Electron Energy Levels Electrons have different energy levels





- Slides: 13

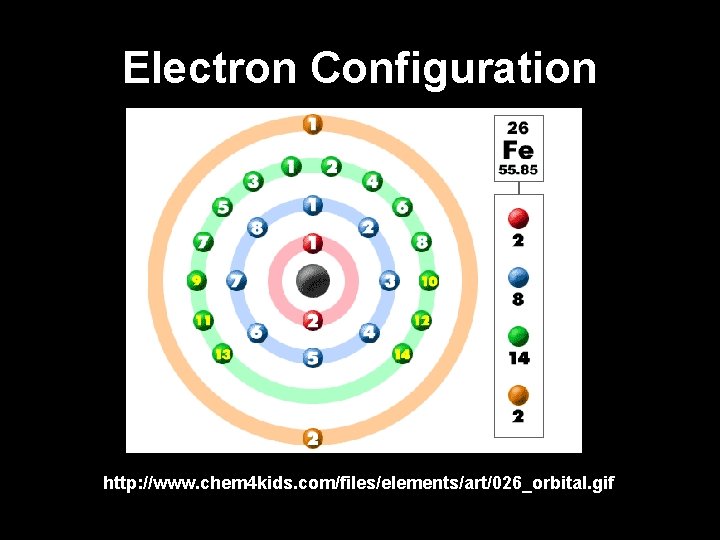
Electron Energy Levels • Electrons have different energy levels – The farther the electron is from the nucleus, the more energy the electron has • Electrons “fill” the energy levels from just outside the nucleus, outward • Each of the seven energy levels have a maximum number of electrons they can hold – Energy level 1 = up to 2 electrons – Energy level 2 = up to 8 electrons – Energy level 3 = up to 18 electrons – Energy level 4 = up to 32 electrons – Etc.

Electron Configuration http: //www. chem 4 kids. com/files/elements/art/026_orbital. gif

Electron Energy Levels • Scientists keep track of the electrons to help predict an element’s behavior • The electron(s) in the highest (outermost) energy level define the chemical properties of the element and participate in chemical reactions (valence electrons) • Elements in the same group (vertical columns of elements in the periodic table with similar properties) have the same number of electrons in their outer energy level

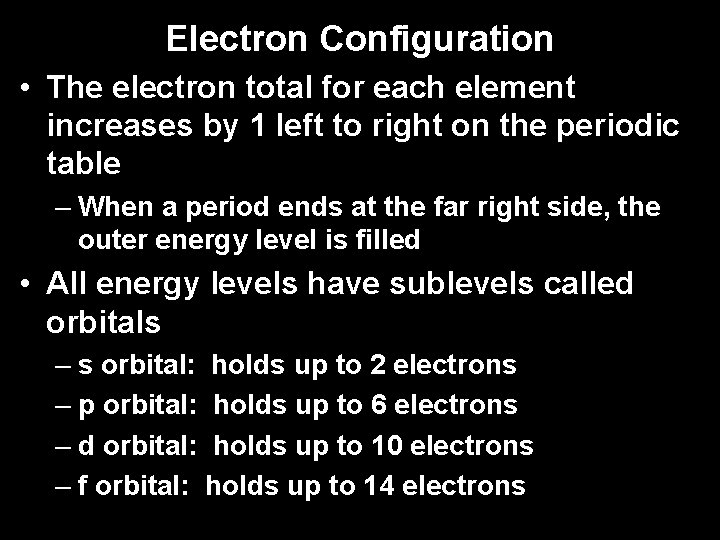
Electron Configuration • The electron total for each element increases by 1 left to right on the periodic table – When a period ends at the far right side, the outer energy level is filled • All energy levels have sublevels called orbitals – s orbital: holds up to 2 electrons – p orbital: holds up to 6 electrons – d orbital: holds up to 10 electrons – f orbital: holds up to 14 electrons

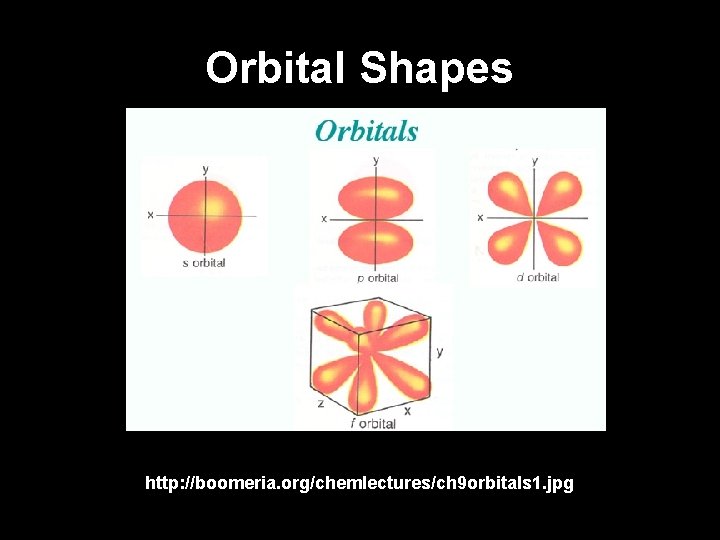
Orbital Shapes http: //boomeria. org/chemlectures/ch 9 orbitals 1. jpg

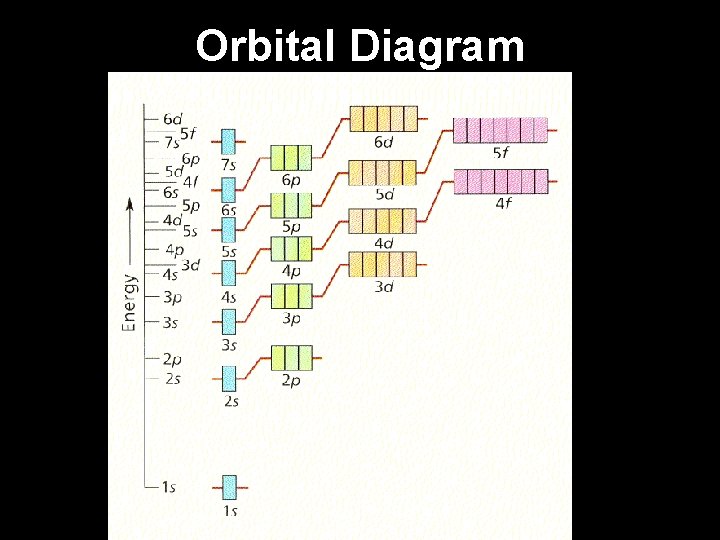
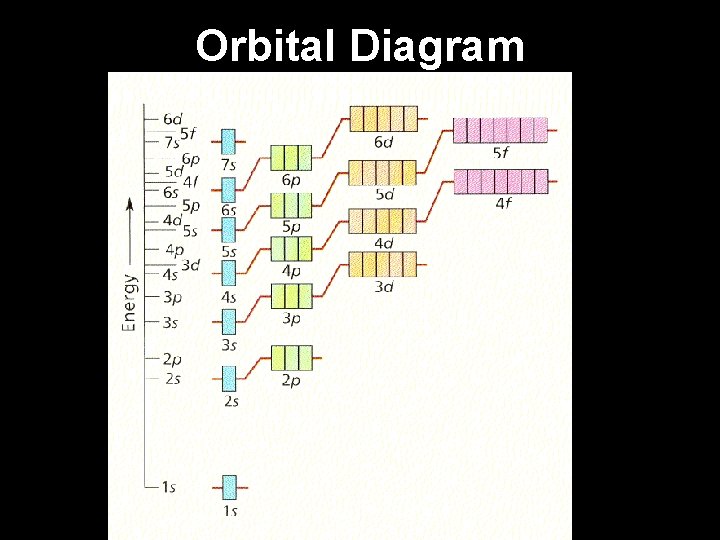
Electron Configuration Steps for determining the electron configuration of an element: 1. Determine the number of electrons the element has. 2. Fill the lowest energy levels first and work your way up from the nucleus. 3. Each orbital “box” gets one up arrow and one down arrow. 4. Up arrows fill first, down arrows last. 5. Write the electron configuration. (energy level number)(orbital) (electrons)

Orbital Diagram

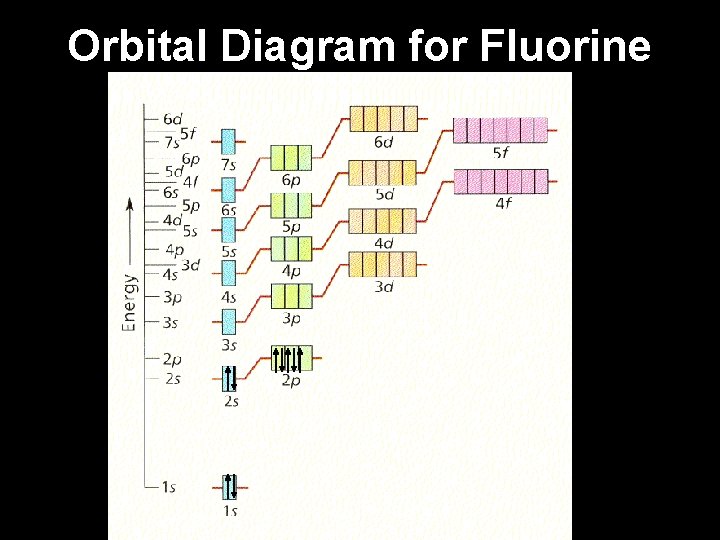
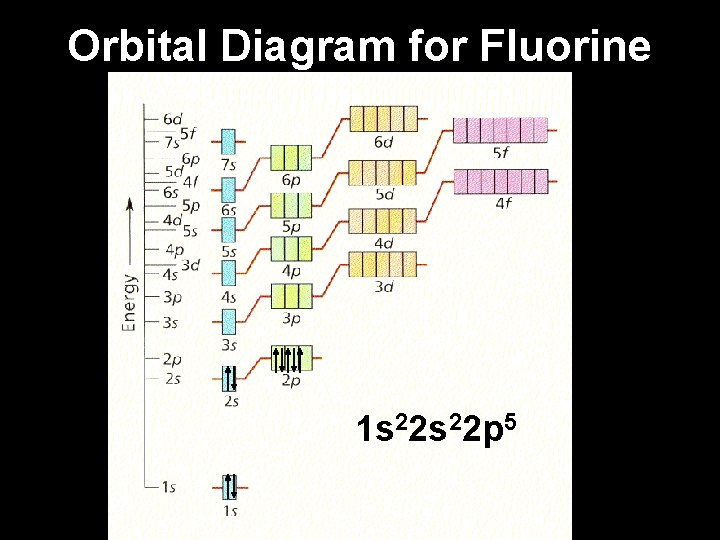
Example: Fluorine 1. Fluorine has 9 electrons. 2. Fill the lowest energy levels first and work your way up from the nucleus. 3. Each orbital “box” gets one up arrow and one down arrow. 4. Up arrows fill first, down arrows last.

Orbital Diagram for Fluorine

Example: Fluorine 1. Fluorine has 9 electrons. 2. Fill the lowest energy levels first and work your way up from the nucleus. 3. Each orbital “box” gets one up arrow and one down arrow. 4. Up arrows fill first, down arrows last. 5. Write the electron configuration.

Orbital Diagram for Fluorine 1 s 22 p 5

Orbital Diagram

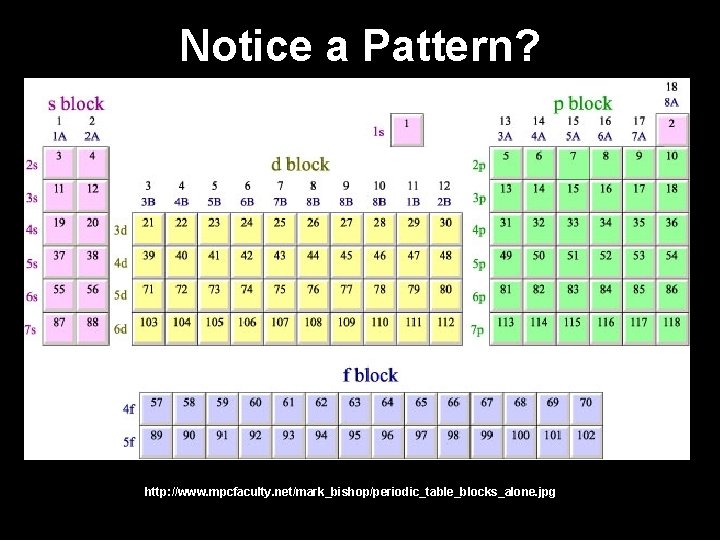
Notice a Pattern? http: //www. mpcfaculty. net/mark_bishop/periodic_table_blocks_alone. jpg