Energy Levels The electron cloud is made up










- Slides: 24


Energy Levels • The electron cloud is made up of different energy levels. • Each energy level can hold only a certain number of electrons: – 1 st energy level can hold only 2 electrons – 2 nd energy level can hold 8 electrons – 3 rd energy level can hold up to 18 electrons

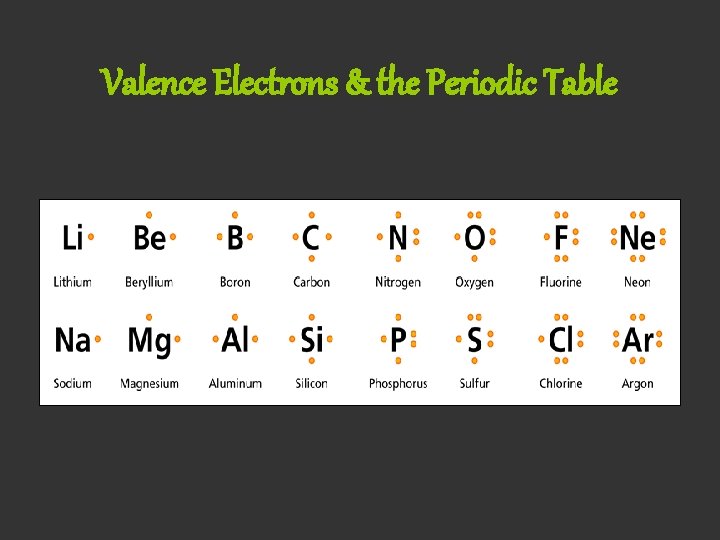
Valence Electrons • The electrons in the outermost energy level of an atom are called valence electrons. • The number of valence electrons in an atom determines whether or not the atom will form chemical bonds with other atoms.

Stable Atoms • Atoms of most elements are more stable when they have eight valence electrons. • Atoms usually react in a way that makes each atom more stable: – either the number of valence electrons increases to 8 (or 2 in the case of hydrogen) – or, the atom gives up loosely held valence electrons

Chemical Bonds • Chemical bonds form when the electrons of one atom interact with the electrons in another atom. • When atoms bond, electrons may be transferred from one atom to another, or they may be shared between atoms.

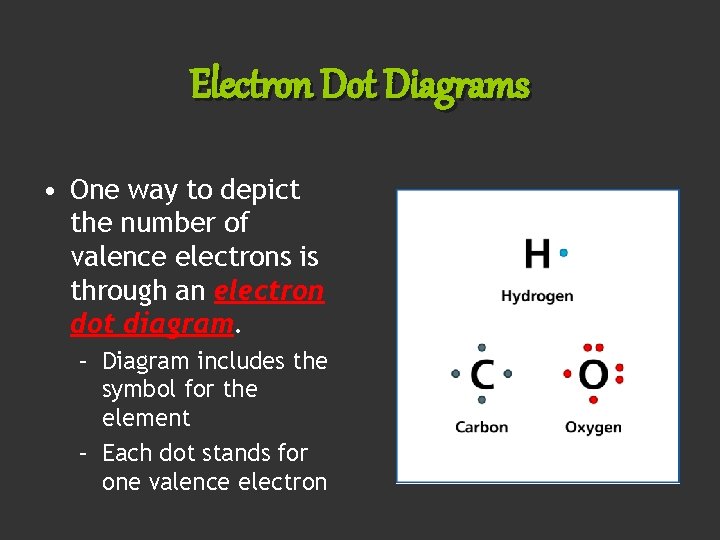
Electron Dot Diagrams • One way to depict the number of valence electrons is through an electron dot diagram. – Diagram includes the symbol for the element – Each dot stands for one valence electron


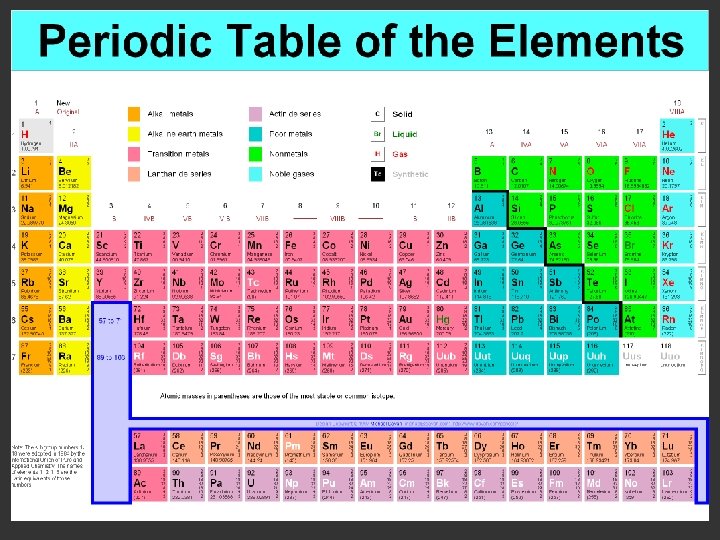
Bonding & the Periodic Table • Periods: – from left to right the atomic number increases – as the atomic number increases, the number of electrons also increases • Groups: – from top to bottom, elements in a group always have the same number of valence electrons – the elements within a group have similar properties because they all have the same number of valence electrons

Valence Electrons & the Periodic Table

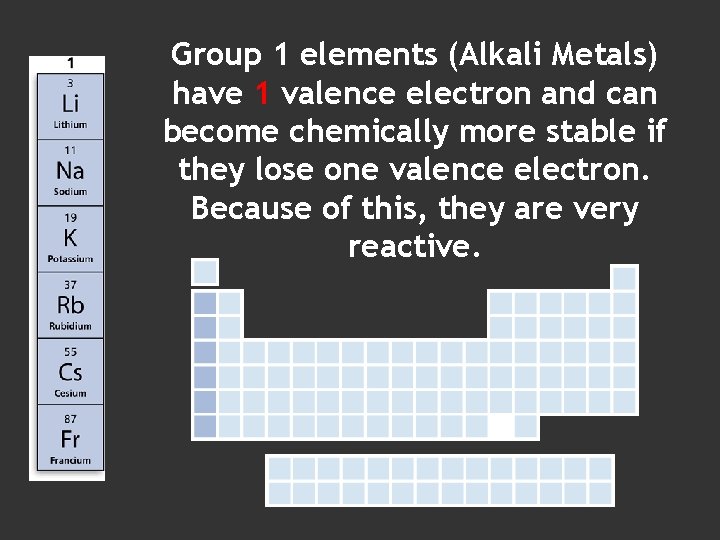
Group 1 elements (Alkali Metals) have 1 valence electron and can become chemically more stable if they lose one valence electron. Because of this, they are very reactive.

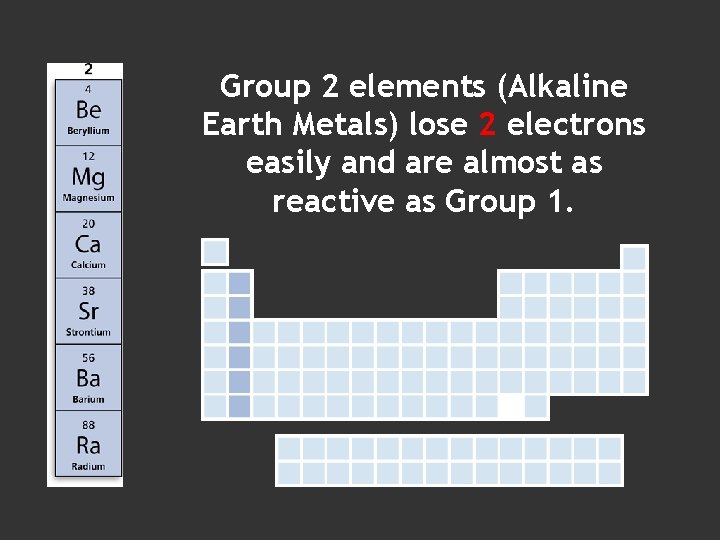
Group 2 elements (Alkaline Earth Metals) lose 2 electrons easily and are almost as reactive as Group 1.

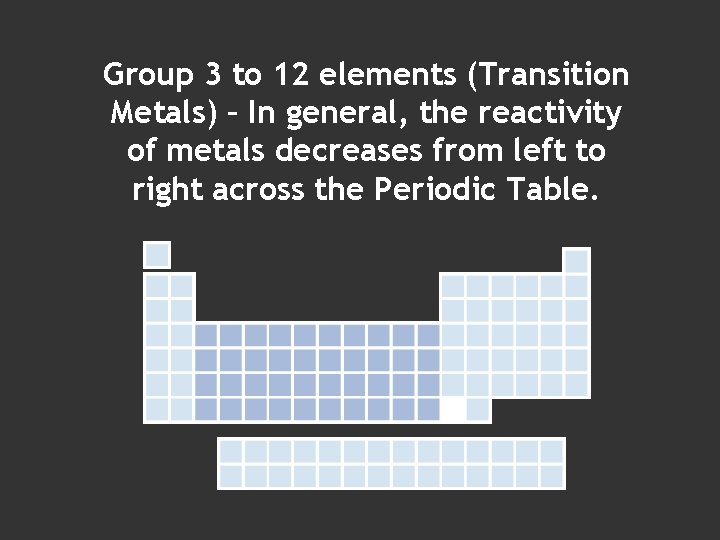
Group 3 to 12 elements (Transition Metals) – In general, the reactivity of metals decreases from left to right across the Periodic Table.

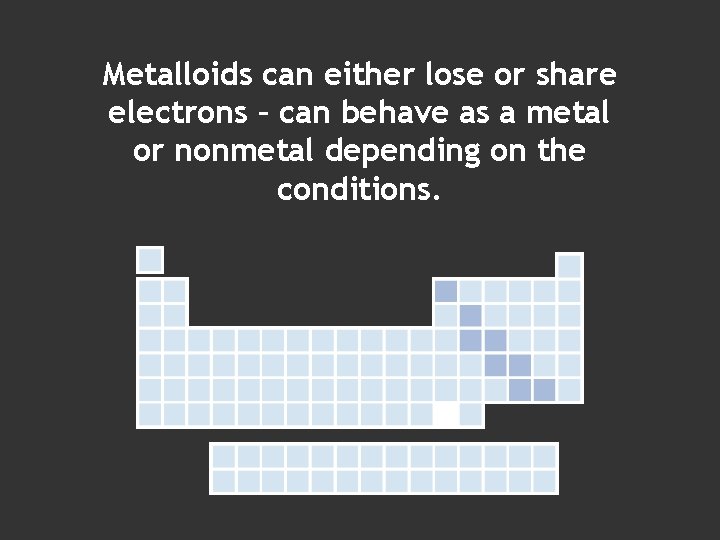
Metalloids can either lose or share electrons – can behave as a metal or nonmetal depending on the conditions.

Nonmetals become more stable when they gain or share electrons – all nonmetals have 4 or more valence electrons. They combine with metals by gaining electrons. They combine with other nonmetals by sharing electrons.

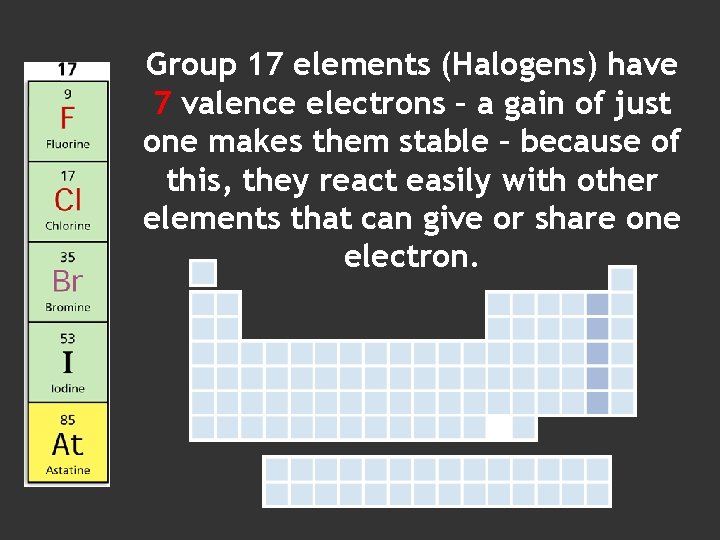
Group 17 elements (Halogens) have 7 valence electrons – a gain of just one makes them stable – because of this, they react easily with other elements that can give or share one electron.

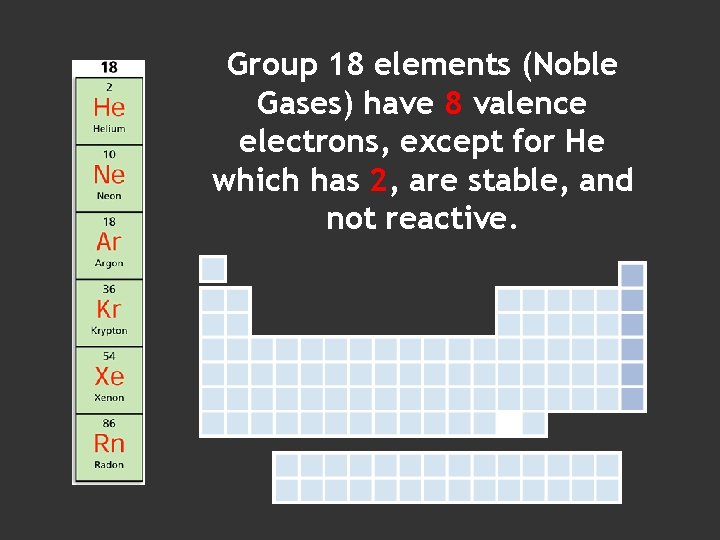
Group 18 elements (Noble Gases) have 8 valence electrons, except for He which has 2, are stable, and not reactive.


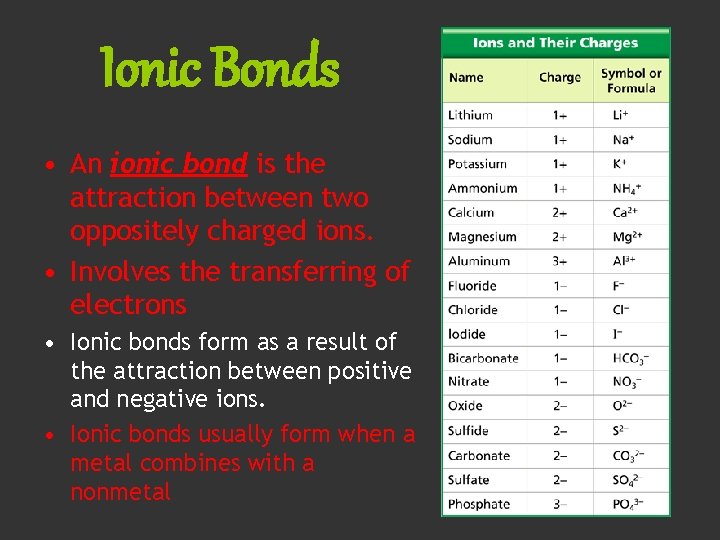
Ionic Bonds • An ionic bond is the attraction between two oppositely charged ions. • Involves the transferring of electrons • Ionic bonds form as a result of the attraction between positive and negative ions. • Ionic bonds usually form when a metal combines with a nonmetal


Covalent Bonds • The chemical bond formed when two atoms share electrons is called a covalent bond. • The force that holds atoms together in a covalent bond is the attraction of each atom’s nucleus for the shared pair of electrons. – Covalent bonds form between atoms of nonmetals


Atoms of some elements pull more strongly on shared electrons than do atoms of other elements. As a result, the electrons are pulled more toward one atom, causing the bonded atoms to have slight electrical charges.

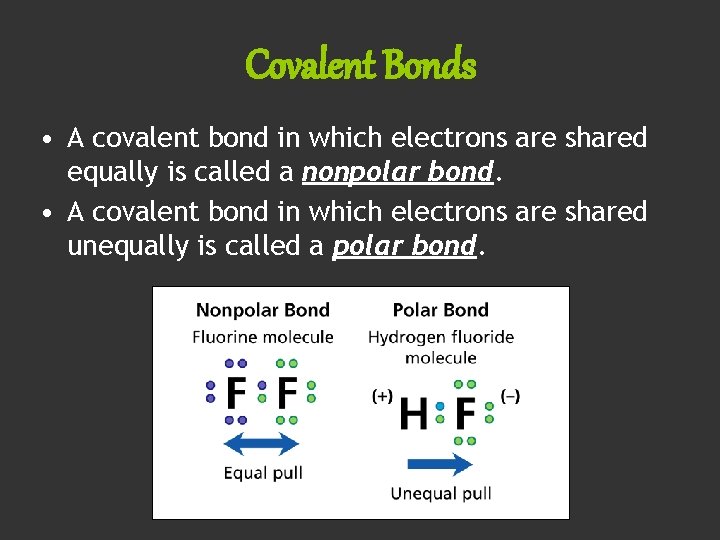
Covalent Bonds • A covalent bond in which electrons are shared equally is called a nonpolar bond. • A covalent bond in which electrons are shared unequally is called a polar bond.

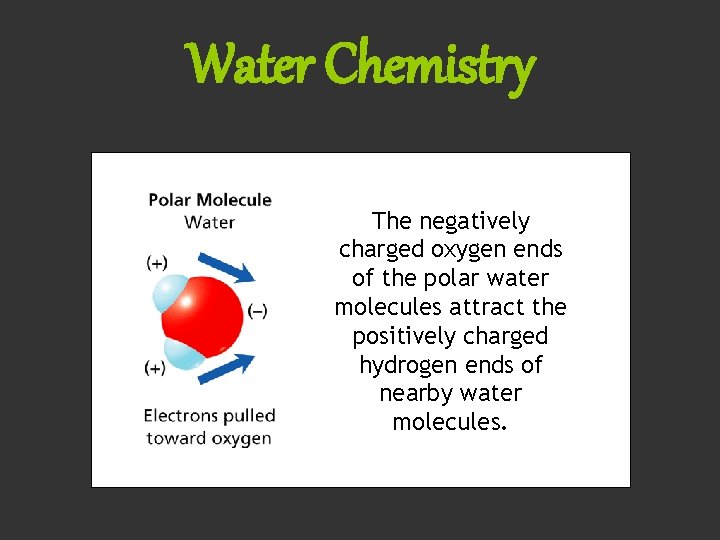
Water Chemistry The negatively charged oxygen ends of the polar water molecules attract the positively charged hydrogen ends of nearby water molecules.