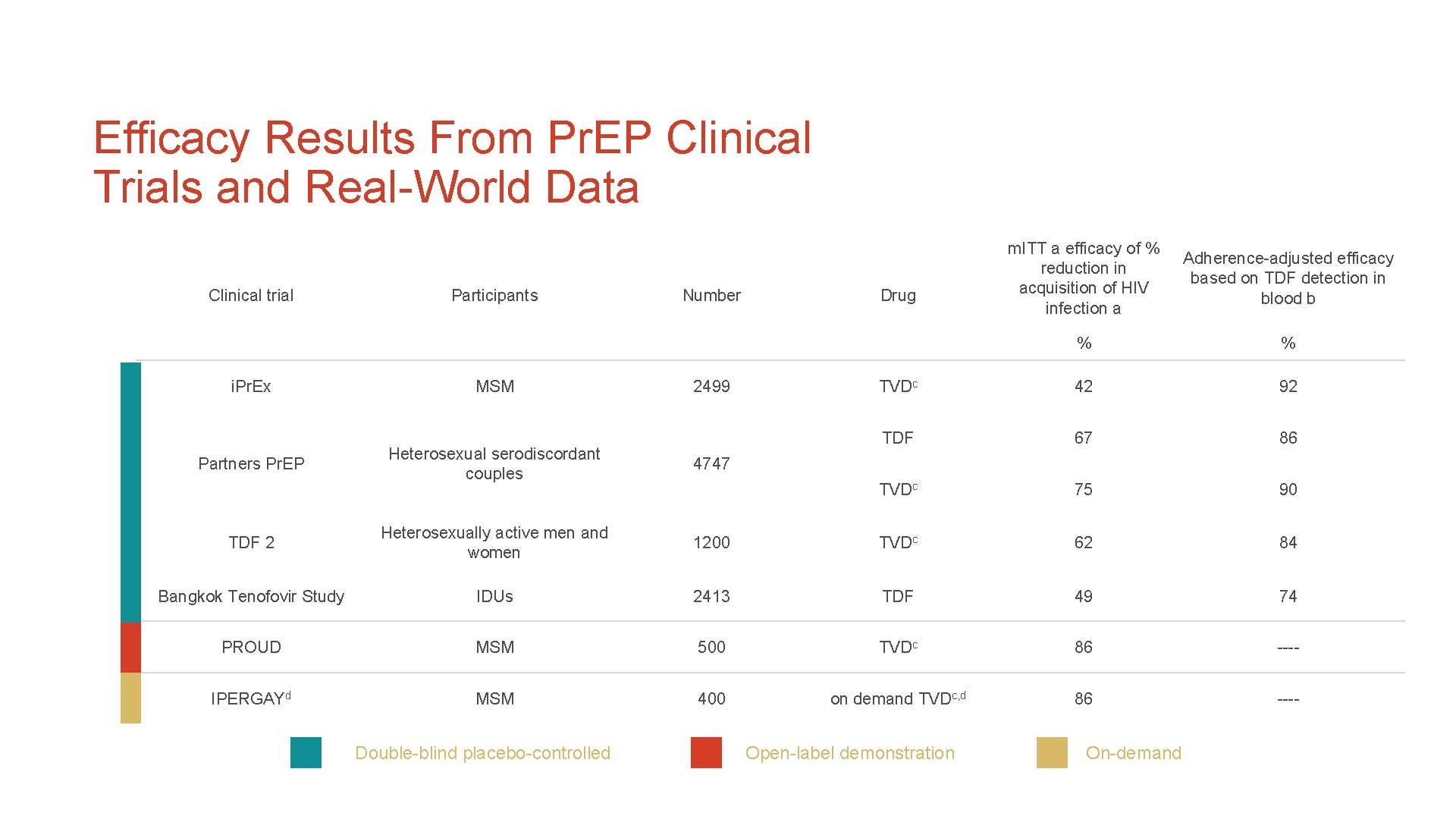
Efficacy Results From Pr. EP Clinical Trials and Real-World Data Clinical trial i. Pr. Ex Participants MSM Number 2499 m. ITT a efficacy of % reduction in acquisition of HIV infection a Adherence-adjusted efficacy based on TDF detection in blood b % % TVDc 42 92 TDF 67 86 TVDc 75 90 Drug Heterosexual serodiscordant couples 4747 TDF 2 Heterosexually active men and women 1200 TVDc 62 84 Bangkok Tenofovir Study IDUs 2413 TDF 49 74 PROUD MSM 500 TVDc 86 ---- IPERGAYd MSM 400 on demand TVDc, d 86 ---- Partners Pr. EP Double-blind placebo-controlled Open-label demonstration On-demand

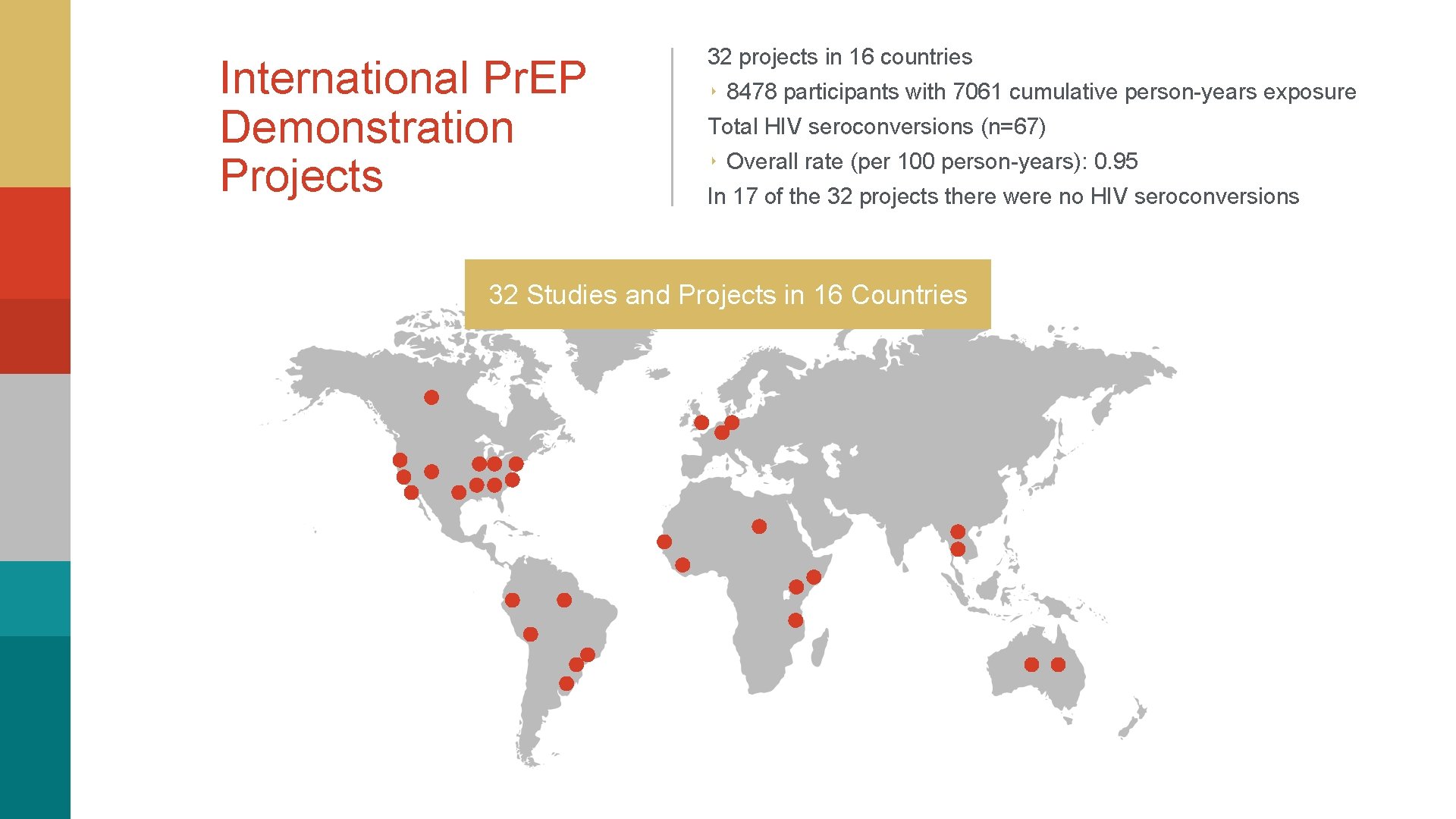
International Pr. EP Demonstration Projects 32 projects in 16 countries ‣ 8478 participants with 7061 cumulative person-years exposure Total HIV seroconversions (n=67) ‣ Overall rate (per 100 person-years): 0. 95 In 17 of the 32 projects there were no HIV seroconversions 32 Studies and Projects in 16 Countries

PROUD Study Pr. EP Use in a Real-World Setting (2012 -2014) ‣ ‣ ‣ ‣ Multicenter United Kingdom Study 13 Sexual Health Clinics Open label study HIV-negative MSM Condomless anal intercourse No HBV Web-based randomization. Follow-up: 3 times monthly for up to 24 months Primary endpoint: HIV infection in the first 12 months Immediate Emtricitabine/Tenofovir DF (n=276) VS Deferred (12 months) Emtricitabine/Tenofovir DF (n=269)

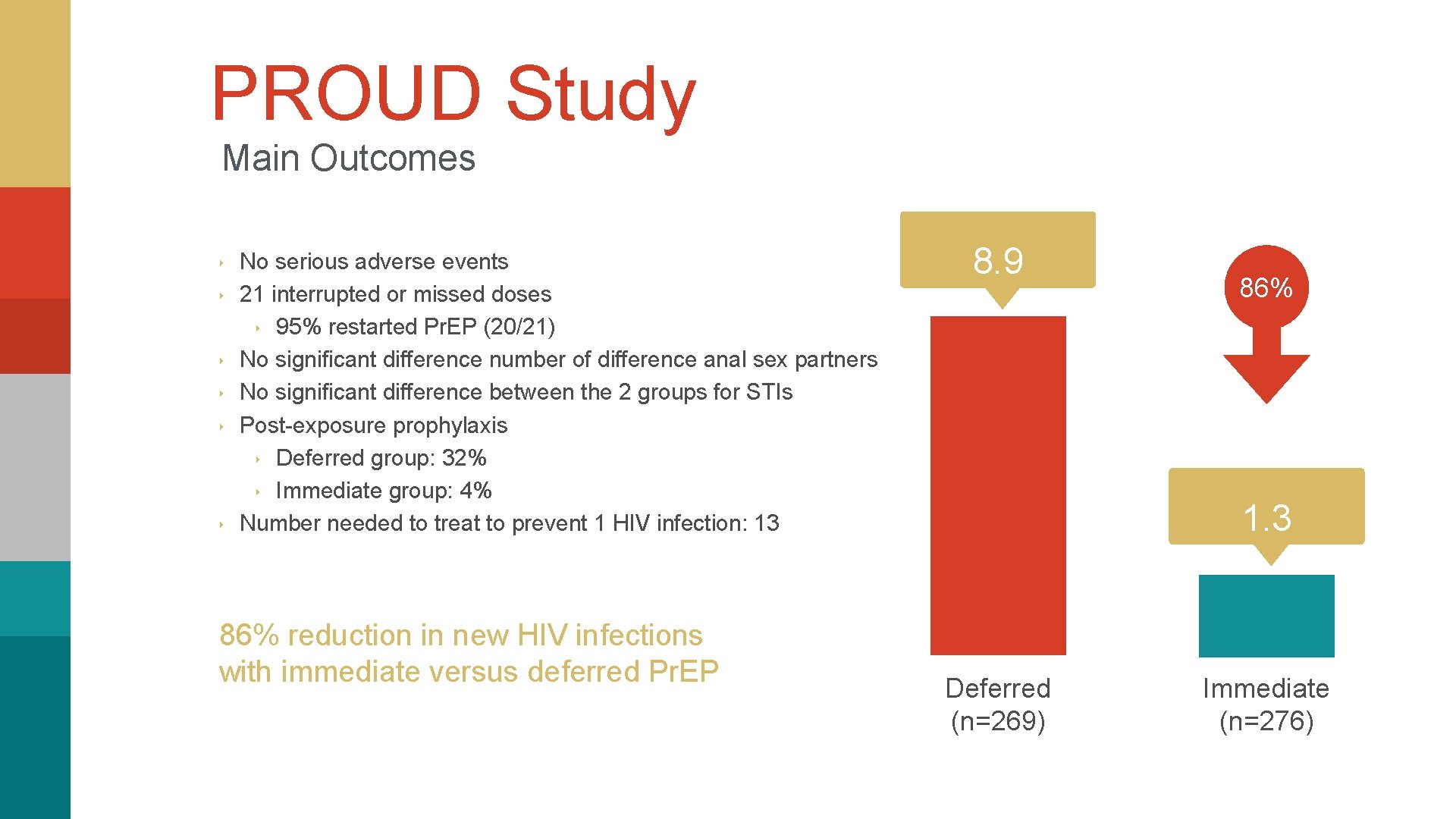
PROUD Study Main Outcomes ‣ ‣ ‣ No serious adverse events 21 interrupted or missed doses ‣ 95% restarted Pr. EP (20/21) No significant difference number of difference anal sex partners No significant difference between the 2 groups for STIs Post-exposure prophylaxis ‣ Deferred group: 32% ‣ Immediate group: 4% Number needed to treat to prevent 1 HIV infection: 13 86% reduction in new HIV infections with immediate versus deferred Pr. EP 8. 9 86% 1. 3 Deferred (n=269) Immediate (n=276)

Tailoring Pr. EP for Key Populations

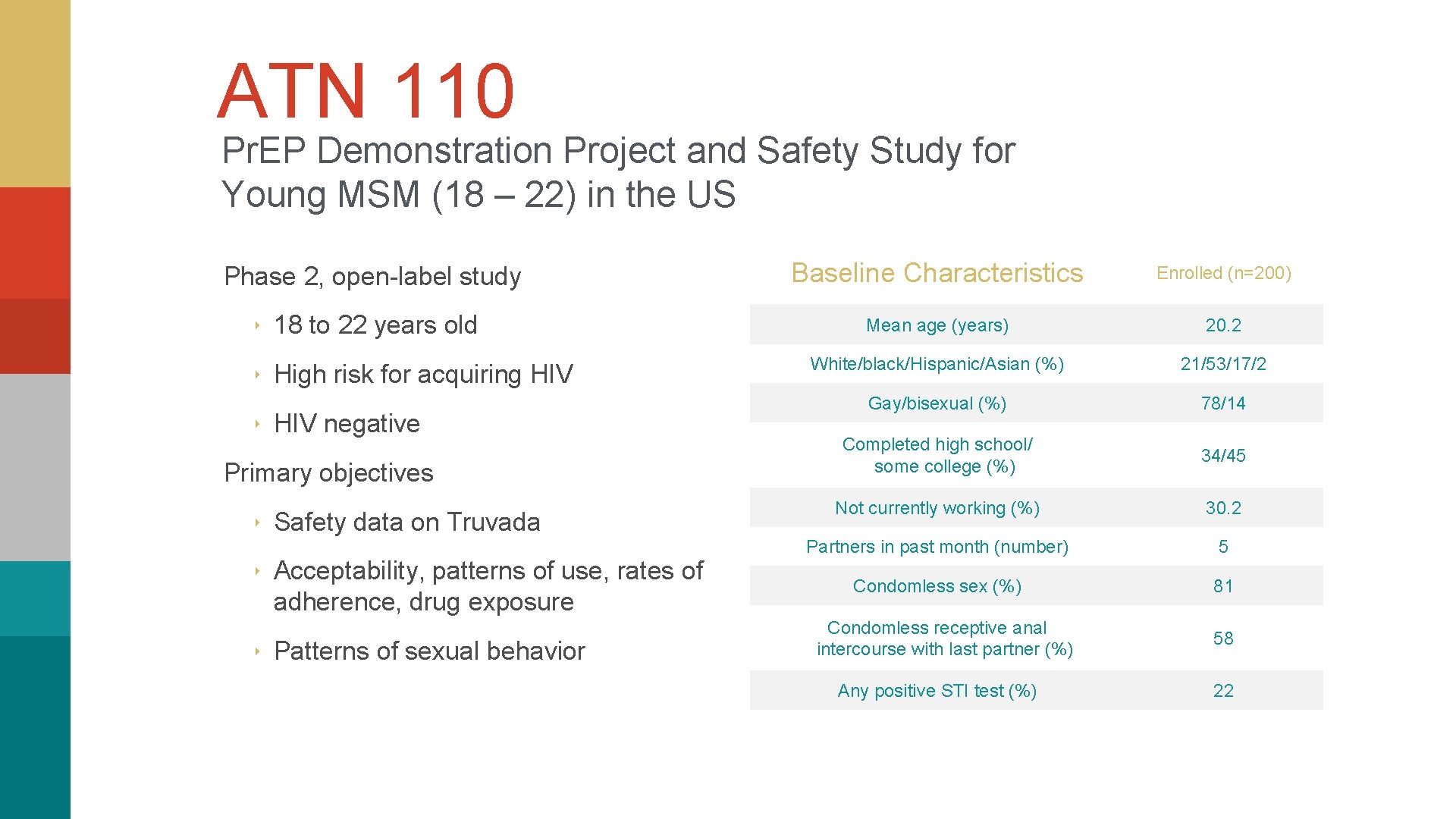
ATN 110 Pr. EP Demonstration Project and Safety Study for Young MSM (18 – 22) in the US Phase 2, open-label study ‣ 18 to 22 years old ‣ High risk for acquiring HIV ‣ HIV negative Primary objectives ‣ Safety data on Truvada ‣ Acceptability, patterns of use, rates of adherence, drug exposure ‣ Patterns of sexual behavior Baseline Characteristics Enrolled (n=200) Mean age (years) 20. 2 White/black/Hispanic/Asian (%) 21/53/17/2 Gay/bisexual (%) 78/14 Completed high school/ some college (%) 34/45 Not currently working (%) 30. 2 Partners in past month (number) 5 Condomless sex (%) 81 Condomless receptive anal intercourse with last partner (%) 58 Any positive STI test (%) 22

ATN 110 Main Outcomes Safety ‣ Discontinued (n=25) ‣ Treatment-related adverse events (n=3) ‣ Nausea, weight loss, headache (all grade 3) HIV seroconversions (n=4) ‣ HIV incidence: 3. 29/100 person-years ‣ No drug resistance Sexual behavior and adherence ‣ STI diagnoses remained constant over time ‣ Adherence decreased for all participants over time, particularly for young black MSM ‣ Higher adherence and tenofovir diphosphate levels among those participating in condomless sex and condomless receptive anal intercourse Young MSM at highest risk of HIV infection may also be most likely to be adherent to Pr. EP

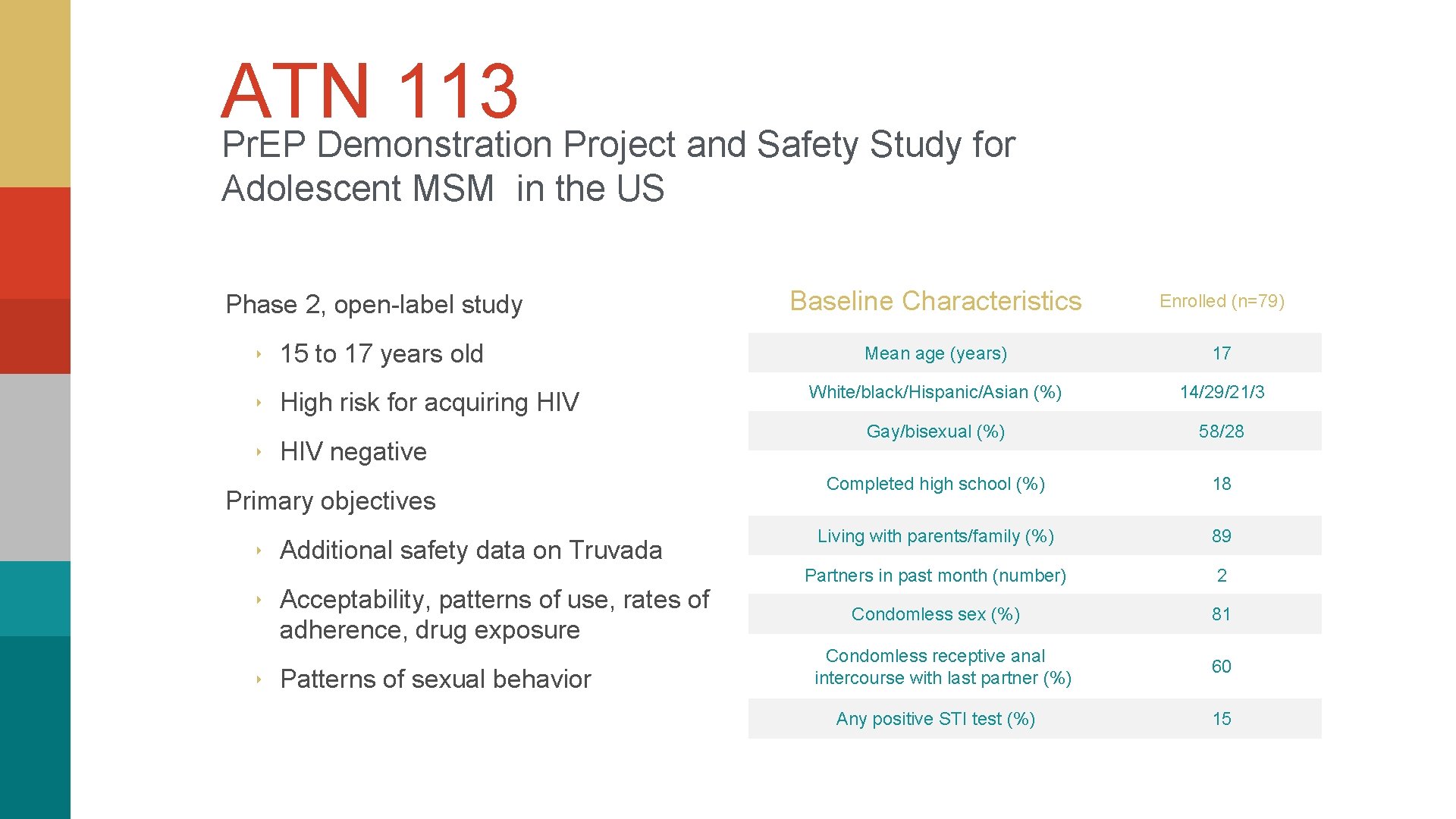
ATN 113 Pr. EP Demonstration Project and Safety Study for Adolescent MSM in the US Phase 2, open-label study ‣ 15 to 17 years old ‣ High risk for acquiring HIV ‣ HIV negative Primary objectives ‣ Additional safety data on Truvada ‣ Acceptability, patterns of use, rates of adherence, drug exposure ‣ Patterns of sexual behavior Baseline Characteristics Enrolled (n=79) Mean age (years) 17 White/black/Hispanic/Asian (%) 14/29/21/3 Gay/bisexual (%) 58/28 Completed high school (%) 18 Living with parents/family (%) 89 Partners in past month (number) 2 Condomless sex (%) 81 Condomless receptive anal intercourse with last partner (%) 60 Any positive STI test (%) 15

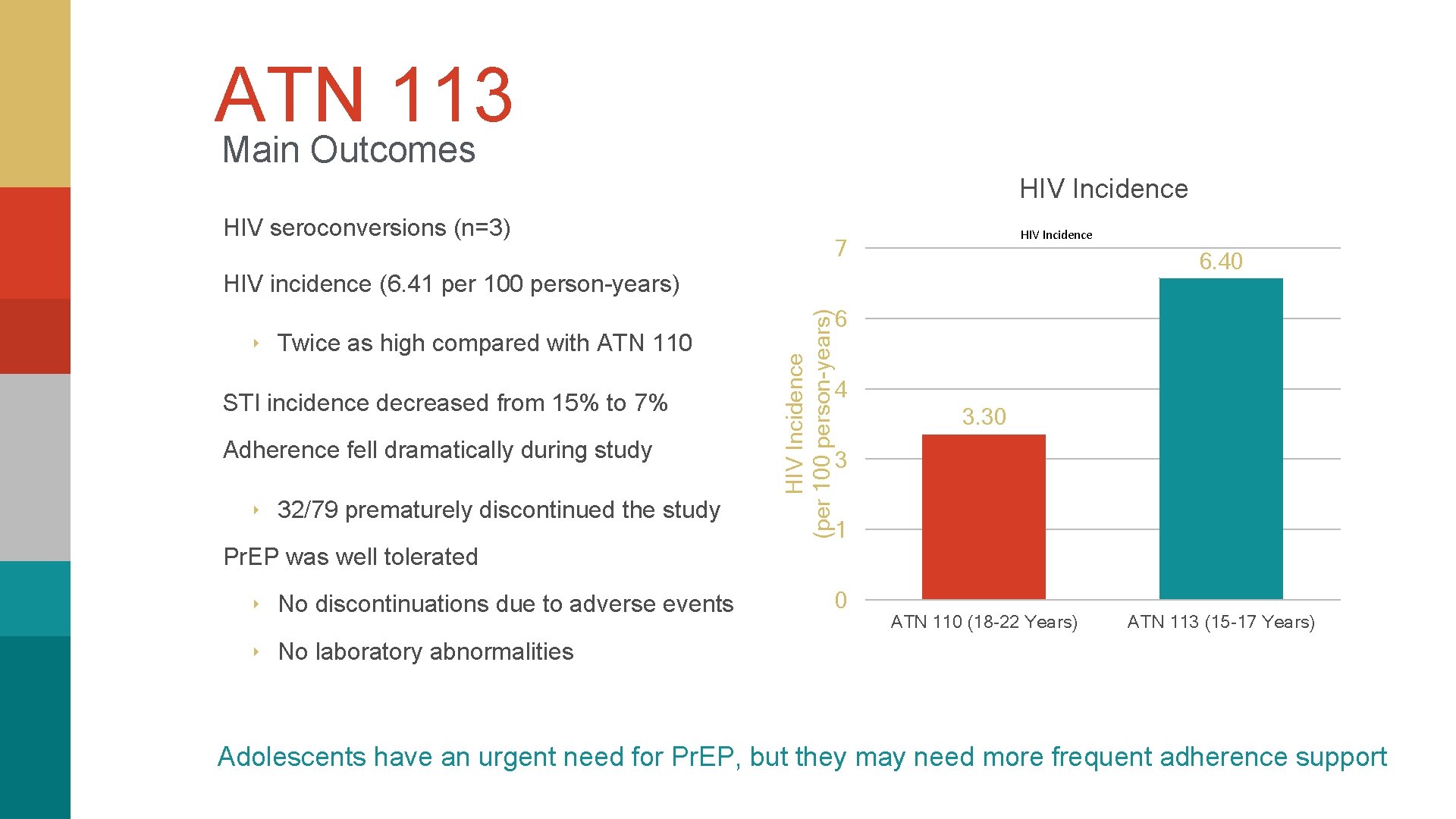
ATN 113 Main Outcomes HIV Incidence HIV seroconversions (n=3) HIV Incidence 7 6. 40 ‣ Twice as high compared with ATN 110 STI incidence decreased from 15% to 7% Adherence fell dramatically during study ‣ 32/79 prematurely discontinued the study HIV Incidence (per 100 person-years) HIV incidence (6. 41 per 100 person-years) 6 4 3. 30 3 1 Pr. EP was well tolerated ‣ No discontinuations due to adverse events 0 ATN 110 (18 -22 Years) ATN 113 (15 -17 Years) ‣ No laboratory abnormalities Adolescents have an urgent need for Pr. EP, but they may need more frequent adherence support

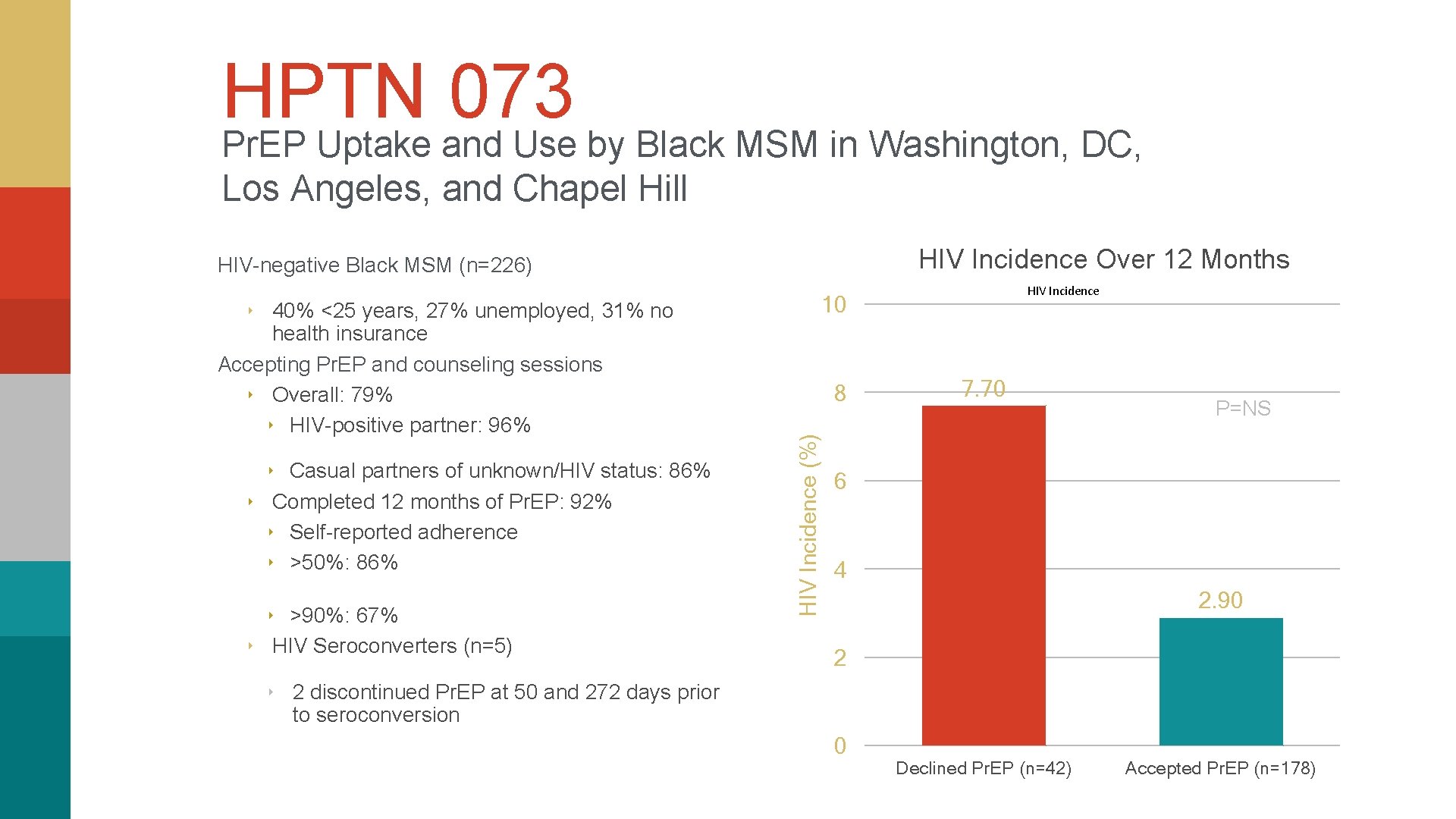
HPTN 073 Pr. EP Uptake and Use by Black MSM in Washington, DC, Los Angeles, and Chapel Hill HIV Incidence Over 12 Months HIV-negative Black MSM (n=226) 10 ‣ 40% <25 years, 27% unemployed, 31% no ‣ Casual partners of unknown/HIV status: 86% ‣ Completed 12 months of Pr. EP: 92% ‣ Self-reported adherence ‣ >50%: 86% ‣ >90%: 67% ‣ HIV Seroconverters (n=5) 8 HIV Incidence (%) health insurance Accepting Pr. EP and counseling sessions ‣ Overall: 79% ‣ HIV-positive partner: 96% HIV Incidence 7. 70 P=NS 6 4 2. 90 2 ‣ 2 discontinued Pr. EP at 50 and 272 days prior to seroconversion 0 Declined Pr. EP (n=42) Accepted Pr. EP (n=178)

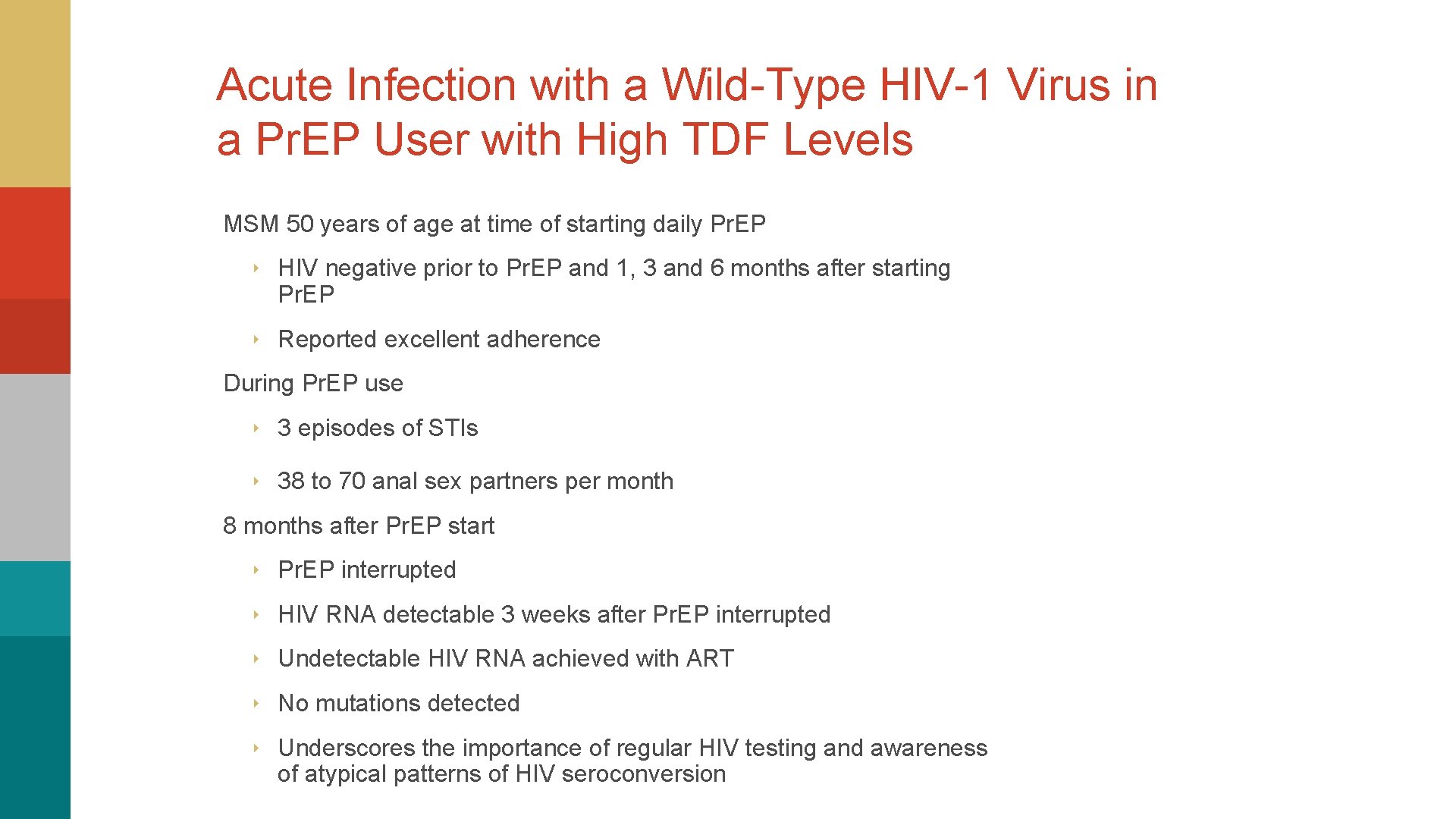
Acute Infection with a Wild-Type HIV-1 Virus in a Pr. EP User with High TDF Levels MSM 50 years of age at time of starting daily Pr. EP ‣ HIV negative prior to Pr. EP and 1, 3 and 6 months after starting Pr. EP ‣ Reported excellent adherence During Pr. EP use ‣ 3 episodes of STIs ‣ 38 to 70 anal sex partners per month 8 months after Pr. EP start ‣ Pr. EP interrupted ‣ HIV RNA detectable 3 weeks after Pr. EP interrupted ‣ Undetectable HIV RNA achieved with ART ‣ No mutations detected ‣ Underscores the importance of regular HIV testing and awareness of atypical patterns of HIV seroconversion

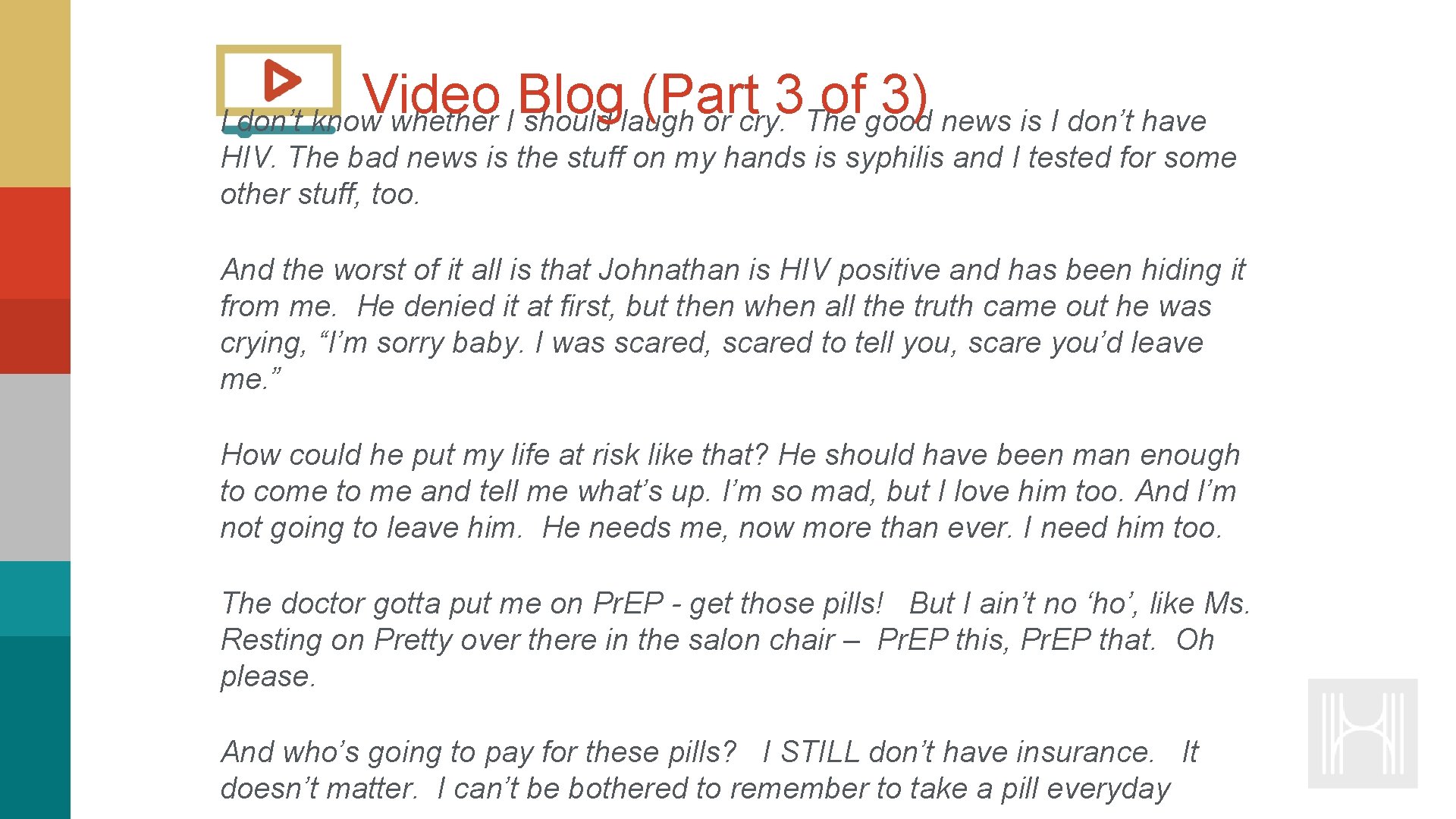
Video Blog (Part 3 of 3) I don’t know whether I should laugh or cry. The good news is I don’t have HIV. The bad news is the stuff on my hands is syphilis and I tested for some other stuff, too. And the worst of it all is that Johnathan is HIV positive and has been hiding it from me. He denied it at first, but then when all the truth came out he was crying, “I’m sorry baby. I was scared, scared to tell you, scare you’d leave me. ” How could he put my life at risk like that? He should have been man enough to come to me and tell me what’s up. I’m so mad, but I love him too. And I’m not going to leave him. He needs me, now more than ever. I need him too. The doctor gotta put me on Pr. EP - get those pills! But I ain’t no ‘ho’, like Ms. Resting on Pretty over there in the salon chair – Pr. EP this, Pr. EP that. Oh please. And who’s going to pay for these pills? I STILL don’t have insurance. It doesn’t matter. I can’t be bothered to remember to take a pill everyday

Eligibility versus Utilization

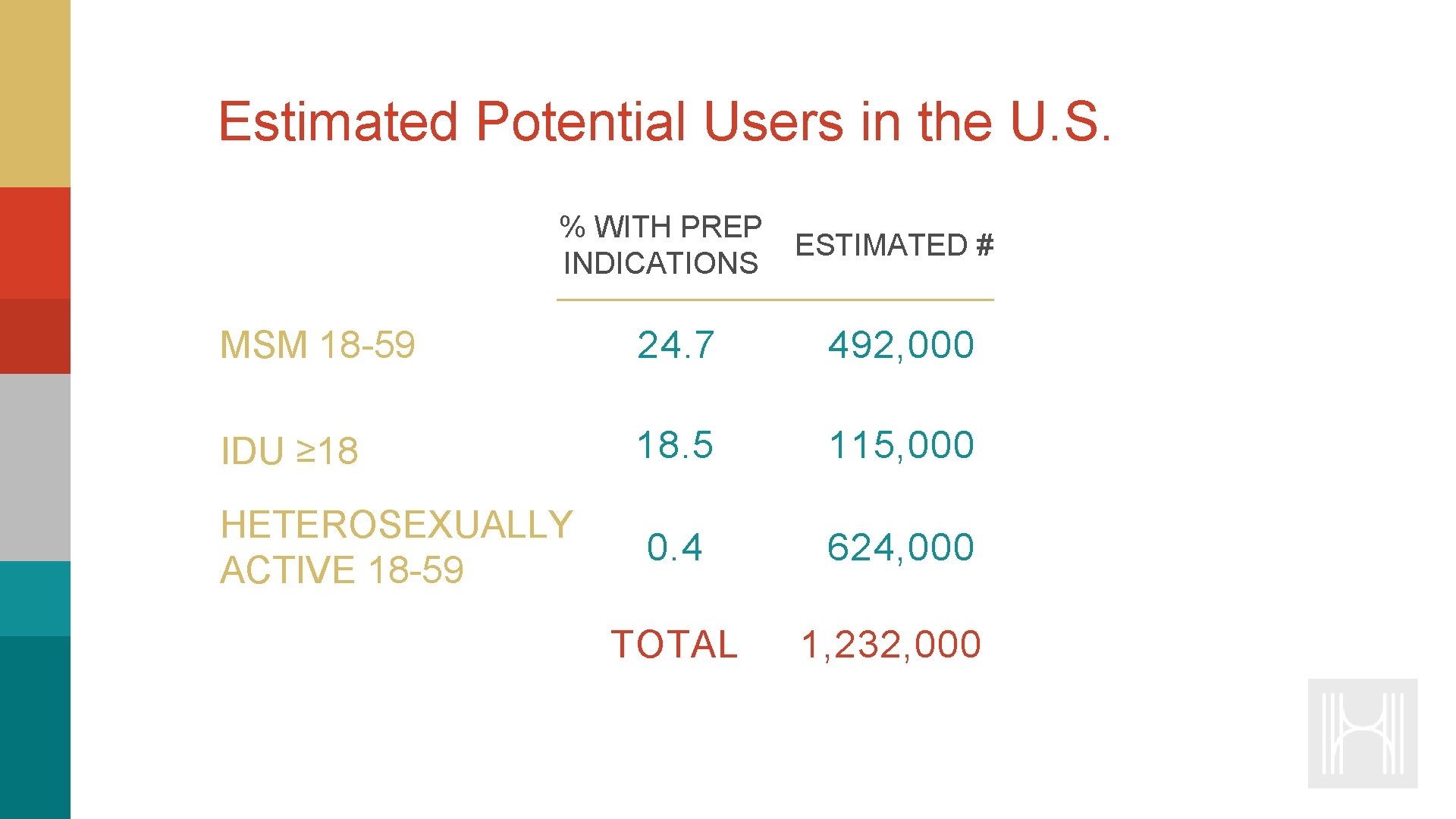
Estimated Potential Users in the U. S. % WITH PREP ESTIMATED # INDICATIONS MSM 18 -59 24. 7 492, 000 IDU ≥ 18 18. 5 115, 000 HETEROSEXUALLY ACTIVE 18 -59 0. 4 624, 000 TOTAL 1, 232, 000

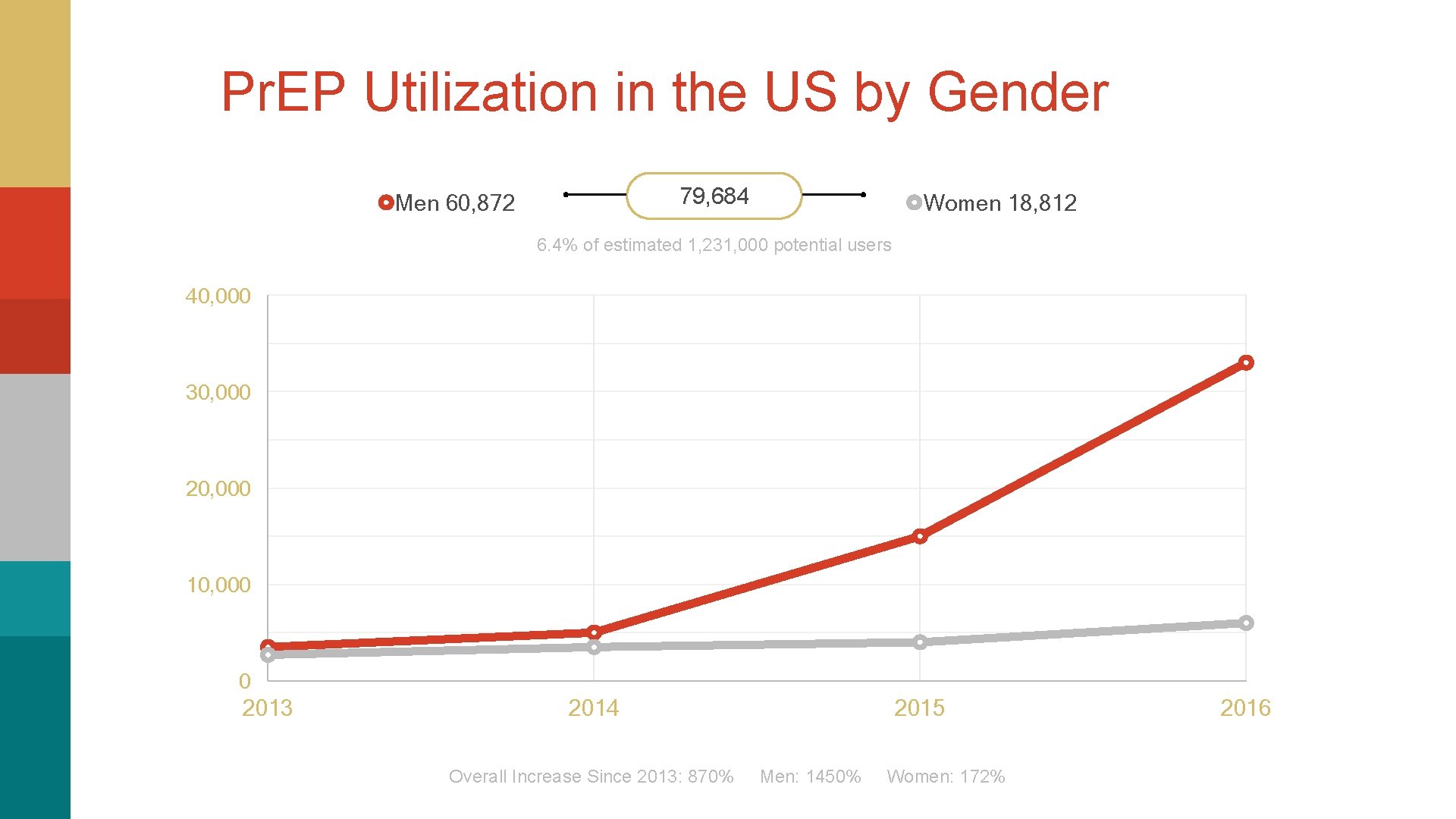
Pr. EP Utilization in the US by Gender 79, 684 Men 60, 872 Women 18, 812 6. 4% of estimated 1, 231, 000 potential users 40, 000 30, 000 20, 000 10, 000 0 2013 2014 2015 Overall Increase Since 2013: 870% Men: 1450% Women: 172% 2016

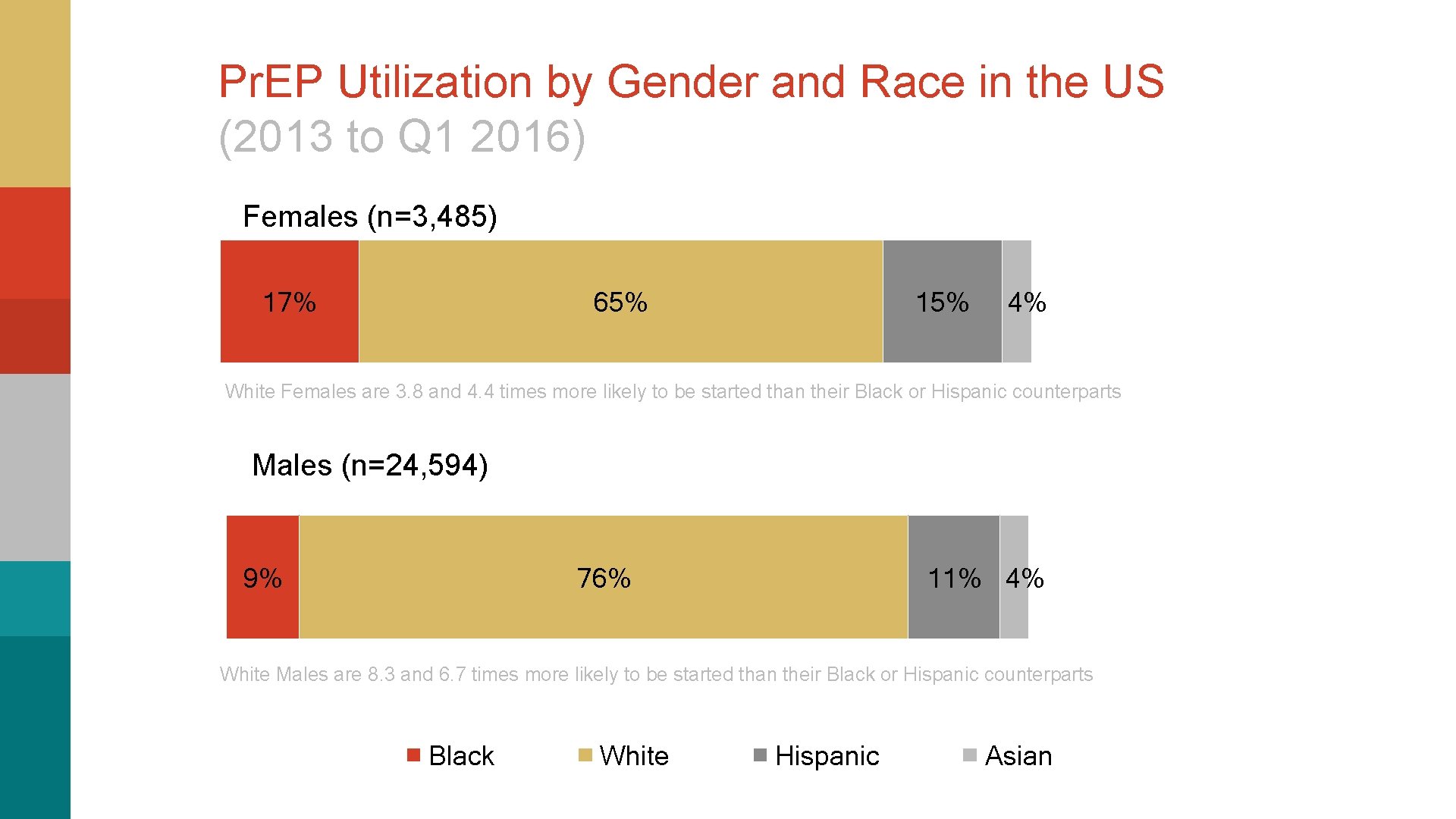
Pr. EP Utilization by Gender and Race in the US (2013 to Q 1 2016) Females (n=3, 485) 17% 65% 15% 4% White Females are 3. 8 and 4. 4 times more likely to be started than their Black or Hispanic counterparts Males (n=24, 594) 9% 76% 11% 4% White Males are 8. 3 and 6. 7 times more likely to be started than their Black or Hispanic counterparts Black White Hispanic Asian

Pr. EP Utilization and HIV Incidence by State HIV Incidence 2015 Starting Pr. EP 2012 -2015

San Francisco Experience: 31% Eligible Using Pr. EP Eligibility and Pr. EP Use in SF HIV-negative persons at substantial risk of HIV acquisition ‣ MSM ‣ >2 nc. AS (non-condom Anal Sex) partners (n=12, 589) ‣ Female partners of HIV- positive MSM (n=653) ‣ Transgender women (n=522) 16, 000 16, 089 14, 000 Number of People ‣ No nc. AS and STI (n=2325) 18, 000 Number of People 12, 000 31% Eligible Using Pr. EP 10, 000 8, 000 6, 000 5, 059 4, 000 2, 000 0 Estimated Total Eligible Reporting Pr. EP Use

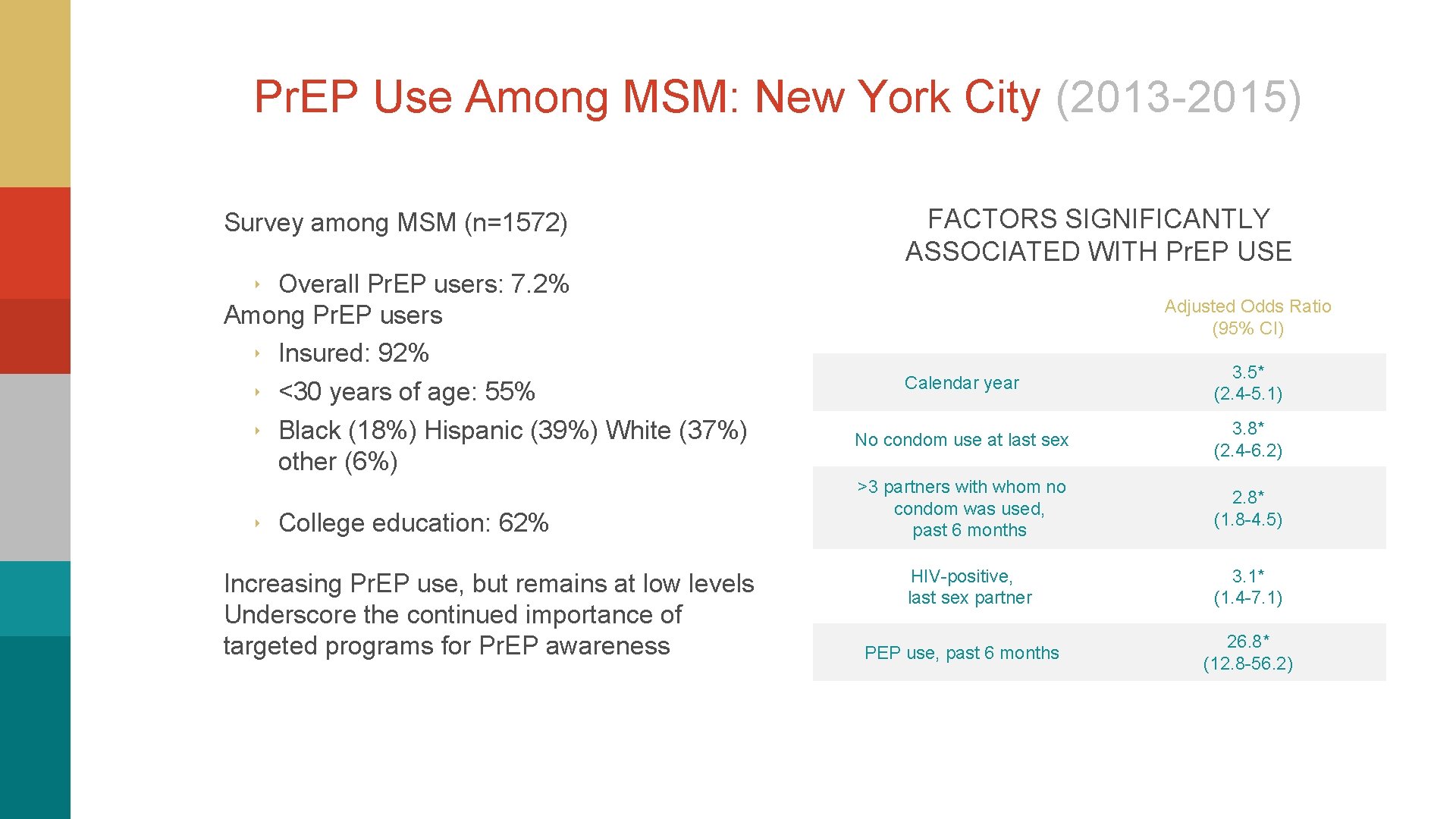
Pr. EP Use Among MSM: New York City (2013 -2015) Survey among MSM (n=1572) FACTORS SIGNIFICANTLY ASSOCIATED WITH Pr. EP USE ‣ Overall Pr. EP users: 7. 2% Among Pr. EP users ‣ Insured: 92% ‣ <30 years of age: 55% ‣ Black (18%) Hispanic (39%) White (37%) other (6%) ‣ College education: 62% Increasing Pr. EP use, but remains at low levels Underscore the continued importance of targeted programs for Pr. EP awareness Adjusted Odds Ratio (95% CI) Calendar year 3. 5* (2. 4 -5. 1) No condom use at last sex 3. 8* (2. 4 -6. 2) >3 partners with whom no condom was used, past 6 months 2. 8* (1. 8 -4. 5) HIV-positive, last sex partner 3. 1* (1. 4 -7. 1) PEP use, past 6 months 26. 8* (12. 8 -56. 2)

Pop-Up Questions #3 What would you share with Jaquis to build his confidence in Pr. EP? (check all that apply) A. Pr. EP is approved by the FDA B. Studies have shown that Pr. EP provides a 92 -100% prevention for HIV, especially when taken 4 to 6 days a week. C. Pr. EP has been studied and found effective in patients like him (young and Black men who have sex with men) D. There almost no cases of study participants discontinuing Pr. EP due to side-effects E. Adherence is of the utmost importance for Pr. EP to be effective F. Additional options for Pr. EP in development

The Pr. EP Pipeline

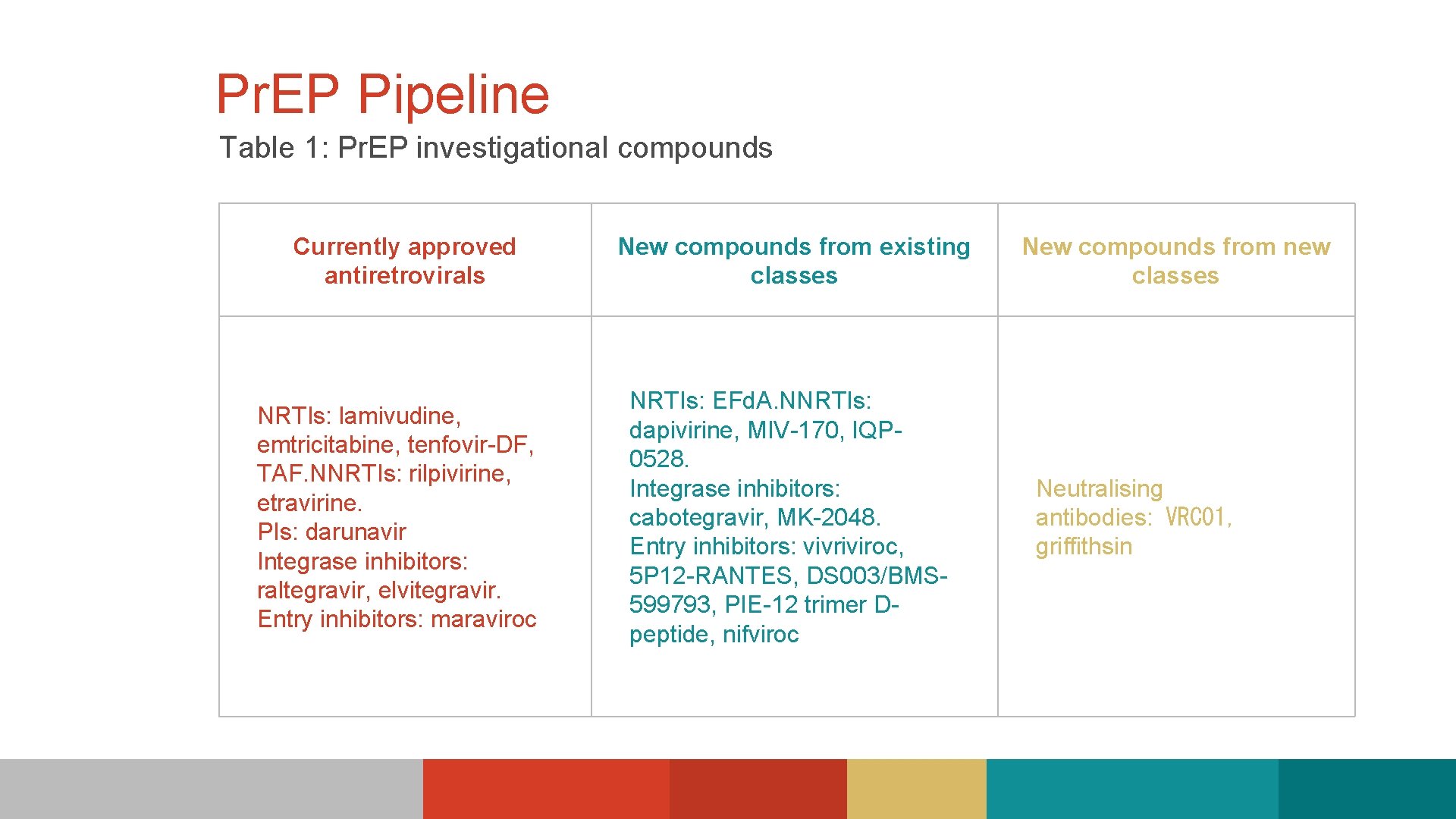
Pr. EP Pipeline Table 1: Pr. EP investigational compounds Currently approved antiretrovirals New compounds from existing classes NRTIs: lamivudine, emtricitabine, tenfovir-DF, TAF. NNRTIs: rilpivirine, etravirine. PIs: darunavir Integrase inhibitors: raltegravir, elvitegravir. Entry inhibitors: maraviroc NRTIs: EFd. A. NNRTIs: dapivirine, MIV-170, IQP 0528. Integrase inhibitors: cabotegravir, MK-2048. Entry inhibitors: vivriviroc, 5 P 12 -RANTES, DS 003/BMS 599793, PIE-12 trimer Dpeptide, nifviroc New compounds from new classes Neutralising antibodies: VRC 01, griffithsin

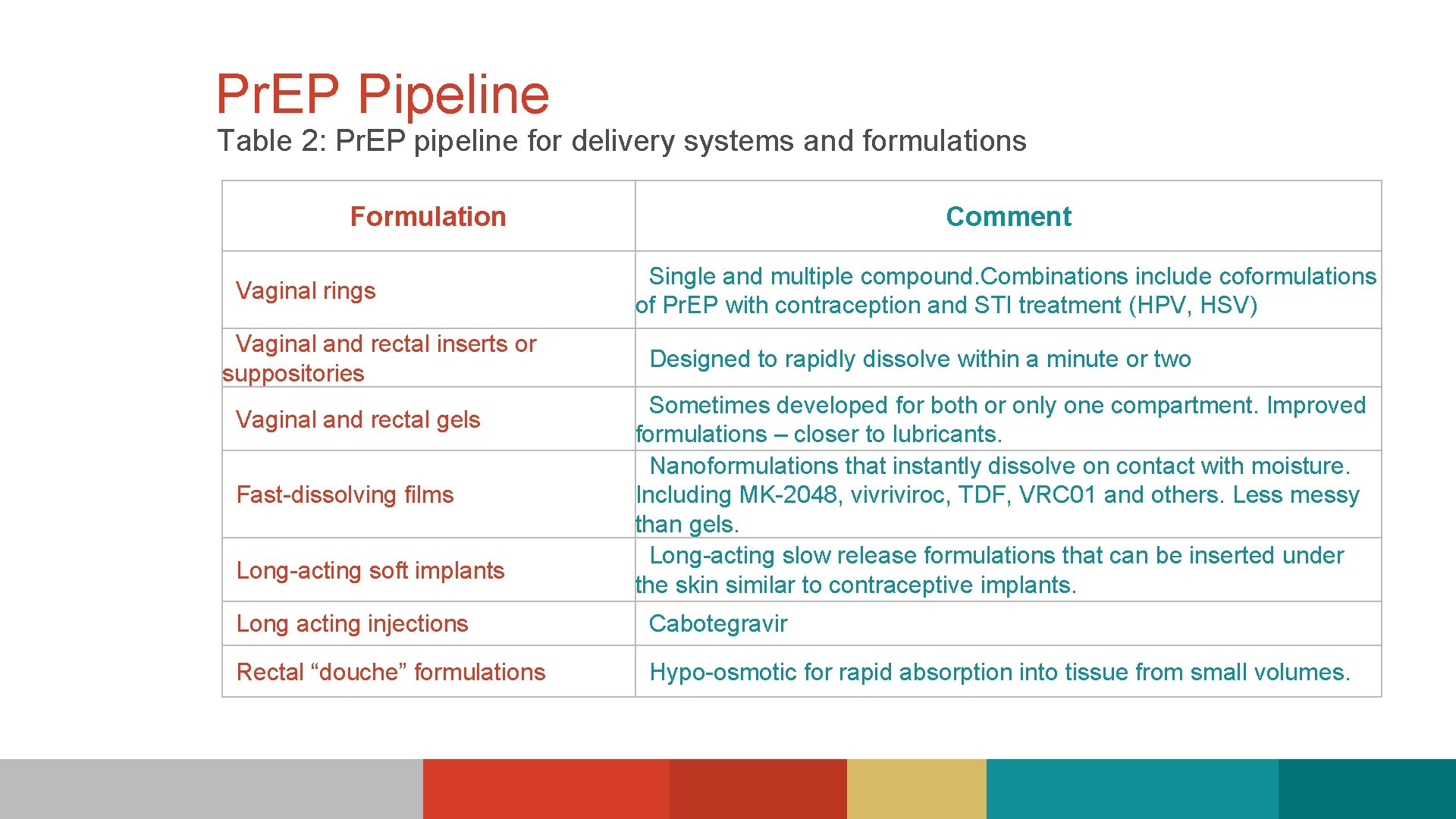
Pr. EP Pipeline Table 2: Pr. EP pipeline for delivery systems and formulations Formulation Vaginal rings Vaginal and rectal inserts or suppositories Vaginal and rectal gels Fast-dissolving films Long-acting soft implants Comment Single and multiple compound. Combinations include coformulations of Pr. EP with contraception and STI treatment (HPV, HSV) Designed to rapidly dissolve within a minute or two Sometimes developed for both or only one compartment. Improved formulations – closer to lubricants. Nanoformulations that instantly dissolve on contact with moisture. Including MK-2048, vivriviroc, TDF, VRC 01 and others. Less messy than gels. Long-acting slow release formulations that can be inserted under the skin similar to contraceptive implants. Long acting injections Cabotegravir Rectal “douche” formulations Hypo-osmotic for rapid absorption into tissue from small volumes.

Clinician Resources ‣ CDC. gov ‣ Clinician Consultation Center NCCC. ucsf. edu ‣ HIVGuidelines. org ‣ US Public Health Service “Preexposure Prophylaxis for the Prevention of HIV Infection in the United States – 2014, A Clinical Practice Guideline” https: //www. cdc. gov/hiv/pdf/prepguidelines 2014. pdf

References ‣ "HIV Incidence: Estimated Annual Infections in the U. S. , 2008 -2014 Overall and by Transmission Route. " NCHHSTP Newsroom. Centers for Disease Control and Prevention, Feb. 2017. Web. 10 Mar. 2017. <https: //www. cdc. gov/nchhstp/newsroom/docs/factsheets/hiv-incidence-fact-sheet_508. pdf>. ‣ Centers for Disease Control and Prevention. HIV Surveillance Report, 2014. Vol. 26. Atlanta: U. S. Department of Health and Human Services, Centers for Disease Control and Prevention; 2015: 1 -123. http: //www. cdc. gov/hiv/library/reports/surveillance/. ‣ H. Irene Hall, Ph. D, MPH; Qian An, MS; Angela B. Hutchinson, Ph. D, MPH; Stephanie Sansom, Ph. D, MPP, ‣ MPH. Estimating the Lifetime Risk of a Diagnosis of the HIV Infection in 33 States, 2004 -2005. J Acquir Immune Defic Syndr. 2008; 49(3): 294 -297. ‣ Hess K, Hu X, Lansky A, et al. Estimating the lifetime risk of a diagnosis of HIV infection in the United States. Program and abstracts from the 23 rd Conference on Retroviruses and Opportunistic Infection; February 22 -25, 2016; Boston, MA. Abstract 52. ‣ Comparisons of disparities and risks of HIV infection in Black and other MSM in Canada, UK, and USA: a meta- analysis. Millett GA 1, Peterson JL, Flores SA, Hart TA, Jeffries WL 4 th, Wilson PA, Rourke SB, Heilig CM, Elford J, Fenton KA, Remis RS. ‣ "Preexposure Prophylaxis For The Prevention of HIV Infection In The United States - 2014, A Clinical Practice Guideline. " Centers for Disease Control and Prevention. US Public Health Service , 2014. Web. 10 Mar. 2017. https: //www. cdc. gov/hiv/pdf/prepguidelines 2014. pdf

References ‣ Grant, R. M. , Lama, J. R. , Anderson, P. L. , Mc. Mahan, V. , Liu, A. Y. , Vargas, L. , & Montoya-Herrera, O. (2010). Preexposurechemoprophylaxis for HIV prevention in men who have sex with men. New England Journal of Medicine, 363(27), 2587 -2599. ‣ Grant RM, Anderson PL, Mc. Mahan V, et al. Uptake of pre-exposure prophylaxis, sexual practices, and HIV incidence in men and transgender women who have sex with men: a cohort study. Lancet Infect Dis. 2014; 14: 820 -829. ‣ Hosek S, Rudy B, Landovitz R, et al. An HIV pre-exposure prophylaxis (prep) demonstration project and safety study for young MSM. J Acquir Immune Defic Syndr. 2016; Sep 13. [Epub ahead of print]. ‣ Hosek S, Landovitz R, Rudy B, et al. An HIV pre-exposure prophylaxis (Pr. EP) demonstration project and safety study for adolescent MSM ages 15 -17 in the United States (ATN 113). JAIDS. 2016; 19(suppl 5): 30. Abstract TUAX 0104 LB. ‣ Wheeler DP, Fields S, Nelson LE, et al. HPTN 073: Pr. EP uptake and use by black men who have sex with men in 3 US cities. Program and abstracts from the 23 rd Conference on Retroviruses and Opportunistic Infection; February 22 -25, 2016; Boston, MA. Abstract 883 LB. ‣ Mc. Callister S, Magnuson D, Guzman R, et al. - HIV-1 seroconversion across 17 international demonstration projects with pre-exposure prophylaxis (PREP) with oral emtricitabine/tenofovir disoproxil fumarate (FTC/TDF). Program and abstracts of American Society of Microbiology Microbe 2016; June 16 -20, 2016; Boston, MA.

References ‣ Mc. Cormack S, Dunn D. Pre-exposure prophylaxis to prevent the acquisition of HIV-1 infection (PROUD): effectiveness results from the pilot phase of a pragmatic open-label randomised trial. Lancet. 2016; 387: 53 -60. ‣ Volk JE, Marcus JL, Phengrasamy T, et al. No new HIV infections with increasing use of HIV preexposure prophylaxis in a clinical practice setting. Clin Infect Dis. 2015; 61: 1601 -1603. ‣ Smith DK, Van Handel M, Wolitski RJ, et al. Vital signs: estimated percentages and numbers of adults with indications for preexposure prophylaxis to prevent HIV acquisition - United States, 2015. MMWR Morb Mortal Wkly Rep. 2015; 64: 1291 -1295. ‣ Bush S, Rawlings K, Magnuson, et al. Utilization of emtricitabine/tenofovir (FTC/TDF) for HIV pre-exposure prophylaxis in the United States by gender (2013 -1 Q 2016). J Int AIDS Soc. 2016; 19(suppl 7): 14 -15. Abstract O 314. ‣ "Diagnoses of HIV Infection in the United States and Dependent Areas, 2015. " HIV Surveillance Reports. Centers for Disease Control and Prevention, Nov. 2016. Web. 13 Mar. 2017. <https: //www. cdc. gov/hiv/pdf/library/reports/surveillance/cdc-hiv-surveillance-report-2015 -vol-27. pdf>. ‣ Mera R, Mc. Callister S, Palmer B, et al. Truvada (TVD) for HIV pre-exposure prophylaxis (Pr. EP) utilization in the United States (2013 -2015). JAIDS. 2016; 19(suppl 5): 30. Abstract TUAX 0105 LB.

References ‣ Grant RM, Liu A, Hecht J, et al. Scale-up of preexposure prophylaxis in San Francisco to impact HIV incidence. Program and abstracts from the 22 nd Conference on Retroviruses and Opportunistic Infection; February 23 -26, 2015; Seattle, WA. Abstract 25. ‣ Scanlin KK, Salcuni PM, Edelstein ZR, et al. Increasing prep use among men who have sex with men, New York City, 2013 -2015. Program and abstracts from the 23 rd Conference on Retroviruses and Opportunistic Infection; February 22 -25, 2016; Boston, MA. Abstract 888. ‣ Acute Infection with a Wild-Type HIV-1 Virus in Pr. EP users with High TDF Levels. ‣ Elske Hoornenborg 1, Godelieve de Bree 2, on behalf of the Amsterdam Pr. EP Project in the HIV Transmission Elimination AMsterdam (H-TEAM) Initiative; 1 Department of Infectious Diseases, Public Health Service of Amsterdam, the Netherlands; 2 Academic Medical Center, Amsterdam Zuidoost, the Netherlands ehoornenborg@ggd. amsterdam. nl

 Dr theo hodge
Dr theo hodge Pcma
Pcma Morehouse medical associates
Morehouse medical associates Wilmington medical associates
Wilmington medical associates Mount auburn medical associates
Mount auburn medical associates Medical faculty in novi sad dean
Medical faculty in novi sad dean Ascaris lumbricoides ova
Ascaris lumbricoides ova Chronic meningitis
Chronic meningitis Escala de hodge
Escala de hodge Penurunan kepala hodge 1 sampai 4
Penurunan kepala hodge 1 sampai 4 Hodge persalinan
Hodge persalinan Planos de hodge
Planos de hodge Drummer hodge summary
Drummer hodge summary Hodge conjecture
Hodge conjecture Planos de hodge
Planos de hodge Literary devices jeopardy
Literary devices jeopardy Sara hodge
Sara hodge What is gross working capital
What is gross working capital Source of capital reserve
Source of capital reserve Multinational capital structure
Multinational capital structure Difference between capital reserve and reserve capital
Difference between capital reserve and reserve capital Regulatory capital vs economic capital
Regulatory capital vs economic capital Regulatory capital vs economic capital
Regulatory capital vs economic capital Constant capital and variable capital
Constant capital and variable capital Multinational cost of capital and capital structure
Multinational cost of capital and capital structure Capital allocation line vs capital market line
Capital allocation line vs capital market line Umd capital region health
Umd capital region health Medical excellence capital
Medical excellence capital Theo thomassen
Theo thomassen Theo góc độ địa lý mạng máy tính
Theo góc độ địa lý mạng máy tính Théo jansen
Théo jansen Theo marzials
Theo marzials Theo jacobi
Theo jacobi Theo bakker huisarts
Theo bakker huisarts Kontak theo de jager
Kontak theo de jager Theo de jager skrywer
Theo de jager skrywer Teor nbapq qtheo
Teor nbapq qtheo Theo timmers
Theo timmers Theo jacobi
Theo jacobi đặt tên con theo 3 gốc rễ
đặt tên con theo 3 gốc rễ Cloud security challenges
Cloud security challenges Theo witte
Theo witte Theo eicher
Theo eicher H
H Theo fens
Theo fens User interface analysis
User interface analysis Where was theo de jager born
Where was theo de jager born Theo schlossnagle
Theo schlossnagle Chuyển số thực sang ieee 754
Chuyển số thực sang ieee 754 Bài trích phúc âm theo thánh gioan
Bài trích phúc âm theo thánh gioan Randomisation en cluster
Randomisation en cluster Bài trích phúc âm theo thánh gioan
Bài trích phúc âm theo thánh gioan Bài trích phúc âm theo thánh gioan
Bài trích phúc âm theo thánh gioan Schönebecker aue wanderweg
Schönebecker aue wanderweg Theo greek god
Theo greek god Theo chocolate makes a sweet difference
Theo chocolate makes a sweet difference Theo ion
Theo ion Theo benson
Theo benson