Electron Energy Level Notes Electron Energy Level Notes









- Slides: 29

Electron Energy Level Notes

Electron Energy Level Notes • Electrons do not travel around the nucleus of an atom in orbits • They are found in energy levels at different distances away from the nucleus. (kind of like shells or layers).

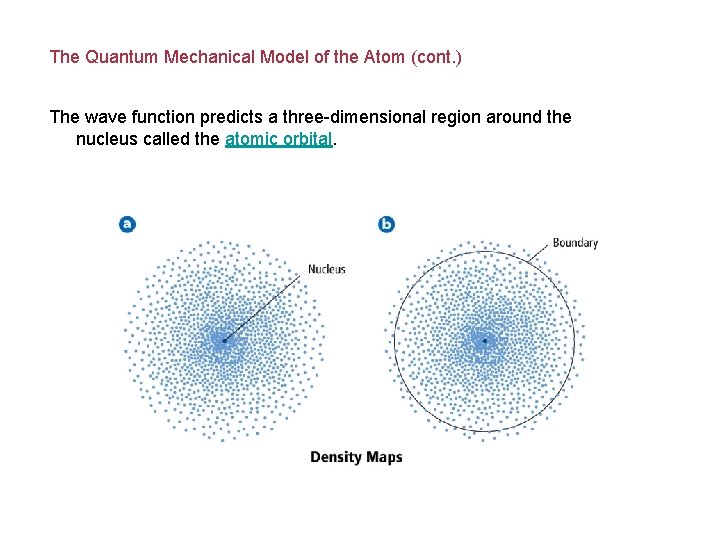
The Quantum Mechanical Model of the Atom (cont. ) The wave function predicts a three-dimensional region around the nucleus called the atomic orbital.

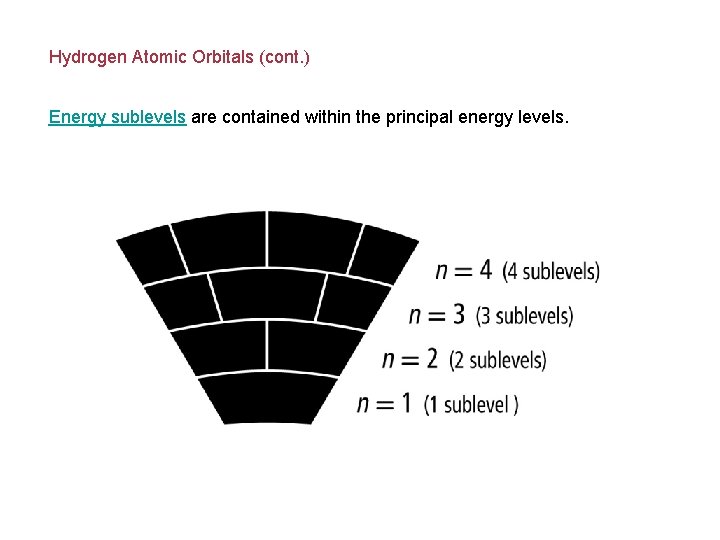
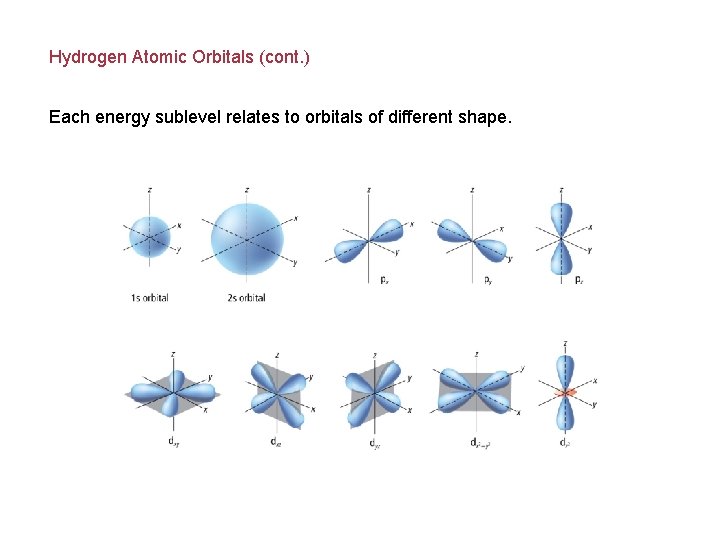
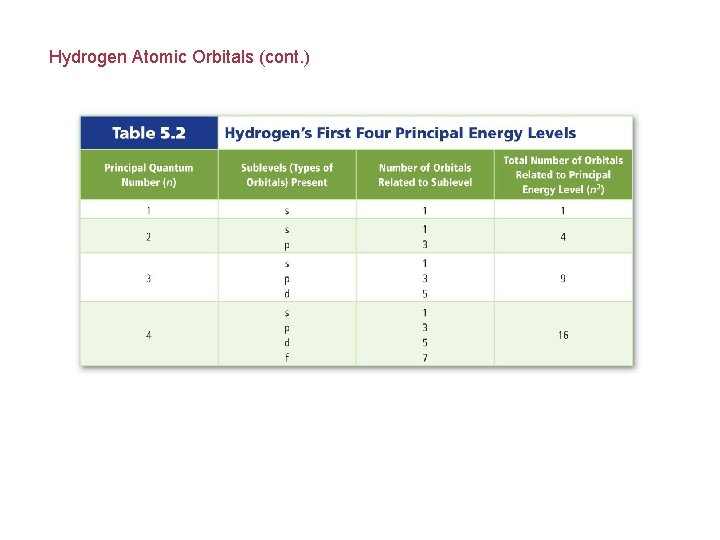
Hydrogen Atomic Orbitals Electrons cannot exist between energy levels (just like the rungs of a ladder). Principal quantum number (n) indicates the relative size and energy of atomic orbitals. n specifies the atom’s major energy levels, called the principal energy levels.

Electron Energy Level Notes • Energy levels are broken up into sublevels: • There at least 4 possible types of sublevels—given labels: s, p, d, or f •

Hydrogen Atomic Orbitals (cont. ) Energy sublevels are contained within the principal energy levels.

Electron Energy Level Notes • Only a certain number of electrons may exist in an energy level, but the number varies. It can be determined by: • # of electrons in level = 2 x (# of energy level)2 • • • Therefore: energy level 1 = 2 (1)2 = 2 energy level 2 = 2 (2)2 = 8 energy level 3 = 18 energy level 4 = 32 etc. . .

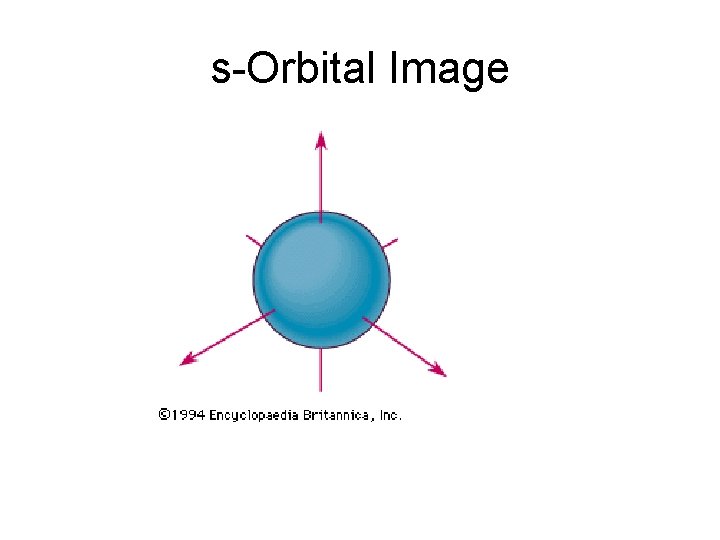
Electron Energy Level Notes • In each energy level, electrons fill sublevels in a certain order • Level 1: • only has one s sublevel (a spherical shape) • 2 electrons may fit in this sublevel--each one has an opposite “spin”, allowing them to take up the same space • Pauli exclusion principle—no more than 2 electrons may be found in the same orbital (“orbital” means a particular location)

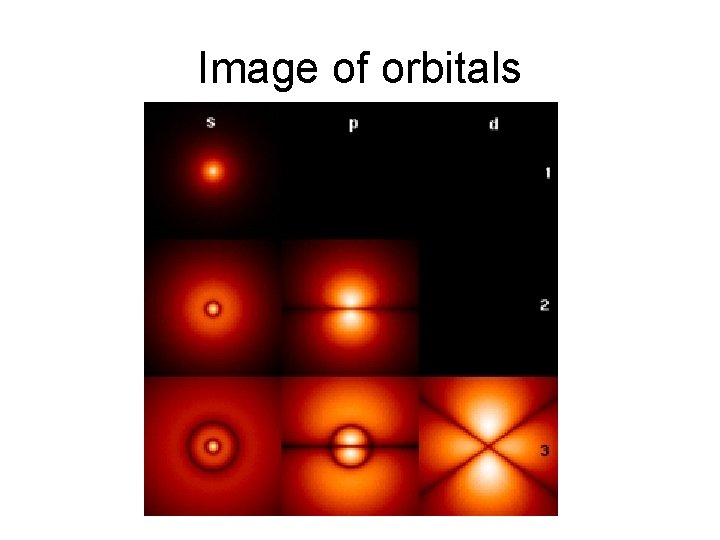
s-Orbital Image

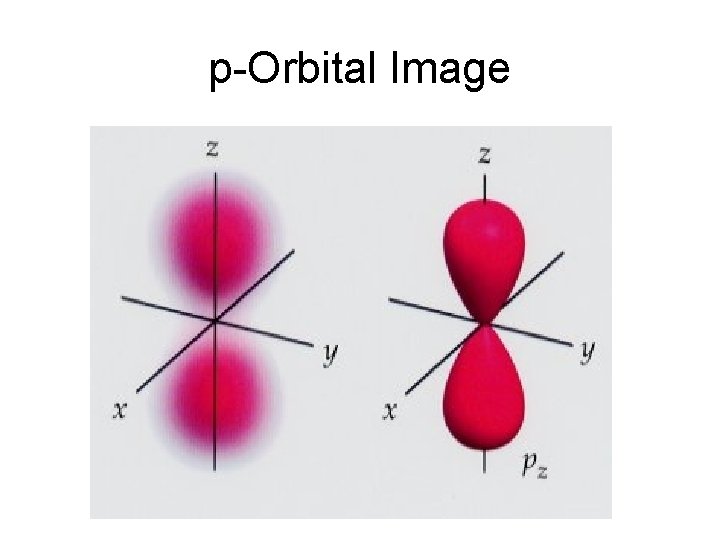
Electron Energy Level Notes • • Level 2: has two sublevels: s and p 2 electrons in s there are 3 different p orbitals, and may hold 2 electrons each— 6 total. (look at shape on p. 154 -imagine how they can fit together) • total of 8 overall in Level 2

p-Orbital Image

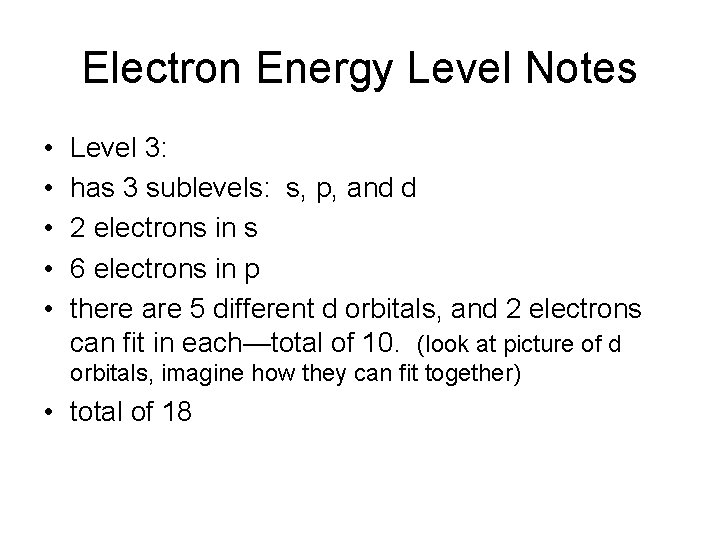
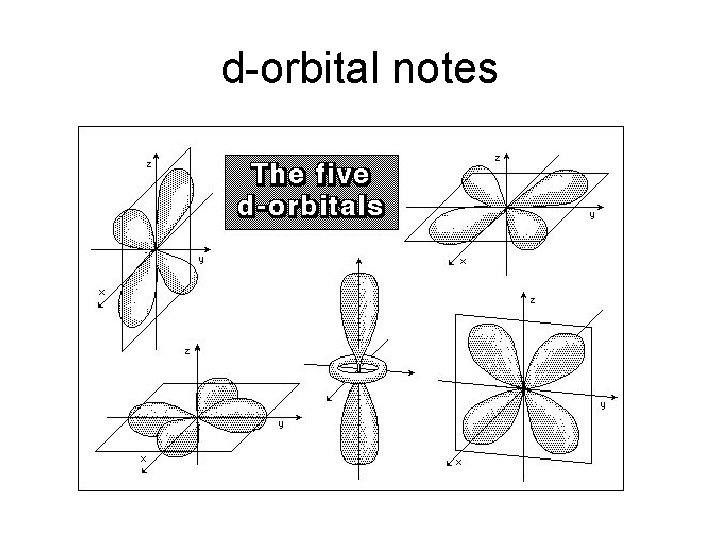
Electron Energy Level Notes • • • Level 3: has 3 sublevels: s, p, and d 2 electrons in s 6 electrons in p there are 5 different d orbitals, and 2 electrons can fit in each—total of 10. (look at picture of d orbitals, imagine how they can fit together) • total of 18

d-orbital notes

Hydrogen Atomic Orbitals (cont. ) Each energy sublevel relates to orbitals of different shape.

Image of orbitals

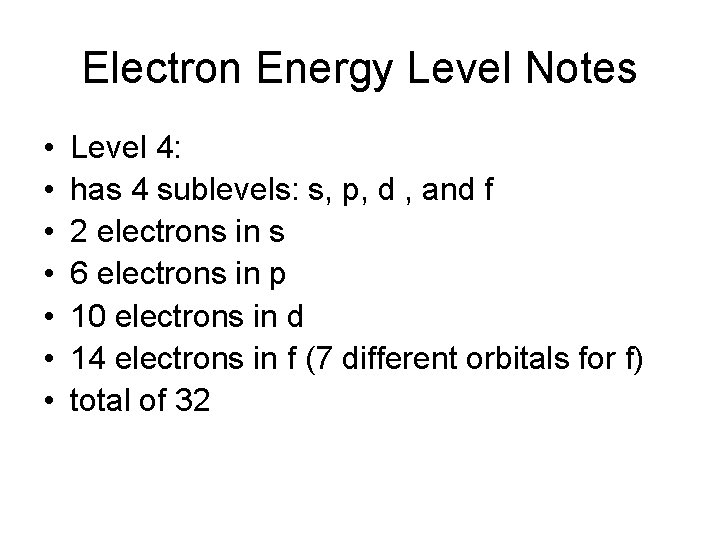
Electron Energy Level Notes • • Level 4: has 4 sublevels: s, p, d , and f 2 electrons in s 6 electrons in p 10 electrons in d 14 electrons in f (7 different orbitals for f) total of 32

Hydrogen Atomic Orbitals (cont. )

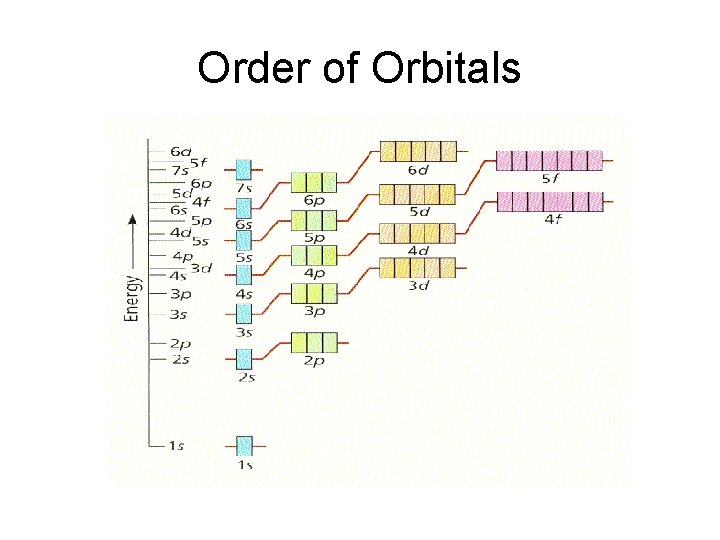
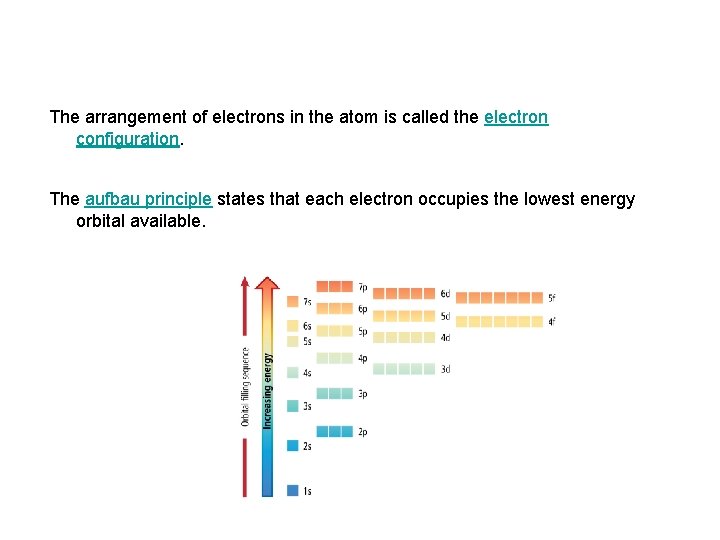
Electron Energy Level Notes • The order that electrons fill up orbitals does not follow the logical order of all 1’s, then all 2’s, then all 3’s, etc. • They follow the order found on pg. 156.

Order of Orbitals

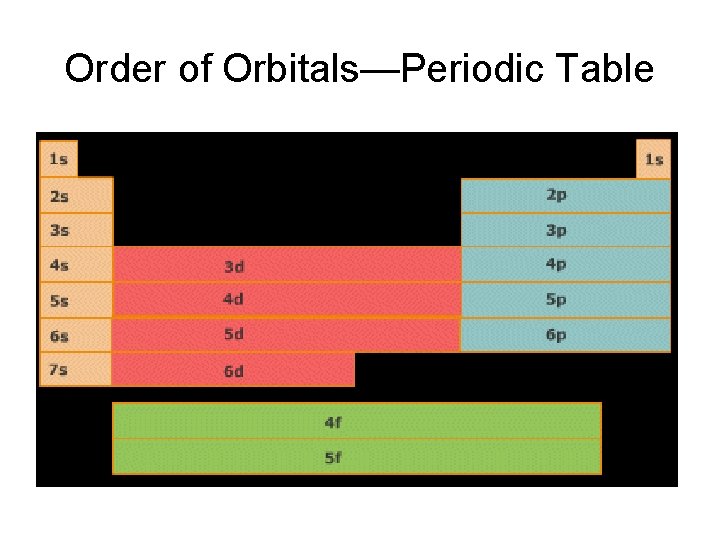
Electron Energy Level Notes • An easy way to remember this is to use the periodic table--it is arranged to show these orbitals are filled.

Order of Orbitals—Periodic Table

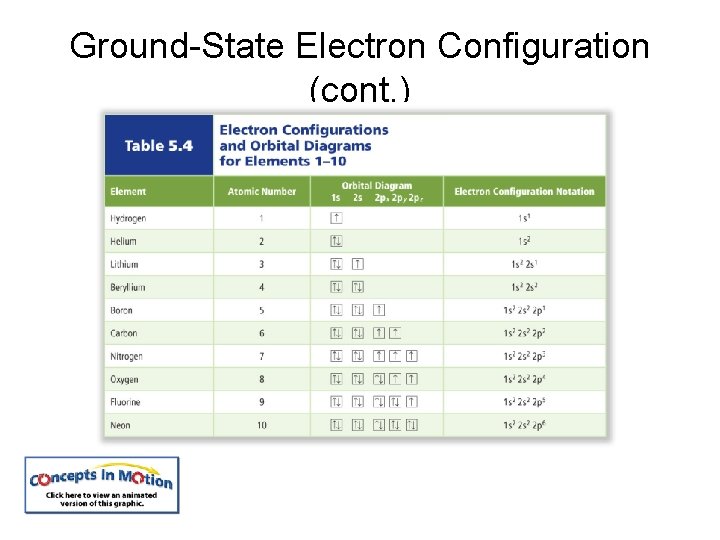
The arrangement of electrons in the atom is called the electron configuration. The aufbau principle states that each electron occupies the lowest energy orbital available.

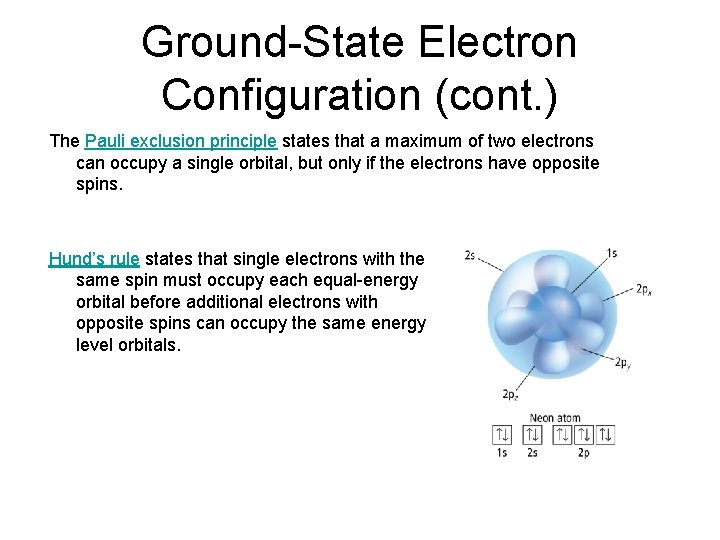
Ground-State Electron Configuration (cont. ) The Pauli exclusion principle states that a maximum of two electrons can occupy a single orbital, but only if the electrons have opposite spins. Hund’s rule states that single electrons with the same spin must occupy each equal-energy orbital before additional electrons with opposite spins can occupy the same energy level orbitals.

Electron Energy Level Notes • Hund’s rule is used for filling orbitals with electrons. It states that only one electron will be put in each orbital of a sublevel until all of them are filled, and after that, they may be paired up until the sublevel is full.

Ground-State Electron Configuration (cont. )

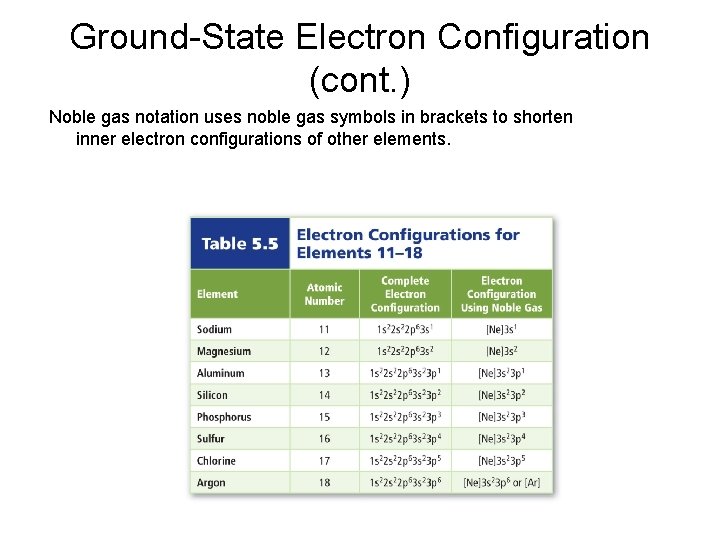
Ground-State Electron Configuration (cont. ) Noble gas notation uses noble gas symbols in brackets to shorten inner electron configurations of other elements.

Ground-State Electron Configuration (cont. ) The electron configurations (for chromium, copper, and several other elements) reflect the increased stability of half-filled and filled sets of s and d orbitals.

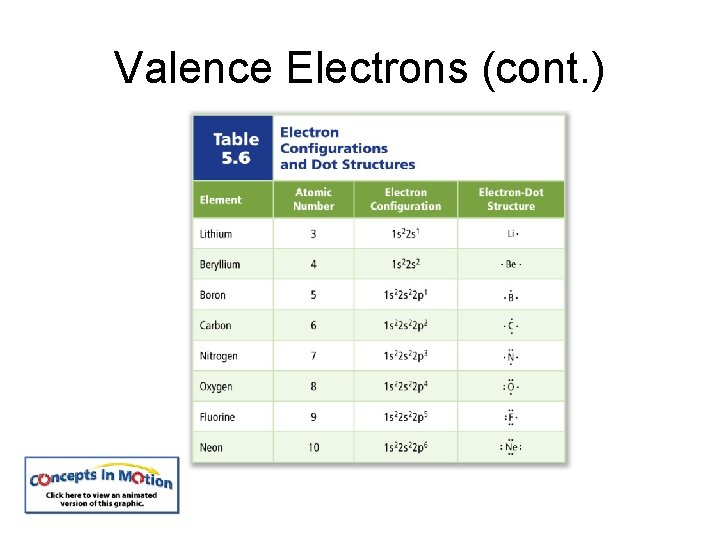
Valence Electrons Valence electrons are defined as electrons in the atom’s outermost orbitals—those associated with the atom’s highest principal energy level. Electron-dot structure consists of the element’s symbol representing the nucleus, surrounded by dots representing the element’s valence electrons.

Valence Electrons (cont. )